Association of Prenatal Alcohol Exposure and Prenatal Maternal Depression with Offspring Low-Grade Inflammation in Early Adolescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Characteristics
2.3. Instruments and Measures
2.3.1. Prenatal Alcohol Exposure
2.3.2. Maternal Self-Report on Prenatal Alcohol Consumption
2.3.3. Prenatal Depressive Symptoms
2.3.4. Assessment of High-Sensitivity CRP
2.4. Covariates
2.5. Confounders
2.6. Statistical Analysis
3. Results
3.1. Descriptive Data
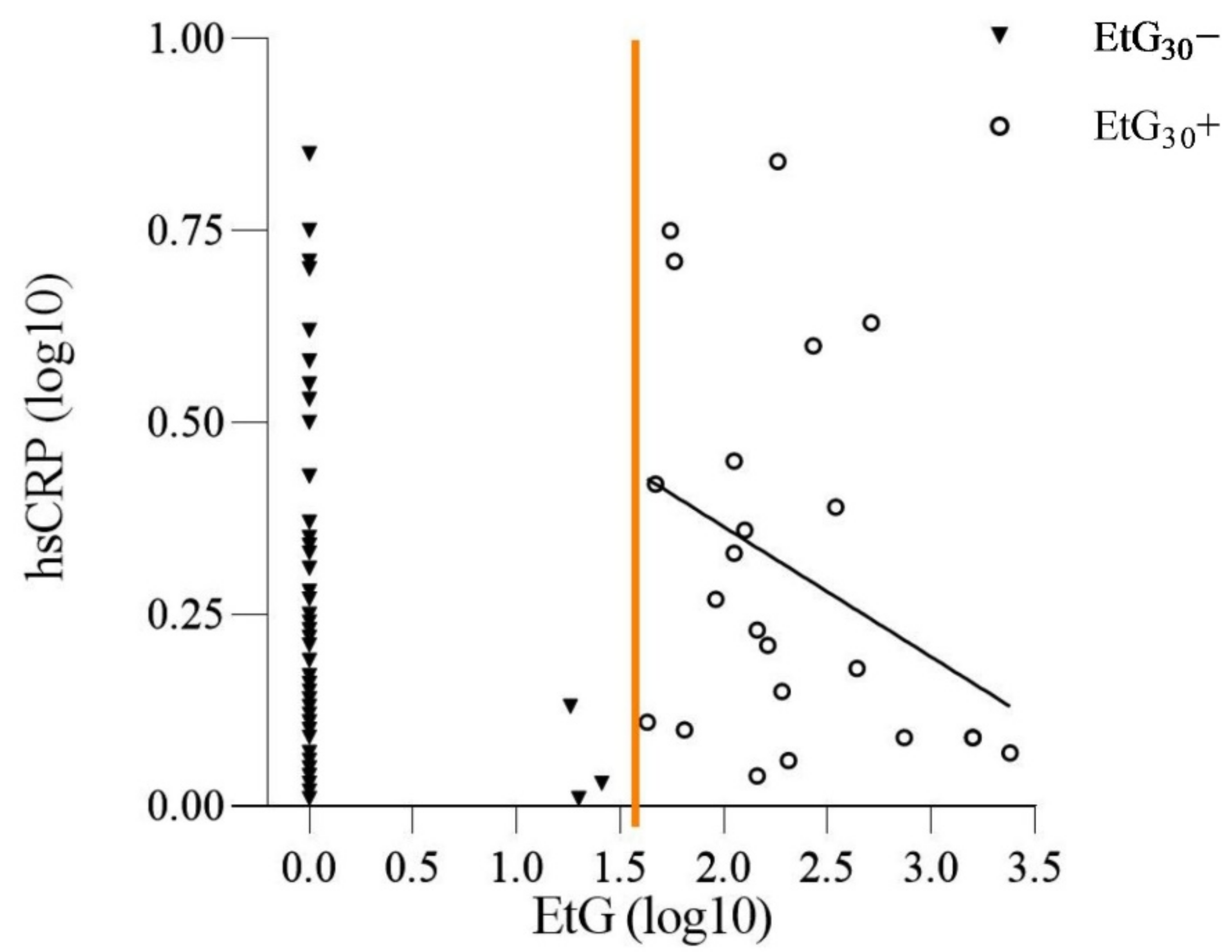
3.2. Prenatal Alcohol Exposure and the Offspring’s hsCRP Level
3.3. Prenatal Maternal Depression and the Offspring’s hsCRP Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckwalter, J.; Stanczyk, F.; McCleary, C.; Bluestein, B.; Buckwalter, D.; Rankin, K.; Chang, L.; Goodwin, T. Pregnancy, the postpartum, and steroid hormones: Effects on cognition and mood. Psychoneuroendocrinology 1999, 24, 69–84. [Google Scholar] [CrossRef]
- Hueston, W.J.; Kasik-Miller, S. Changes in functional health status during normal pregnancy. J. Fam. Pract. 1998, 47, 209–212. [Google Scholar]
- DiPietro, J.A.; Novak, M.F.S.X.; Costigan, K.A.; Atella, L.D.; Reusing, S.P. Maternal Psychological Distress During Pregnancy in Relation to Child Development at Age Two. Child Dev. 2006, 77, 573–587. [Google Scholar] [CrossRef]
- Safi-Stibler, S.; Gabory, A. Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin. Cell Dev. Biol. 2020, 97, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A conceptual framework for the developmental origins of health and disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Du Clos, T.W.; Mold, C. C-reactive protein: An activator of innate immunity and a modulator of adaptive immunity. Immunol. Res. 2004, 30, 261–277. [Google Scholar] [CrossRef]
- Murphy, K.M.; Weaver, C.; Mowat, A.; Berg, L.; Chaplin, D.; Janeway, C.A.; Travers, P.; Walport, M. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2017. [Google Scholar]
- Schmit, X.; Vincent, J.L. The time course of blood C-reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection 2008, 36, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Chung, C.S.; Grutkoski, P.S.; Song, G.Y. Mechanisms of immune resolution. Crit. Care Med. 2003, 31, S558–S571. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N. Stress and inflammation—The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Rohleder, N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef]
- Ridker, P.M.; Bassuk, S.S.; Toth, P.P. C-reactive protein and risk of cardiovascular disease: Evidence and clinical application. Curr. Atheroscler. Rep. 2003, 5, 341–349. [Google Scholar] [CrossRef]
- McDade, T.W.; Williams, S.; Snodgrass, J.J. What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 2007, 44, 899–925. [Google Scholar] [CrossRef] [PubMed]
- Brindle, E.; Fujita, M.; Shofer, J.; O’Connor, K.A. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J. Immunol. Methods 2010, 362, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.R.; Silva, S.O.; Soares, S.C. The Use of High Sensitivity C-Reactive Protein in Cardiovascular Disease Detection. J. Pharm. Pharm. Sci. 2018, 21, 496–503. [Google Scholar] [CrossRef]
- Danesh, J.; Whincup, P.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Gallimore, J.R.; Pepys, M.B. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ 2000, 321, 199–204. [Google Scholar] [CrossRef]
- Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Carrizo, T.R.; Díaz, E.I.; Áleman, M.N.; Bazán, M.C.; Abregu, A.V. Estado proinflamatorio en niños obesos. Rev. Chil. Pediatría 2018, 89, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Todendi, P.F.; Possuelo, L.G.; Klinger, E.I.; Reuter, C.P.; Burgos, M.S.; Moura, D.J.; Fiegenbaum, M.; Valim, A.R. Low-grade inflammation markers in children and adolescents: Influence of anthropometric characteristics and CRP and IL6 polymorphisms. Cytokine 2016, 88, 177–183. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Ortega, F.B.; Warnberg, J.; Sjöström, M. Associations of low-grade inflammation with physical activity, fitness and fatness in prepubertal children; the European Youth Heart Study. Int. J. Obes. 2007, 31, 1545–1551. [Google Scholar] [CrossRef]
- Lund, M.A.V.; Thostrup, A.H.; Frithioff-Bøjsøe, C.; Lausten-Thomsen, U.; Hedley, P.L.; Pedersen, O.; Christiansen, M.; Hansen, T.; Holm, J.C. Low-grade inflammation independently associates with cardiometabolic risk in children with overweight/obesity. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1544–1553. [Google Scholar] [CrossRef]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Burd, L.; Blair, J.; Dropps, K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J. Perinatol. 2012, 32, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Burd, L.; Hofer, R. Biomarkers for detection of prenatal alcohol exposure: A critical review of fatty acid ethyl esters in meconium. Birth Defects Res. Part A Clin. Mol. Teratol. 2008, 82, 487–493. [Google Scholar] [CrossRef]
- Barr, T.; Helms, C.; Grant, K.; Messaoudi, I. Opposing effects of alcohol on the immune system. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2016, 65, 242–251. [Google Scholar] [CrossRef]
- Szabo, G.; Saha, B. Alcohol’s Effect on Host Defense. Alcohol Res. Curr. Rev. 2015, 37, 159–170. [Google Scholar]
- Simons, S.S.; Beijers, R.; Cillessen, A.H.; de Weerth, C. Development of the cortisol circadian rhythm in the light of stress early in life. Psychoneuroendocrinology 2015, 62, 292–300. [Google Scholar] [CrossRef]
- Kane, C.J.; Drew, P.D. Inflammatory responses to alcohol in the CNS: Nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J. Leukoc. Biol. 2016, 100, 951–959. [Google Scholar] [CrossRef]
- Terasaki, L.S.; Gomez, J.; Schwarz, J.M. An examination of sex differences in the effects of early-life opiate and alcohol exposure. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 20150123. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Wesley, B.; Adeyiga, O.; Smith, D.M.; Da-Silva, A.; Rajguru, S. Alcohol modulates cytokine secretion and synthesis in human fetus: An in vivo and in vitro study. Alcohol 2000, 21, 207–213. [Google Scholar] [CrossRef]
- Bodnar, T.S.; Raineki, C.; Wertelecki, W.; Yevtushok, L.; Plotka, L.; Granovska, I.; Zymak-Zakutnya, N.; Pashtepa, A.; Wells, A.; Honerkamp-Smith, G.; et al. Immune network dysregulation associated with child neurodevelopmental delay: Modulatory role of prenatal alcohol exposure. J. Neuroinflamm. 2020, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, T.S.; Raineki, C.; Wertelecki, W.; Yevtushok, L.; Plotka, L.; Zymak-Zakutnya, N.; Honerkamp-Smith, G.; Wells, A.; Rolland, M.; Woodward, T.S.; et al. Altered maternal immune networks are associated with adverse child neurodevelopment: Impact of alcohol consumption during pregnancy. Brain Behav. Immun. 2018, 73, 205–215. [Google Scholar] [CrossRef]
