Pristine and Magnetic Kenaf Fiber Biochar for Cd2+ Adsorption from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Biochar Preparation
2.2.2. Magnetic Biochar Preparation
2.3. Characterizations
2.4. Adsorption Study
2.5. Kinetics, Isotherm, and Thermodynamic Analysis
3. Results and Discussion
3.1. Biochar Characteristic
3.1.1. Ultimate and Physical Properties
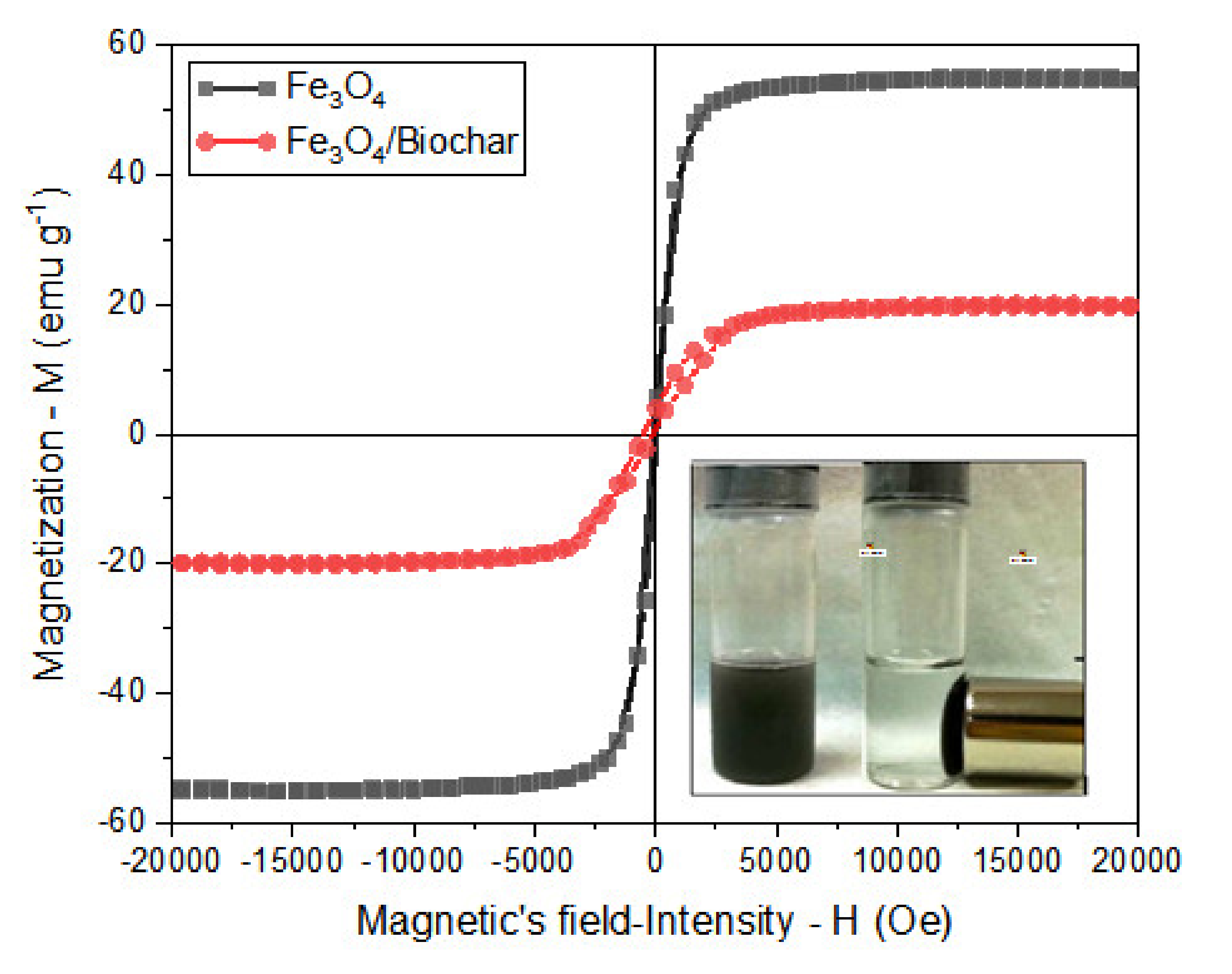
3.1.2. Magnetic Properties
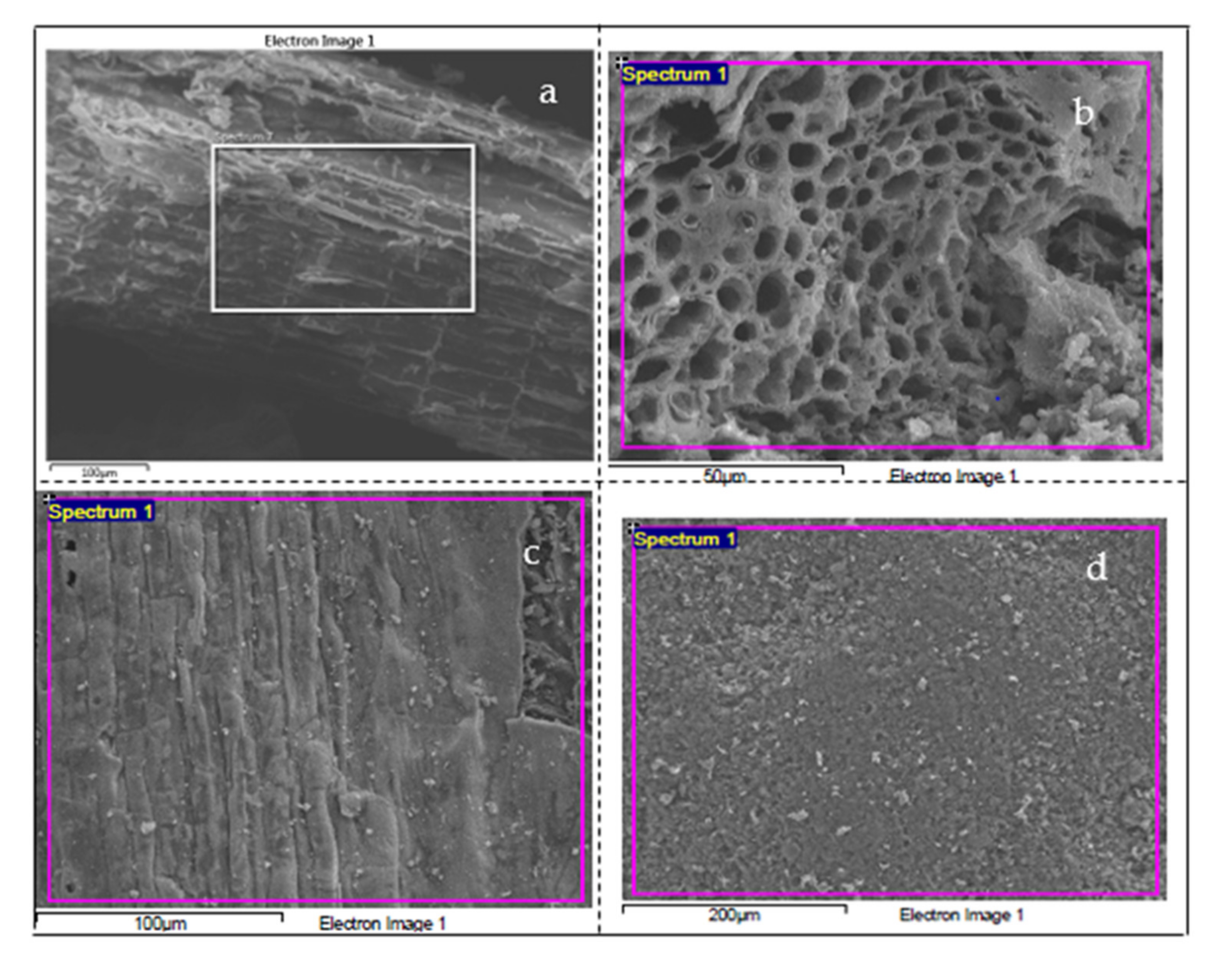
3.1.3. Microstructure Analysis
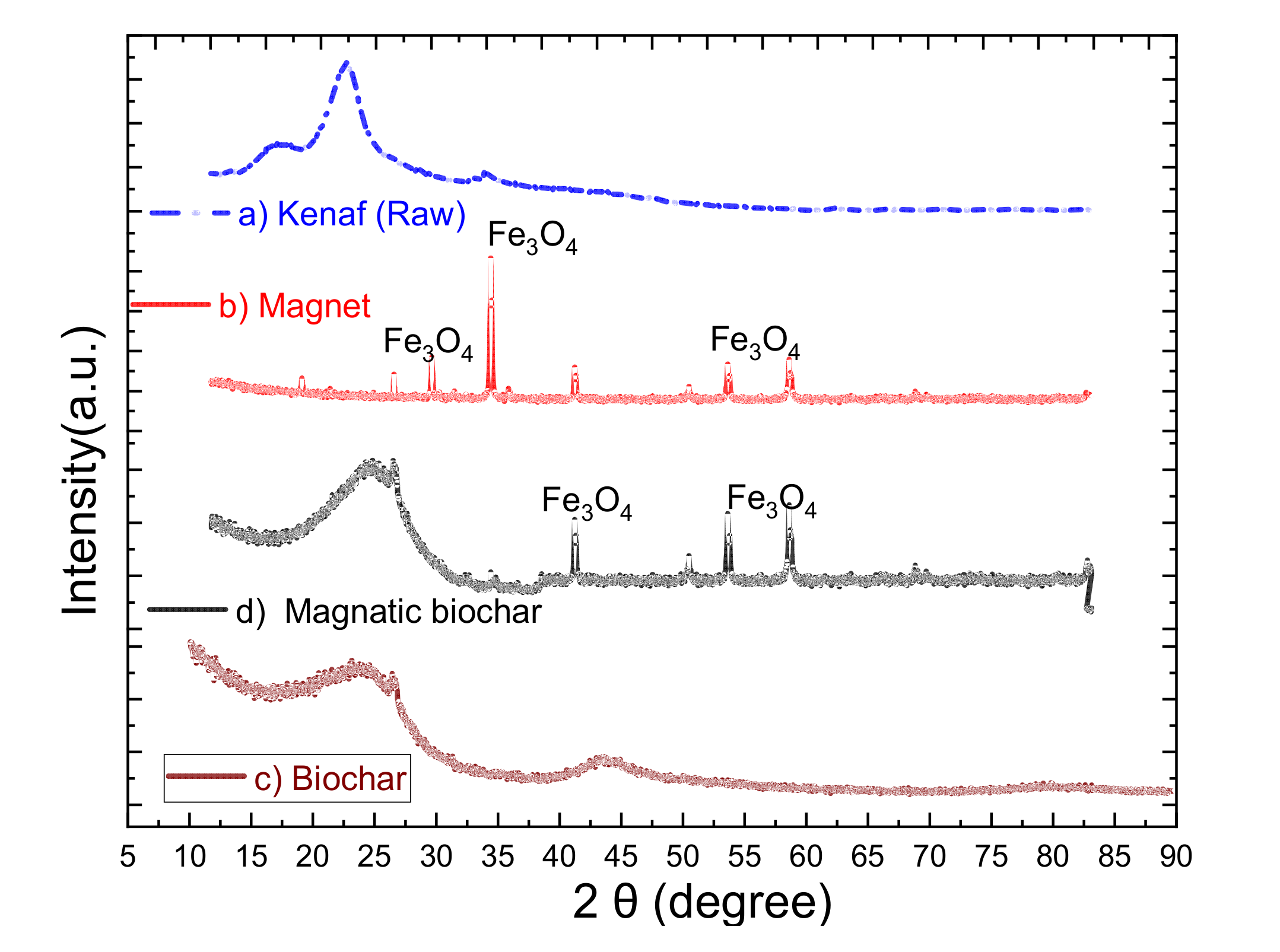
3.1.4. XRD Analysis
3.1.5. Surface Functional Group
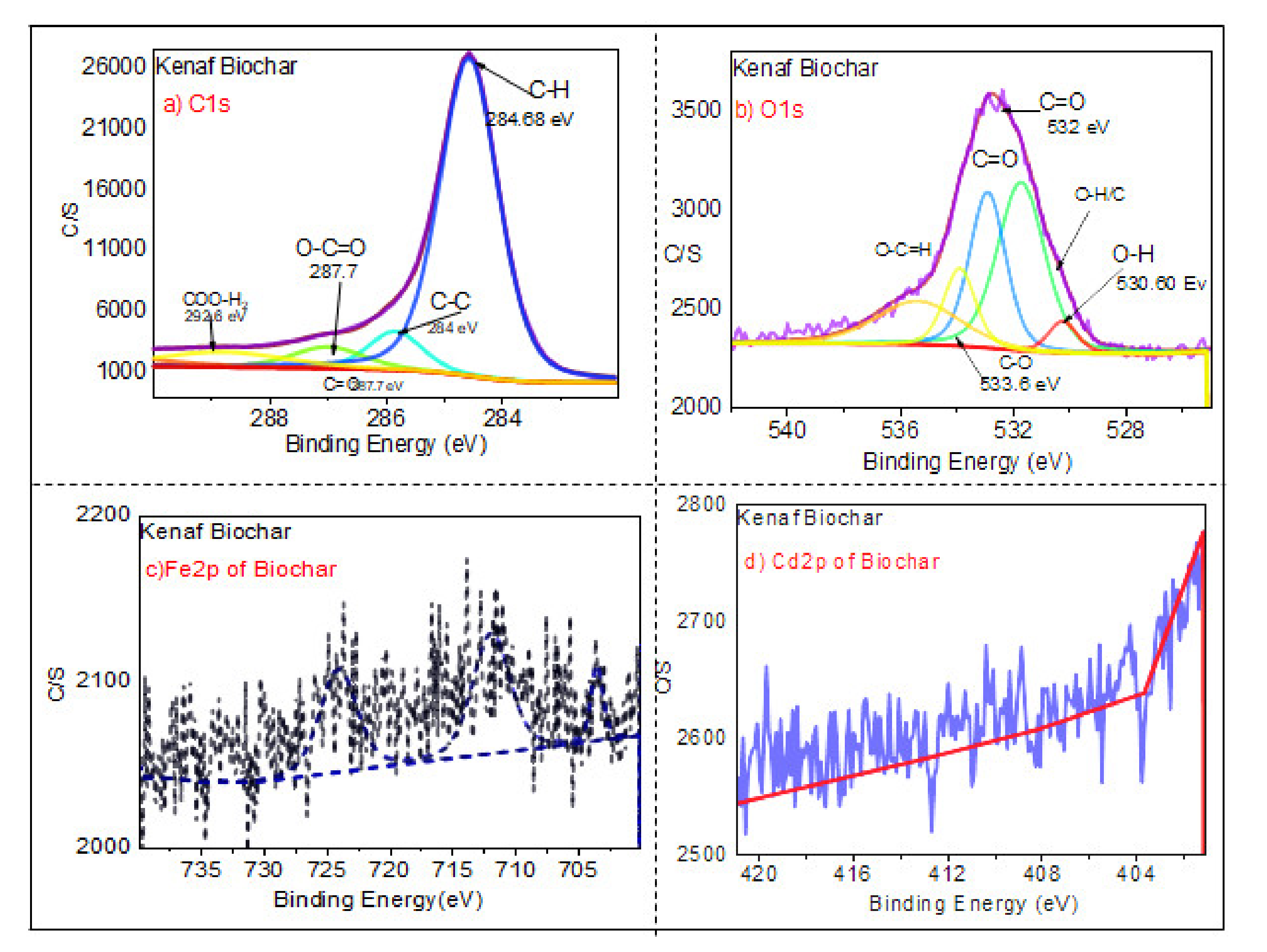
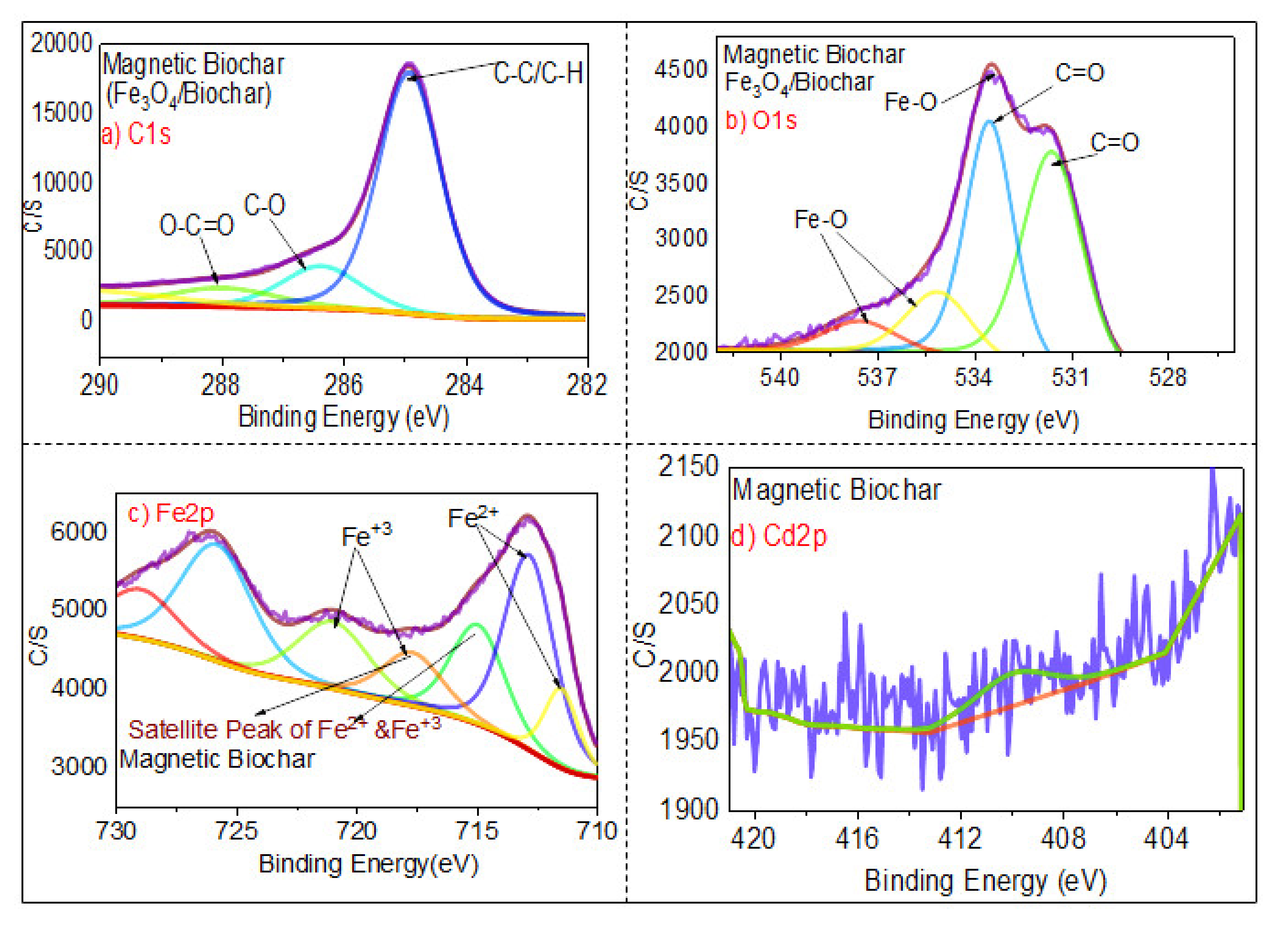
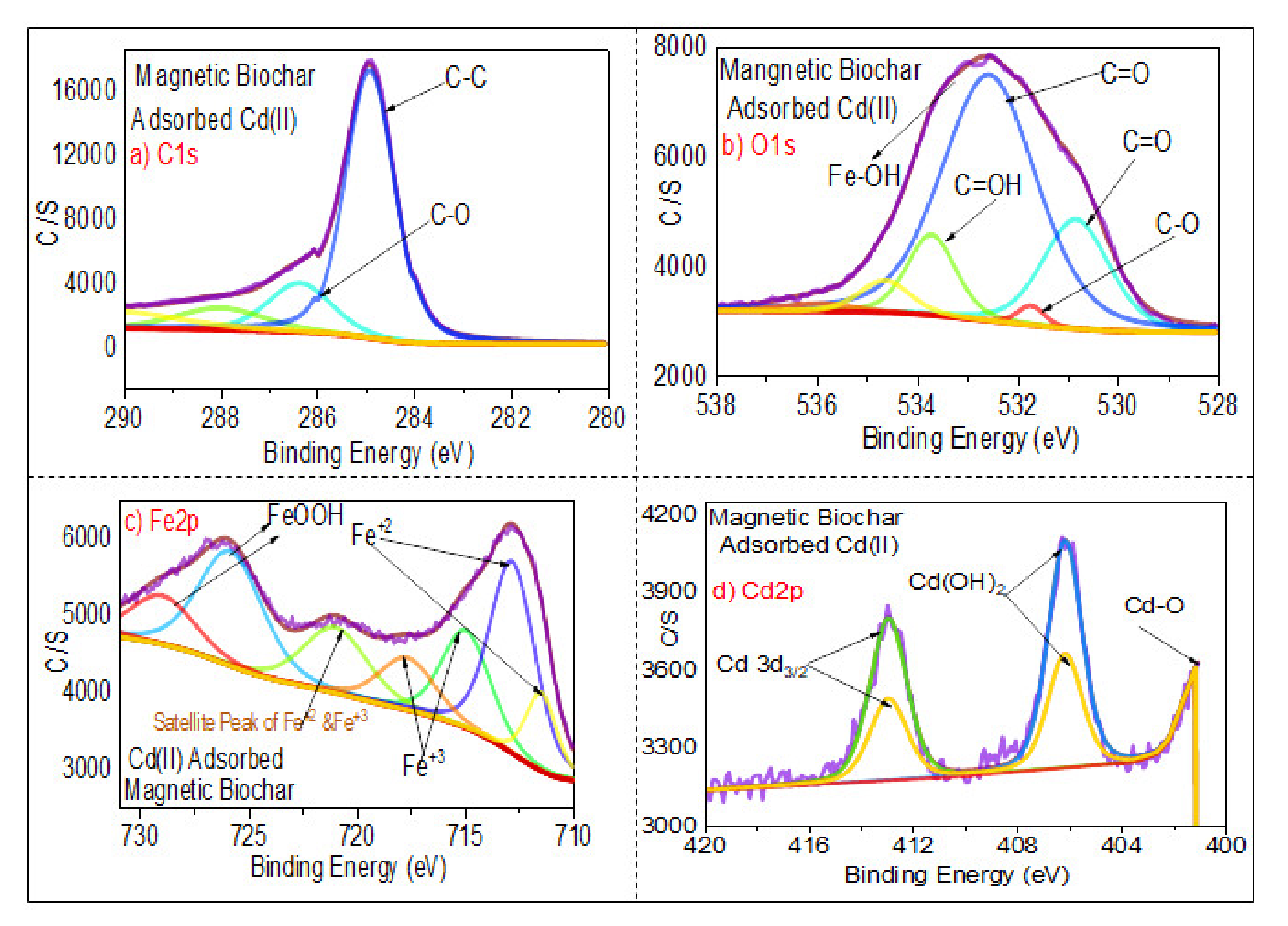
3.1.6. XPS Analysis
3.2. Adsorption Kinetic Analysis
3.3. Adsorption Isotherm Analysis
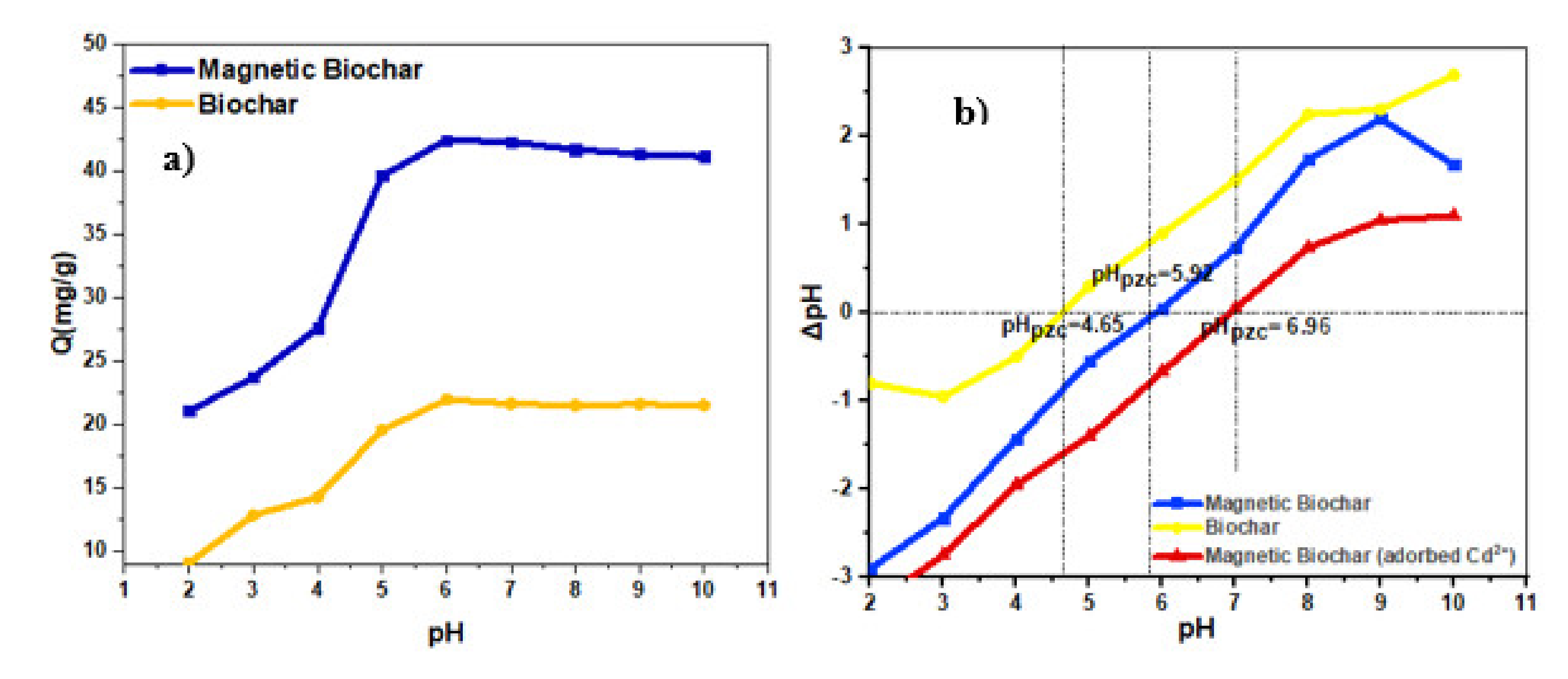
3.4. Effect of pH
3.5. Effect of Temperature
3.6. Performance Assessment of Magnetic Biochar in Comparison with Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, M.; Tabassum, S.; Shah, N.S.; Khan, J.A.; Shah, L.A.; Rehman, F.; Khan, S.U.; Khan, H.M.; Ullah, M. Acid fuchsin dosimeter: A potential dosimeter for food irradiation dosimetry. J. Food Meas. Charact. 2018, 13, 707–715. [Google Scholar] [CrossRef]
- Saeed, A.; Harun, N.; Sufian, S.; Siyal, A.; Zulfiqar, M.; Bilad, M.; Vagananthan, A.; Al-Fakih, A.; Ghaleb, A.; Almahbashi, N. Eucheuma cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye. Sustainability 2020, 12, 10318. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Abubakar, S.; Nasara, M.A.; Jagaba, S.M.; Chamah, H.M.; Lawal, I.M. Defluoridation of Drinking Water by Activated Carbon Prepared from Tridax Procumbens Plant (A Case Study of Gashaka Village, Hong L. G. A., Adamawa State, Nigeria). Int. J. Comput. Theor. Chem. 2019, 7, 1. [Google Scholar] [CrossRef]
- Waalkes, M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Nasef, M.M.; Afolabi, H.K.; Ghaleb, A.A.S. Removal of Cadmium from Aqueous Solution by Optimized Magnetic Biochar Using Response Surface Methodology. ICCOEE 2021 2021, 119–126. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Cadmium Toxicity and Treatment. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Kavand, M.; Eslami, P.; Razeh, L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. J. Water Process. Eng. 2020, 34, 101151. [Google Scholar] [CrossRef]
- Hezam Saeed, A.A.; Harun, N.Y.; Sufian, S.; Bin Aznan, M.F. Effect of Adsorption Parameter on the Removal of Nickel (II) by Low-Cost Adsorbent Extracted From Corn Cob. Int. J. Adv. Res. Eng. Technol. 2020, 11, 981–989. [Google Scholar]