- Raineki, C.; Bodnar, T.S.; Holman, P.J.; Baglot, S.L.; Lan, N.; Weinberg, J. Effects of early-life adversity on immune function are mediated by prenatal environment: Role of prenatal alcohol exposure. Brain Behav. Immun. 2017, 66, 210–220. [Google Scholar] [CrossRef]
- Field, T. Prenatal depression effects on early development: A review. Infant Behav. Dev. 2011, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Field, T. Prenatal Depression Risk Factors, Developmental Effects and Interventions: A Review. J. Pregnancy Child Health 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Eichler, A.; Walz, L.; Grunitz, J.; Grimm, J.; Van Doren, J.; Raabe, E.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kornhuber, J.; et al. Children of Prenatally Depressed Mothers: Externalizing and Internalizing Symptoms are Accompanied by Reductions in Specific Social-Emotional Competencies. J. Child Fam. Stud. 2017, 26, 3135–3144. [Google Scholar] [CrossRef]
- Gentile, S. Untreated depression during pregnancy: Short- and long-term effects in offspring. A systematic review. Neuroscience 2017, 342, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Madlala, S.S.; Kassier, S.M. Antenatal and postpartum depression: Effects on infant and young child health and feeding practices. S. Afr. J. Clin. Nutr. 2018, 31, 1–7. [Google Scholar] [CrossRef]
- Pacheco, A.; Figueiredo, B. Mother’s depression at childbirth does not contribute to the effects of antenatal depression on neonate’s behavioral development. Infant Behav. Dev. 2012, 35, 513–522. [Google Scholar] [CrossRef]
- Moss, K.M.; Dobson, A.J.; Mishra, G.D. Testing the role of the timing and chronicity of maternal depressive symptoms in the associations with child behaviour and development. Paediatr. Perinat. Epidemiol. 2020, 34, 678–686. [Google Scholar] [CrossRef]
- Szekely, E.; Neumann, A.; Sallis, H.; Jolicoeur-Martineau, A.; Verhulst, F.C.; Meaney, M.J.; Pearson, R.M.; Levitan, R.D.; Kennedy, J.L.; Lydon, J.E.; et al. Maternal Prenatal Mood, Pregnancy-Specific Worries, and Early Child Psychopathology: Findings From the DREAM BIG Consortium. J. Am. Acad. Child Adolesc. Psychiatry 2020, 60, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Donado, C.; Friedrich, Y.; Kossowsky, J.; Locher, C.; Koechlin, H. Exposure to Parental Depressive Symptoms: A Longitudinal Analysis on the Association with Adolescents’ Depressive Symptoms and Adjustment Problems. J. Dev. Behav. Pediatrics 2020, 41, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Tirumalaraju, V.; Suchting, R.; Evans, J.; Goetzl, L.; Refuerzo, J.; Neumann, A.; Anand, D.; Ravikumar, R.; Green, C.E.; Cowen, P.J.; et al. Risk of Depression in the Adolescent and Adult Offspring of Mothers With Perinatal Depression: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e208783. [Google Scholar] [CrossRef]
- Christian, L.M. Effects of stress and depression on inflammatory immune parameters in pregnancy. Am. J. Obstet. Gynecol. 2014, 211, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.T.; Pawlby, S.; Sharp, D.; Zunszain, P.A.; Pariante, C.M. Prenatal maternal depression is associated with offspring inflammation at 25 years: A prospective longitudinal cohort study. Transl. Psychiatry 2016, 6, e936. [Google Scholar] [CrossRef]
- Reulbach, U.; Bleich, S.; Knorr, J.; Burger, P.; Fasching, P.A.; Kornhuber, J.; Beckmann, M.W.; Goecke, T.W. Prä-, peri- und postpartale Depressivität. Erste Erkenntnisse aus FRAMES (Franconian Maternal Health Evaluation Study). Neurol. Psychiatr. 2009, 77, 708–713. [Google Scholar] [CrossRef]