- Chowdhury, P.; Elkamel, A.; Ray, A.K. Photocatalytic Processes for the Removal of Toxic Metal Ions. Heavy Met. Water Presence Remov. Saf. 2014, 25–43. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, M.; Chen, J.; Ding, Z.; Wang, X.; Mu, Y.; Meng, C. Modification of synthetic zeolite X by thiourea and its adsorption for Cd (II). Mater. Lett. 2019, 236, 233–235. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Saimon, N.N.; Ali, M.W.; Kidam, K.; Jusoh, Y.M.; Jusoh, M.; Zakaria, Z.Y. Effect of particle size on the explosive characteristics of grain (Wheat) starch in a closed cylindrical vessel. Chem. Eng. Trans. 2018, 63, 571–576. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total. Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.F.M.A.; Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Foo, K.Y. Semi-aerobic stabilized landfill leachate treatment by ion exchange resin: Isotherm and kinetic study. Appl. Water Sci. 2015, 7, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Bogusz, A.; Oleszczuk, P. Effect of biochar addition to sewage sludge on cadmium, copper and lead speciation in sewage sludge-amended soil. Chemosphere 2020, 239, 124719. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Hayder, G.; Baloo, L.; Ghaleb, A.A.S.; Lawal, I.M.; Abubakar, S.; Al-dhawi, B.N.S.; Almahbashi, N.M.Y.; Umaru, I. Degradation of Cd, Cu, Fe, Mn, Pb and Zn by Moringa-oleifera, zeolite, ferric-chloride, chitosan and alum in an industrial effluent. Ain Shams Eng. J. 2020, 12, 57–64. [Google Scholar] [CrossRef]
- Al-Mekhlafi, A.-B.; Isha, A.; Chileshe, N.; Abdulrab, M.; Saeed, A.; Kineber, A. Modelling the Relationship between the Nature of Work Factors and Driving Performance Mediating by Role of Fatigue. Int. J. Environ. Res. Public Health 2021, 18, 6752. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Methodologies for removal of heavy metal ions from wastewater: An overview. Interdiscip. Environ. Rev. 2017, 18, 124–142. [Google Scholar] [CrossRef]
- Baloo, L.; Isa, M.H.; Bin Sapari, N.; Jagaba, A.H.; Wei, L.J.; Yavari, S.; Razali, R.; Vasu, R. Adsorptive removal of methylene blue and acid orange 10 dyes from aqueous solutions using oil palm wastes-derived activated carbons. Alex. Eng. J. 2021, 60, 5611–5629. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.-V.N.; Arun, J.; Gopinath, K.P. Production, characterization, activation and environmental applications of engineered biochar: A review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Kılıç, M.; Çepelioğullar; Pütün, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2015, 46, 406–433. [Google Scholar] [CrossRef]