- Goecke, T.W.; Burger, P.; Fasching, P.A.; Bakdash, A.; Engel, A.; Haberle, L.; Voigt, F.; Faschingbauer, F.; Raabe, E.; Maass, N.; et al. Meconium Indicators of Maternal Alcohol Abuse during Pregnancy and Association with Patient Characteristics. BioMed Res. Int. 2014, 2014, 702848. [Google Scholar] [CrossRef]
- Eichler, A.; Grunitz, J.; Grimm, J.; Walz, L.; Raabe, E.; Goecke, T.W.; Beckmann, M.W.; Kratz, O.; Heinrich, H.; Moll, G.H.; et al. Did you drink alcohol during pregnancy? Inaccuracy and discontinuity of women’s self-reports: On the way to establish meconium ethyl glucuronide (EtG) as a biomarker for alcohol consumption during pregnancy. Alcohol 2016, 54, 39–44. [Google Scholar] [CrossRef]
- Eichler, A.; Heinrich, H.; Moll, G.H.; Beckmann, M.W.; Goecke, T.W.; Fasching, P.A.; Muschler, M.-R.; Bouna-Pyrrou, P.; Lenz, B.; Kornhuber, J. Digit ratio (2D:4D) and behavioral symptoms in primary-school aged boys. Early Hum. Dev. 2018, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Eichler, A.; Stonawski, V.; Kriebel, J.; Wahl, S.; Gallati, S.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; et al. Prenatal Alcohol Exposure Is Associated With Adverse Cognitive Effects and Distinct Whole-Genome DNA Methylation Patterns in Primary School Children. Front. Behav. Neurosci. 2018, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Stonawski, V.; Frey, S.; Golub, Y.; Moll, G.H.; Heinrich, H.; Eichler, A. Affektive Belastungen der Mutter in der Schwangerschaft und assoziierte epigenetische Veränderungen beim Kind: Eine Übersicht. Z. Kinder Jugendpsychiatrie Psychother. 2018, 46, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.; Stemmler, M.; Golub, Y.; Schwenke, E.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; Moll, G.H.; Kornhuber, J.; et al. The association between prenatal alcohol consumption and preschool child stress system disturbance. Dev. Psychobiol. 2020, 63, 687–697. [Google Scholar] [CrossRef]
- Bakdash, A.; Burger, P.; Goecke, T.W.; Fasching, P.A.; Reulbach, U.; Bleich, S.; Hastedt, M.; Rothe, M.; Beckmann, M.W.; Pragst, F.; et al. Quantification of fatty acid ethyl esters (FAEE) and ethyl glucuronide (EtG) in meconium from newborns for detection of alcohol abuse in a maternal health evaluation study. Anal. Bioanal. Chem. 2010, 396, 2469–2477. [Google Scholar] [CrossRef]
- Eichler, A.; Hudler, L.; Grunitz, J.; Grimm, J.; Raabe, E.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; Moll, G.H.; et al. Effects of prenatal alcohol consumption on cognitive development and ADHD-related behaviour in primary-school age: A multilevel study based on meconium ethyl glucuronide. J. Child Psychol. Psychiatry Allied Discip. 2018, 59, 110–118. [Google Scholar] [CrossRef]
- Himes, S.K.; Dukes, K.A.; Tripp, T.; Petersen, J.; Raffo, C.; Burd, L.; Odendaal, H.; Elliott, A.J.; Hereld, D.; Signore, C.; et al. Clinical Sensitivity and Specificity of Meconium Fatty Acid Ethyl Ester, Ethyl Glucuronide, and Ethyl Sulfate for Detecting Maternal Drinking During Pregnancy. Clin. Chem. 2015, 61, 523–532. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Cox, J.L.; Chapman, G.; Murray, D.; Jones, P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J. Affect. Disord. 1996, 39, 185–189. [Google Scholar] [CrossRef]
- Murray, D.; Cox, J.L. Screening for depression during pregnancy with the edinburgh depression scale (EDDS). J. Reprod. Infant Psychol. 1990, 8, 99–107. [Google Scholar] [CrossRef]
- Gibson, J.; McKenzie-McHarg, K.; Shakespeare, J.; Price, J.; Gray, R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr. Scand. 2009, 119, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Apgar, V. A Proposal for a New Method of Evaluation of the Newborn Infant. Anesth. Analg. 1953, 32, 260–267. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- O’Connor, M.J.; Paley, B. Psychiatric conditions associated with prenatal alcohol exposure. Dev. Disabil. Res. Rev. 2009, 15, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Evans, S.H.; Anastasio, E.J. Misuse of analysis of covariance when treatment effect and covariate are confounded. Psychol. Bull. 1968, 69, 225–234. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatrics 2017, 171, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Pichini, S.; Busardò, F.P.; Garcia-Algar, O. Only Total Abstinence From Alcohol Consumption During Pregnancy Guarantees Absolute Absence of Any Deleterious Effect in the Fetus and Child. J. Stud. Alcohol Drugs 2020, 81, 220–221. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Deak, T.; Schiml-Webb, P.A. Early attachment-figure separation and increased risk for later depression: Potential mediation by proinflammatory processes. Neurosci. Biobehav. Rev. 2010, 34, 782–790. [Google Scholar] [CrossRef][Green Version]
- Nusslock, R.; Miller, G.E. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol. Psychiatry 2016, 80, 23–32. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Andersson, N.W.; Li, Q.; Mills, C.W.; Ly, J.; Nomura, Y.; Chen, J. Influence of prenatal maternal stress on umbilical cord blood cytokine levels. Arch. Women’s Ment. Health 2016, 19, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Kumsta, R.; Nelson, E.L.; Hellhammer, D.H.; Wadhwa, P.D.; Wust, S. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev. Psychobiol. 2008, 50, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Antonson, A.M.; Evans, M.V.; Galley, J.D.; Chen, H.J.; Rajasekera, T.A.; Lammers, S.M.; Hale, V.L.; Bailey, M.T.; Gur, T.L. Unique maternal immune and functional microbial profiles during prenatal stress. Sci. Rep. 2020, 10, 20288. [Google Scholar] [CrossRef] [PubMed]
- Golub, Y.; Kuitunen-Paul, S.; Panaseth, K.; Stonawski, V.; Frey, S.; Steigleder, R.; Grimm, J.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; et al. Salivary and hair cortisol as biomarkers of emotional and behavioral symptoms in 6–9 year old children. Physiol. Behav. 2019, 209, 112584. [Google Scholar] [CrossRef]
- Alink, L.R.; van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J.; Mesman, J.; Juffer, F.; Koot, H.M. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev. Psychobiol. 2008, 50, 427–450. [Google Scholar] [CrossRef]
- Abernethy, C.; McCall, K.E.; Cooper, G.; Favretto, D.; Vaiano, F.; Bertol, E.; Mactier, H. Determining the pattern and prevalence of alcohol consumption in pregnancy by measuring biomarkers in meconium. Arch. Dis. Childhood. Fetal Neonatal Ed. 2017, 103, F216–F220. [Google Scholar] [CrossRef] [PubMed]
- Bearer, C.F.; Jacobson, J.L.; Jacobson, S.W.; Barr, D.; Croxford, J.; Molteno, C.D.; Viljoen, D.L.; Marais, A.S.; Chiodo, L.M.; Cwik, A.S. Validation of a new biomarker of fetal exposure to alcohol. J. Pediatrics 2003, 143, 463–469. [Google Scholar] [CrossRef]
- Muggli, E.; O’Leary, C.; Donath, S.; Orsini, F.; Forster, D.; Anderson, P.J.; Lewis, S.; Nagle, C.; Craig, J.M.; Elliott, E.; et al. “Did you ever drink more?” A detailed description of pregnant women’s drinking patterns. BMC Public Health 2016, 16, 683. [Google Scholar] [CrossRef]