- Komkiene, J.; Baltrenaite, E. Biochar as adsorbent for removal of heavy metal ions [Cadmium (II), Copper (II), Lead (II), Zinc (II)] from aqueous phase. Environ. Sci. Technol. 2016, 13, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Harun, N.Y.; Han, T.J.; Vijayakumar, T.; Saeed, A.; Afzal, M. Ash Deposition Characteristics of Industrial Biomass Waste and Agricultural Residues. Mater. Today Proc. 2019, 19, 1712–1721. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Nasef, M.M. Physicochemical characterization of different agricultural residues in malaysia for bio char production. Int. J. Civ. Eng. Technol. 2019, 10, 213–225. [Google Scholar]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Afolabi, H.K.; Al-Qadami, E.H.H.; Roslan, F.A.S.; Rahim, S.A.; Ghaleb, A.A.S. Production and Characterization of Rice Husk Biochar and Kenaf Biochar for Value-Added Biochar Replacement for Potential Materials Adsorption. Ecol. Eng. Env. Tech. 2021, 22, 1–8. [Google Scholar]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem. Eng. J. 2019, 360, 212–220. [Google Scholar] [CrossRef]
- Thompson, K.; Shimabuku, K.K.; Kearns, J.; Knappe, D.; Summers, R.S.; Cook, S.M. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef] [PubMed]
- Qambrani, N.A.; Rahman, M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.; Kim, S.-H.; Kang, S.-W.; Jeong, C.Y.; Jeon, J.-R.; Park, K.H.; Cho, J.-S.; Delaune, R.D.; Seo, D.-C. Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Franco, M.A.; Gómez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, B.; Diao, J. Adsorption of cadmium by biochar produced from pyrolysis of corn stalk in aqueous solution. Water Sci. Technol. 2016, 74, 1335–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Yue, X.; Li, F.; Xiao, R.; Zhang, Y.; Gu, D. Preparation of rice straw-derived biochar for efficient cadmium removal by modification of oxygen-containing functional groups. Sci. Total. Environ. 2018, 631–632, 795–802. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process. Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Petrović, J.; Stojanović, M.; Milojković, J.V.; Petrović, M.; Šoštarić, T.; Laušević, M.D.; Mihajlović, M.L. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb 2+ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Zulfani, N. Heavy Metals Capture from Water Sludge by Kenaf Fibre Activated Carbon in Batch Adsorption. J. Ecol. Eng. 2020, 21, 102–115. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Harun, N.Y.; Bilad, M.; Afzal, M.; Parvez, A.; Roslan, F.; Rahim, S.A.; Vinayagam, V.; Afolabi, H. Moisture Content Impact on Properties of Briquette Produced from Rice Husk Waste. Sustainability 2021, 13, 3069. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Rizwan, M.; Lin, Q.; Chen, H.; Li, Y.; Li, G.; Zhao, X.; Tian, Y. Synthesis, characterization and application of magnetic and acid modified biochars following alkaline pretreatment of rice and cotton straws. Sci. Total. Environ. 2020, 714, 136532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, C.; Zhang, L.; Liu, H.; Cao, B.; Liu, L.; Gong, W. Adsorption studies of cadmium onto magnetic Fe3O4@FePO4 and its preconcentration with detection by electrothermal atomic absorption spectrometry. Talanta 2018, 181, 352–358. [Google Scholar] [CrossRef]
- Mohammed, H.G.; Albarody, T.M.B.; Mustapha, M.; Sultan, N.; Al-Jothery, H. Investigate the effect of process parameters of magnetic inductively assisted spark plasma sintering (SPS) of iron oxide (Fe3O4) on microstructure behaviour—Part I. Mater. Today Proc. 2021, 42, 2106–2112. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Salih, G.H.A.; Noor, A.; Hafiz, M.F.U.B.M.; Yaro, N.S.A.; Saeed, A.A.H.; Lawal, I.M.; Birniwa, A.H.; Kilaco, A.U. Palm Oil Clinker as a Waste by-Product: Utilization and Circular Economy Potential. IntechOpen 2021. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, I.K.; Tsang, D.C.; Cao, X.; Lin, D.; Wang, L.; Graham, N.J.; Alessi, D.; Komárek, M.; Ok, Y.S.; et al. Multifunctional iron-biochar composites for the removal of potentially toxic elements, inherent cations, and hetero-chloride from hydraulic fracturing wastewater. Environ. Int. 2019, 124, 521–532. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, B.; Theng, B.K.; Wu, P.; Liu, F.; Wang, S.; Lee, X.; Chen, M.; Li, L.; Zhang, X. Formation and mechanisms of nano-metal oxide-biochar composites for pollutants removal: A review. Sci. Total. Environ. 2021, 767, 145305. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Albarody, T.; Susilawati, S.; Gohari, S.; Doyan, A.; Prayogi, S.; Bilad, M.; Alebrahim, R.; Saeed, A. Process Optimization of In Situ Magnetic-Anisotropy Spark Plasma Sintering of M-Type-Based Barium Hexaferrite BaFe12O19. Materials 2021, 14, 2650. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions. J. Environ. Manag. 2014, 146, 444–450. [Google Scholar] [CrossRef]
- Frišták, V.; Micháleková-Richveisová, B.; Víglašová, E.; Ďuriška, L.; Galamboš, M.; Moreno-Jimenéz, E.; Pipíška, M.; Soja, G. Sorption separation of Eu and As from single-component systems by Fe-modified biochar: Kinetic and equilibrium study. J. Iran. Chem. Soc. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Eng. J. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Qiu, W.; Islam, S.; Song, Z. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere 2020, 246, 125701. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Harun, N.; Sufian, S.; Bilad, M.; Nufida, B.; Ismail, N.; Zakaria, Z.; Jagaba, A.; Ghaleb, A.; Al-Dhawi, B. Modeling and Optimization of Biochar Based Adsorbent Derived from Kenaf Using Response Surface Methodology on Adsorption of Cd2+. Water 2021, 13, 999. [Google Scholar] [CrossRef]
- Wulandari, I.O.; Santjojo, D.J.D.H.; Shobirin, R.A.; Sabarudin, A. Characteristics and magnetic properties of chitosan-coated Fe3O4 nanoparticles prepared by ex-situ co-precipitation method. Rasayan J. Chem. 2017, 10, 1348–1358. [Google Scholar]
- Singh, H.; Du, J.; Singh, P.; Mavlonov, G.T.; Yi, T.H. Development of superparamagnetic iron oxide nanoparticles via direct conjugation with ginsenosides and its in-vitro study. J. Photochem. Photobiol. B Biol. 2018, 185, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zh. Fiz. Chim. 1941, 15, 296–332. [Google Scholar]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chen, D.; Yu, X.; Song, C.; Pang, X.; Huang, J.; Li, Y. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol. 2016, 218, 1303–1306. [Google Scholar] [CrossRef]
- Claoston, N.; Samsuri, A.; Husni, M.A.; Amran, M.M. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Kang, D.; Yu, X.; Ge, M.; Lin, M.; Yang, X.; Jing, Y. Insights into adsorption mechanism for fluoride on cactus-like amorphous alumina oxide microspheres. Chem. Eng. J. 2018, 345, 252–259. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q.; Yang, H.; Alam, E.; Gao, B.; Gu, D. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: Characterization, kinetics and mechanisms. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 517, 63–71. [Google Scholar] [CrossRef]
- Matos, T.; Schultz, J.; Khan, M.; Zanoelo, E.; Mangrich, A.; Araújo, B.R.; Navickiene, S.; Romao, L. Using Magnetized (Fe3O4 / Biochar Nanocomposites) and Activated Biochar as Adsorbents to Remove Two Neuro-Active Pesticides from Waters. J. Braz. Chem. Soc. 2017. [Google Scholar] [CrossRef]
- Li, H.-B.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.-B.; Yin, J.; Liu, S.-G.; Liu, Q.; Yang, Y.-S.; Zheng, Y.-M. Facile one-pot synthesis of urchin-like Fe–Mn binary oxide nanoparticles for effective adsorption of Cd(ii) from water. RSC Adv. 2016, 6, 103438–103445. [Google Scholar] [CrossRef]
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 2020, 261, 114157. [Google Scholar] [CrossRef]
- Din, S.U.; Azeez, A.; Abdin, Z.U.; Haq, S.; Hafeez, M.; Imran, M.; Hussain, S.; Alarfaji, S.S. Investigation on Cadmium Ions Removal from Water by a Nanomagnetite Based Biochar Derived from Eleocharis Dulcis. J. Inorg. Organomet. Polym. Mater. 2020, 31, 415–425. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. Role of silica mid-layer in thermal and chemical sta-bility of hierarchical Fe3O4-SiO2-TiO2 nanoparticles for improvement of lead adsorption: Kinetics, thermodynamic and deep XPS investigation. Mater. Sci. Eng. 2020, 262, 114690. [Google Scholar] [CrossRef]