- Joya, X.; Friguls, B.; Ortigosa, S.; Papaseit, E.; Martínez, S.E.; Manich, A.; Garcia-Algar, O.; Pacifici, R.; Vall, O.; Pichini, S. Determination of maternal-fetal biomarkers of prenatal exposure to ethanol: A review. J. Pharm. Biomed. Anal. 2012, 69, 209–222. [Google Scholar] [CrossRef]
- Ygberg, S.; Nilsson, A. The developing immune system—From foetus to toddler. Acta Paediatr. 2012, 101, 120–127. [Google Scholar] [CrossRef]
- Dukes, K.; Tripp, T.; Willinger, M.; Odendaal, H.; Elliott, A.J.; Kinney, H.C.; Robinson, F.; Petersen, J.M.; Raffo, C.; Hereld, D.; et al. Drinking and smoking patterns during pregnancy: Development of group-based trajectories in the Safe Passage Study. Alcohol 2017, 62, 49–60. [Google Scholar] [CrossRef]
- Lamy, S.; Hennart, B.; Houivet, E.; Dulaurent, S.; Delavenne, H.; Benichou, J.; Allorge, D.; Marret, S.; Thibaut, F. Assessment of tobacco, alcohol and cannabinoid metabolites in 645 meconium samples of newborns compared to maternal self-reports. J. Psychiatr. Res. 2017, 90, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Shield, K.; Koren, G.; Rehm, J.; Popova, S. A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self-reports versus meconium testing: A systematic literature review and meta-analysis. BMC Pregnancy Childbirth 2014, 14, 127. [Google Scholar] [CrossRef]
- Todorow, M.; Moore, T.E.; Koren, G. Investigating the effects of low to moderate levels of prenatal alcohol exposure on child behaviour: A critical review. J. Popul. Ther. Clin. Pharmacol. 2010, 17, e323–e330. [Google Scholar]
- Freeman, J.; Condon, C.; Hamilton, S.; Mutch, R.C.; Bower, C.; Watkins, R.E. Challenges in Accurately Assessing Prenatal Alcohol Exposure in a Study of Fetal Alcohol Spectrum Disorder in a Youth Detention Center. Alcohol. Clin. Exp. Res. 2019, 43, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Muggli, E.; Cook, B.; O’Leary, C.; Forster, D.; Halliday, J. Increasing accurate self-report in surveys of pregnancy alcohol use. Midwifery 2015, 31, e23–e28. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Shen, X.; Stevenson, A.J.; Conole, E.L.S.; Harris, M.A.; Barbu, M.C.; Hawkins, E.L.; Adams, M.J.; Hillary, R.F.; Lawrie, S.M.; et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav. Immun. 2021, 92, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chaiton, M.; O’Loughlin, J.; Karp, I.; Lambert, M. Depressive Symptoms and C-Reactive Protein Are Not Associated in a Population-Based Sample of Adolescents. Int. J. Behav. Med. 2010, 17, 216–222. [Google Scholar] [CrossRef]
- Steptoe, A.; Hamer, M.; Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef]
- Buglione-Corbett, R.; Deligiannidis, K.M.; Leung, K.; Zhang, N.; Lee, M.; Rosal, M.C.; Moore Simas, T.A. Expression of inflammatory markers in women with perinatal depressive symptoms. Arch. Women’s Ment. Health 2018, 21, 671–679. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. Is depression an inflammatory disorder? Curr. Psychiatry Rep. 2011, 13, 467–475. [Google Scholar] [CrossRef]
- Scrandis, D.A.; Langenberg, P.; Tonelli, L.H.; Sheikh, T.M.; Manogura, A.C.; Alberico, L.A.; Hermanstyne, T.; Fuchs, D.; Mighty, H.; Hasday, J.D.; et al. Prepartum Depressive Symptoms Correlate Positively with C-Reactive Protein Levels and Negatively with Tryptophan Levels: A Preliminary Report. Int. J. Child. Health Hum. Dev. 2008, 1, 167–174. [Google Scholar] [PubMed]