- Wu, H.; Gao, G.; Zhou, X.; Zhang, Y.; Guo, S. Control on the formation of Fe3O4nanoparticles on chemically reduced graphene oxide surfaces. CrystEngComm 2012, 14, 499–504. [Google Scholar] [CrossRef]
- Yang, T.; He, R.; Nie, G.; Wang, W.; Zhang, G.; Hu, Y.; Wu, L. Creation of Hollow Calcite Single Crystals with CQDs: Synthesis, Characterization, and Fast and Efficient Decontamination of Cd(II). Sci. Rep. 2018, 8, 17603. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, P.; Athapaththu, S.; Elkamel, A.; Ray, A.K. Visible-solar-light-driven photo-reduction and removal of cadmium ion with Eosin Y-sensitized TiO2 in aqueous solution of triethanolamine. Sep. Purif.Technol. 2017, 174, 109–115. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Lee, S.Y.; Mafize, A.A.; Kahar, N.A.M.A.; Johari, K.; Rabat, N.E. Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers 2020, 12, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu (II) and Cd (II) from aqueous solutions by ferro-manganese binary oxide–biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Tong, X.-J.; Li, J.-Y.; Yuan, J.; Xu, R.-K. Adsorption of Cu(II) by biochars generated from three crop straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Rao, K.S.; Anand, S.; Venkateswarlu, P.J.B. Adsorption of cadmium (II) ions from aqueous solution by Tectona grandis LF (teak leaves powder). BioResources 2010, 5, 438–454. [Google Scholar]
- Jefferson, W.A.; Hu, C.; Liu, H.; Qu, J. Reaction of aqueous Cu–Citrate with MnO2 birnessite: Characterization of Mn dissolution, oxidation products and surface interactions. Chemosphere 2015, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Osasona, I.; Aiyedatiwa, K.; Johnson, J.; Faboya, O.L. Activated carbon from spent brewery barley husks for cadmium ion adsorption from aqueous solution. Indones. J. Chem. 2018, 18, 145–152. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Ebrahim, S.E.; M-Ridha, M.J. Competitive biosorption of Pb (II), Cr (III), and Cd (II) from synthetic wastewater onto heterogeneous anaerobic biomass in single, binary, and ternary batch systems. Desalin. Water Treat. 2014, 52, 5629–5638. [Google Scholar] [CrossRef]
- Usman, A.; Sallam, A.; Zhang, M.; Vithanage, M.; Ahmad, M.; Al-Farraj, A.; Ok, Y.S.; Abduljabbar, A.; Al-Wabel, M. Sorption Process of Date Palm Biochar for Aqueous Cd (II) Removal: Efficiency and Mechanisms. Water Air Soil Pollut. 2016, 227, 449. [Google Scholar] [CrossRef]
- Doi, A.; Khosravi, M.; Ejtemaei, M.; Nguyen, T.A.; Nguyen, A.V. Specificity and affinity of multivalent ions adsorption to kaolinite surface. Appl. Clay Sci. 2020, 190, 105557. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, P.E.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Kumar, A.; Sarswat, A.; Franco, M.A.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Environ. Health Sci. Eng. 2015, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, M.; Mubarak, N.; Sahu, J.N.; Abdullah, E.C. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Hussain, N.; Chantrapromma, S.; Suwunwong, T.; Phoungthong, K. Cadmium (II) removal from aqueous solution using magnetic spent coffee ground biochar: Kinetics, isotherm and thermodynamic adsorption. Mater. Res. Express 2020, 7, 085503. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Zhang, L. Functionalization of magnetic hollow porous oval shape NiFe2O4 as a highly selective sorbent for the simultaneous determination of five heavy metals in real samples. Talanta 2016, 161, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Trakal, L.; Veselská, V.; Šafařík, I.; Vítková, M.; Číhalová, S.; Komárek, M. Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef]
- Son, E.-B.; Poo, K.-M.; Chang, J.-S.; Chae, K.-J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total. Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Ruthiraan, M.; Abdullah, E.C.; Mubarak, N.; Noraini, M. A promising route of magnetic based materials for removal of cadmium and methylene blue from waste water. J. Environ. Chem. Eng. 2017, 5, 1447–1455. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, L.; Wu, R.-R.; Zhang, S.-M.; Xiao, R.-B. Adsorption Behavior and Relative Distribution of Cd2+ Adsorption Mechanisms by the Magnetic and Nonmagnetic Biochars Derived from Chicken Manure. Int. J. Environ. Res. Public Heal. 2020, 17, 1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Liang, W.; Huang, H.; Jiang, S.; Guo, D.; Li, M.; Zhang, Z.; Ali, A.; Wang, J.J. Removal of cadmium(II) cations from an aqueous solution with aminothiourea chitosan strengthened magnetic biochar. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Buema, G.; Lupu, N.; Chiriac, H.; Herea, D.D.; Favier, L.; Ciobanu, G.; Litu, L.F.; Harja, M. Fly ash magnetic adsorbent for cadmium ion removal from an aqueous solutions. Spring 2021, 185, 42–50. [Google Scholar] [CrossRef]





| Model | Equilibrium | Linearized | Ref. |
|---|---|---|---|
| Langmuir | [64] | ||
| Freundlich | [65] | ||
| Temkin | [66] | ||
| Pseudo-first-order | [67] | ||
| Pseudo-second-order | [68] |