- Matthey, S.; Ross-Hamid, C. Repeat testing on the Edinburgh Depression Scale and the HADS-A in pregnancy: Differentiating between transient and enduring distress. J. Affect. Disord. 2012, 141, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lahti, K.; Vanska, M.; Qouta, S.R.; Diab, S.Y.; Perko, K.; Punamaki, R.L. Maternal experience of their infants’ crying in the context of war trauma: Determinants and consequences. Infant Ment. Health J. 2019, 40, 186–203. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Total n = 122 | EtG n = 104 | Self-Report n = 122 | EPDS n = 122 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EtG30− (n = 81) | EtG30+ (n = 23) | Self-Report− (n = 93) | Self-Report+ (n = 29) | EPDS < 10 (n = 87) | EPDS ≥ 10 (n = 35) | |||||||||||
| M (SD) | M (SD) | M (SD) | t(df) | p | d | M (SD) | M (SD) | t(df) | p | d | M (SD) | M (SD) | t(df) | p | d | |
| EtG level | 93.08 (332.77) | 0.75 (3.92) | 418.22 (613.59) | c −29.71 (102) | 0.000 ** | 1.47 | 106.43 (375.13) | 48.58 (99.37) | c −0.76 (102) | 0.450 | 0.17 | 104.52 (363.62) | 38.39 (77.46) | c −0.42 (102) | 0.679 | 0.21 |
| EPDS score | 6.41 (4.68) | 5.44 (4.72) | 6.26 (3.76) | −0.76 (102) | 0.447 | 0.18 | 6.51 (4.60) | 6.10 (5.02) | 0.40 (120) | 0.688 | 0.08 | 3.97 (2.51) | 12.49 (2.95) | −16.09 (120) | 0.000 ** | 3.23 |
| hsCRP (mg/L) b | 0.91 (1.28) | 0.79 (1.12) | 1.43 (1.63) | a 1.77 (102) | 0.088 + | 0.52 | 0.94 (1.34) | 0.82 (1.06) | −0.43 (120) | 0.665 | 0.09 | 0.99 (1.35) | 0.73 (1.07) | −1.00 (120) | 0.317 * | 0.20 |
| Birth Weight | 3446.23 (516.87) | 3404.26 (443.24) | 3681.74 (508.44) | −2.56 (102) | 0.012 * | 0.61 | 3405.64 (535.37) | 3576.38 (435.58) | −1.56 (120) | 0.121 | 0.33 | 3419.83 (456.88) | 3511.86 (645.42) | a 0.77 (120) | 0.445 * | 0.18 |
| APGAR | 9.36 (0.74) | 9.40 (0.68) | 9.38 (0.64) | −0.13 (100) | 0.896 * | 0.03 | 9.37 (0.74) | 9.29 (0.76) | −0.47 (118) | 0.639 | 0.11 | 09.44 (0.67) | 9.14 (0.87) | a −1.83 (118) | 0.073 +* | 0.41 |
| Age (Mother at Delivery) | 32.52 (4.61) | 32.73 (4.89) | 32.26 (4.47) | −0.41 (102) | 0.681 * | 0.10 | 31.94 (4.69) | 34.38 (3.89) | −2.54 (120) | 0.012 * | 0.61 | 33.05 (4.38) | 31.20 (4.98) | −2.02 (120) | 0.061 + | 0.41 |
| Age (Offspring) | 13.32 (0.33) | 13.27 (0.29) | 13.43 (0.39) | a 1.89 (102) | 0.068+ | 0.51 | 13.31 (0.32) | 13.36 (0.36) | −0.67 (120) | 0.506 | 0.15 | 13.30 (0.32) | 13.37 (0.34) | −1.11 (120) | 0.269 * | 0.22 |
| BMI percentile | 51.21 (30.01) | 51.46 (28.59) | 57.26 (31.48) | −0.84 (101) | 0.404 * | 0.20 | 50.92 (29.12) | 52.10 (33.24) | −0.18 (119) | 0.855 | 0.04 | 51.79 (29.51) | 49.77 (31.61) | −0.33 (119) | 0.739 * | 0.07 |
| SES index | 12.02 (1.86) | 12.09 (1.86) | 11.91 (1.83) | –0.39 (102) | 0.693 * | 0.10 | 11.86 (1.93) | 12.52 (1.53) | a 1.89 (120) | 0.063 + | 0.38 | 12.16 (1.84) | 11.66 (1.90) | −1.36 (120) | 0.177 * | 0.27 |
| Total n = 122 | EtG30 n = 104 | Self-Report n = 122 | EPDS n = 122 | |||||||||||||
| EtG30− (n = 81) | EtG30+ (n = 23) | Self-Report− (n = 93) | Self-Report+ (n = 29) | EPDS < 10 (n = 87) | EPDS ≥ 10 (n = 35) | |||||||||||
| n | n | n | Χ2(df) | p | Φ d | n | n | Χ2(df) | p | Φ d | n | n | Χ2(df) | p | Φ d | |
| Sex assigned at birth (female/male) | 59/63 | 36/45 | 14/9 | 1.94 (1) | 0.164 | 0.14 | 47/46 | 12/17 | 0.74 (1) | 0.389 | 0.08 | 37/50 | 22/13 | 4.13 (1) | 0.042 * | 0.18 |
| Prenatal Maternal Smoking (yes/no) | 10/112 | 8/73 | 2/21 | 0.03 (1) | 0.865 | 0.02 | 8/85 | 2/27 | 0.09 (1) | 0.770 | 0.03 | 10/77 | 0/35 | 4.38 (1) | 0.036 * | 0.19 |