| Adsorbent | C% | H% | N% | O*% | Fe% | ash | O/C | H/C | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Width (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kenaf (Raw) | 39.2 | 5.12 | 0.35 | 43.6 | N/A | 16.32 | 1.12 | 0.130 | 4.50 | 0.009819 | 2.07 |
| Biochar (550 °C) | 67.52 | 1.27 | 1.23 | 19.18 | N/A | 10.80 | 0.284 | 0.0188 | 117.70 | 0.063884 | 2.17098 |
| Magnetic Biochar | 58.32 | 1.12 | 1.06 | 25.75 | 8.34 | 13.75 | 0.442 | 0.019 | 175.55 | 0.102366 | 2.33242 |
| T(K) | Qe (mg g−1) | InKL | ΔG° (KJ mole −1) | ΔH° (kJ mole −1) | ΔS° (KJ mole −1) |
|---|---|---|---|---|---|
| 298 | 44.60 | 1.60 | −3.96 | 8.69 | 0.042 |
| 308 | 45.80 | 1.73 | −4.42 | ||
| 318 | 47.30 | 1.82 | −4.70 | ||
| Adsorbent | SSA (m2 g−1) | pH | Cd2+ Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|
| Magnetic oak wood biochar | 8.80 | 5 | 7.40 | [94] |
| (iron oxide/tangerine peel biochar) | - | 4 | 15.5 | [95] |
| Magnetic coconut shell biochar | 834 | 4.8 | 4.77 | [96] |
| Magnetic spent coffee ground biochar | 3.60 | 7 | 10.42 | [97] |
| Magnetic hollow porous oval shape NiFe2O4 | - | 5 | 16.65 | [98] |
| Grape husk/FeSO4.7H2O | 127 | 5 | 38.3 | [99] |
| Algal-magnetic biochar | 63.33 | 5 | 19.40 | [100] |
| Fe3O4@FePO4 | - | 7 | 13.51 | [49] |
| Magnetic Douglas fir biochar | 460 | 5 | 11 | [58] |
| (Mangosteen peel/Fe2o3) biochar | - | 7 | 45.662 | [101] |
| Magnetic chicken manure biochar | 5.44 | 6 | 8.1 | [102] |
| Chitosan modified magnetic biochar composite | 112.33 | 6 | 93.72 | [103] |
| Corn straw waste @ferric nitrate | 313.90 | 2–10 | 46.15 | [59] |
| Fly ash/Fe3O4 | - | 5 | 9.65 | [104] |
| Fe3O4/kenaf fiber biochar | 175.55 | 6 | 47.90 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Bilad, M.R.; Zakaria, Z.Y.; Jagaba, A.H.; Ghaleb, A.A.S.; Mohammed, H.G. Pristine and Magnetic Kenaf Fiber Biochar for Cd2+ Adsorption from Aqueous Solution. Int. J. Environ. Res. Public Health 2021, 18, 7949. https://doi.org/10.3390/ijerph18157949
Saeed AAH, Harun NY, Sufian S, Bilad MR, Zakaria ZY, Jagaba AH, Ghaleb AAS, Mohammed HG. Pristine and Magnetic Kenaf Fiber Biochar for Cd2+ Adsorption from Aqueous Solution. International Journal of Environmental Research and Public Health. 2021; 18(15):7949. https://doi.org/10.3390/ijerph18157949
Chicago/Turabian StyleSaeed, Anwar Ameen Hezam, Noorfidza Yub Harun, Suriati Sufian, Muhammad Roil Bilad, Zaki Yamani Zakaria, Ahmad Hussaini Jagaba, Aiban Abdulhakim Saeed Ghaleb, and Haetham G. Mohammed. 2021. "Pristine and Magnetic Kenaf Fiber Biochar for Cd2+ Adsorption from Aqueous Solution" International Journal of Environmental Research and Public Health 18, no. 15: 7949. https://doi.org/10.3390/ijerph18157949