| Anti-Inflammatory Medicine (yes/no) | 12/110 | 5/76 | 2/21 | 0.18 (1) | 0.670 | 0.04 | 10/83 | 2/27 | 0.37 (1) | 0.543 | 0.06 | 7/80 | 5/30 | 1.09 (1) | 0.295 | 0.09 |
| Migration Background (yes/no) | 13/109 | 9/72 | 2/21 | 0.11 (1) | 0.740 | 0.03 | 9/84 | 4/25 | 0.39 (1) | 0.531 | 0.06 | 10/77 | 3/32 | 0.22 (1) | 0.636 | 0.04 |
| Potential Confounders | hsCRP (mg/L) | |
|---|---|---|
| r | p | |
| Birth Weight | 0.07 | 0.467 |
| APGAR | 0.05 | 0.606 |
| Age (Mother at Delivery) | 0.08 | 0.390 |
| Age (Offspring) | −0.12 | 0.176 |
| BMI | 0.39 | <0.001 ** |
| SES | 0.02 | 0.858 |
| t | p | |
| Sex | 0.07 | 0.945 |
| Prenatal Maternal Smoking | 0.75 | 0.458 |
| Anti-Inflammatory Medicine | 0.02 | 0.987 |
| Migration Background | −0.36 | 0.723 |
| Risk Groups | n | Self+ | Self− | χ2(1) p φ |
|---|---|---|---|---|
| EtG+ | 23 | 8 (34.8%) | 15 (65.2%) | 2.28 |
| EtG− | 81 | 16 (19.8%) | 65 (80.2%) | 0.131 |
| n | 24 | 80 | 0.15 | |
| EPDS+ | EPDS− | |||
| EtG+ | 23 | 5 (21.7%) | 18 (78.3%) | 0.41 |
| EtG− | 81 | 13 (16.0%) | 68 (84.0%) | 0.524 |
| n | 18 | 86 | 0.06 | |
| EPDS+ | EPDS− | |||
| EPDS+ | 35 | 7 (20.0%) | 28 (80.0%) | 0.39 |
| EPDS− | 87 | 22 (25.3%) | 65 (74.7%) | 0.535 |
| n | 29 | 93 | −0.06 |
| ANCOVA Models | F(df/df) | p | ηp2 |
|---|---|---|---|
| EtG30 | |||
| Total Model | 10.49 (2/100) | <0.001 ** | 0.17 |
| EtG30 | 4.52 (1/100) | <0.036 * | 0.04 |
| Current BMI | 14.95 (1/100) | <0.001 ** | 0.13 |
| Self-Report | |||
| Total Model | 10.88 (2/118) | <0.001 ** | 0.16 |
| Self-Report | 0.08 (1/118) | 0.780 | 0.00 |
| Current BMI | 21.72 (1/118) | <0.001 ** | 0.16 |
| EPDS | |||
| Total Model | 11.33 (2/118) | <0.001 ** | 0.16 |
| EPDS | 0.84 (1/118) | <0.360 | 0.01 |
| Current BMI | 21.54 (1/118) | <0.001 ** | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maschke, J.; Roetner, J.; Bösl, S.; Plank, A.-C.; Rohleder, N.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; Moll, G.H.; et al. Association of Prenatal Alcohol Exposure and Prenatal Maternal Depression with Offspring Low-Grade Inflammation in Early Adolescence. Int. J. Environ. Res. Public Health 2021, 18, 7920. https://doi.org/10.3390/ijerph18157920
Maschke J, Roetner J, Bösl S, Plank A-C, Rohleder N, Goecke TW, Fasching PA, Beckmann MW, Kratz O, Moll GH, et al. Association of Prenatal Alcohol Exposure and Prenatal Maternal Depression with Offspring Low-Grade Inflammation in Early Adolescence. International Journal of Environmental Research and Public Health. 2021; 18(15):7920. https://doi.org/10.3390/ijerph18157920
Chicago/Turabian StyleMaschke, Janina, Jakob Roetner, Sophia Bösl, Anne-Christine Plank, Nicolas Rohleder, Tamme W. Goecke, Peter A. Fasching, Matthias W. Beckmann, Oliver Kratz, Gunther H. Moll, and et al. 2021. "Association of Prenatal Alcohol Exposure and Prenatal Maternal Depression with Offspring Low-Grade Inflammation in Early Adolescence" International Journal of Environmental Research and Public Health 18, no. 15: 7920. https://doi.org/10.3390/ijerph18157920
APA StyleMaschke, J., Roetner, J., Bösl, S., Plank, A.-C., Rohleder, N., Goecke, T. W., Fasching, P. A., Beckmann, M. W., Kratz, O., Moll, G. H., Lenz, B., Kornhuber, J., Eichler, A., & IMAC-Mind-Consortium. (2021). Association of Prenatal Alcohol Exposure and Prenatal Maternal Depression with Offspring Low-Grade Inflammation in Early Adolescence. International Journal of Environmental Research and Public Health, 18(15), 7920. https://doi.org/10.3390/ijerph18157920







