In Situ Neutral System Synthesis, Spectroscopic, and Biological Interpretations of Magnesium(II), Calcium(II), Chromium(III), Zinc(II), Copper(II) and Selenium(IV) Sitagliptin Complexes
Abstract
:1. Introduction
2. Experimental
2.1. Chemical Experiments
2.2. Biological Experiments
2.2.1. Experimental Animals
2.2.2. Drugs and Chemicals
2.2.3. Experimental Induction of Diabetes Mellitus
2.2.4. Blood Samples Collection
2.2.5. Serum Blood Glucose, C-peptide, Insulin, and HbA1c
2.2.6. Preparation of Pancreatic Tissue Homogenates
2.2.7. Determination of Oxidative Stress Markers
2.2.8. Histological Analysis of Pancreas Tissues
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemistry Part
3.1.1. Microanalytical and Molar Conductance Data
3.1.2. Electronic Spectra and Magnetic Measurements
3.1.3. Infrared Spectra
3.1.4. 1H-NMR Spectra
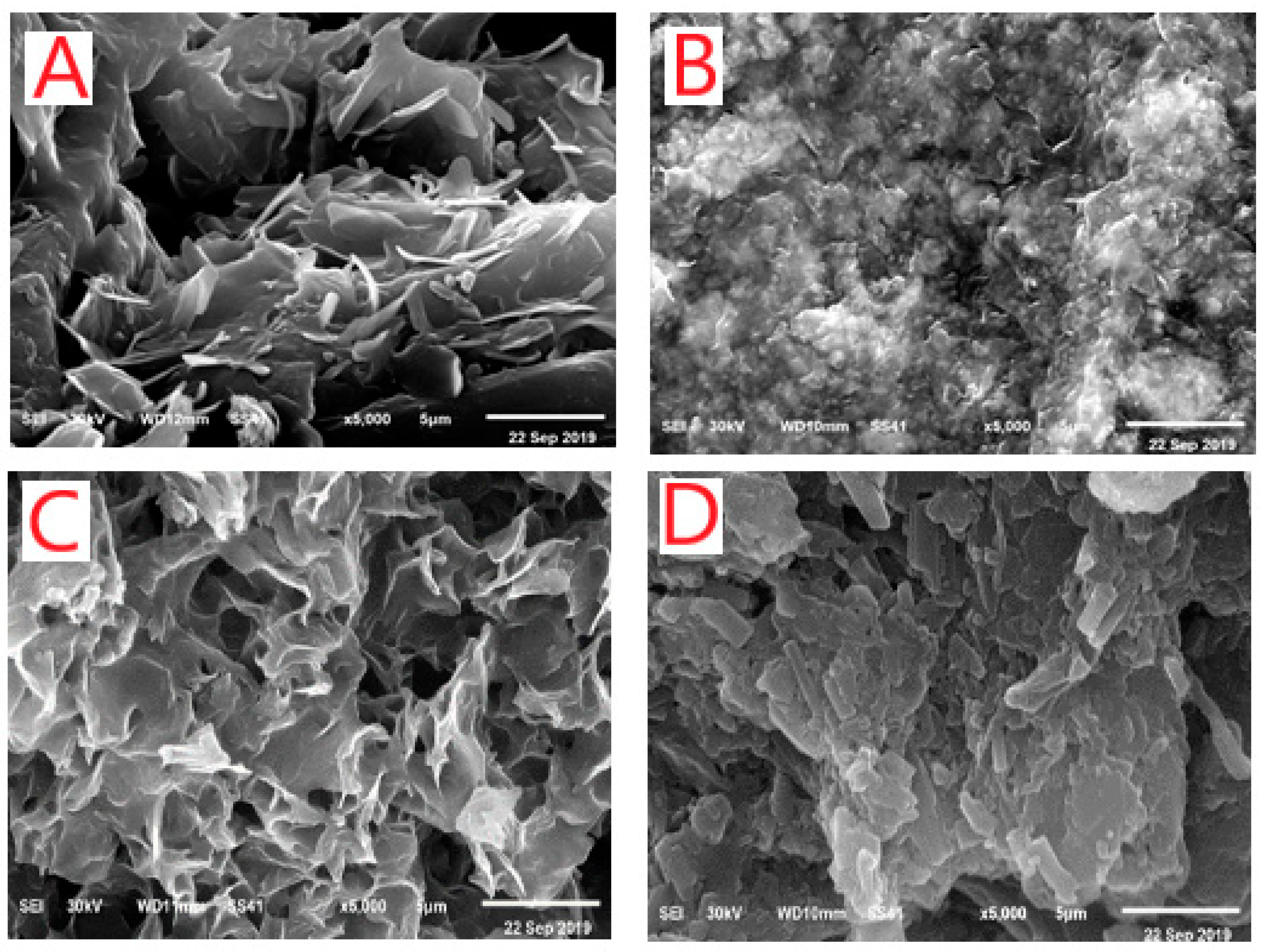
3.1.5. X-ray Powder Diffraction Patterns, SEM and TEM Morphological Studies
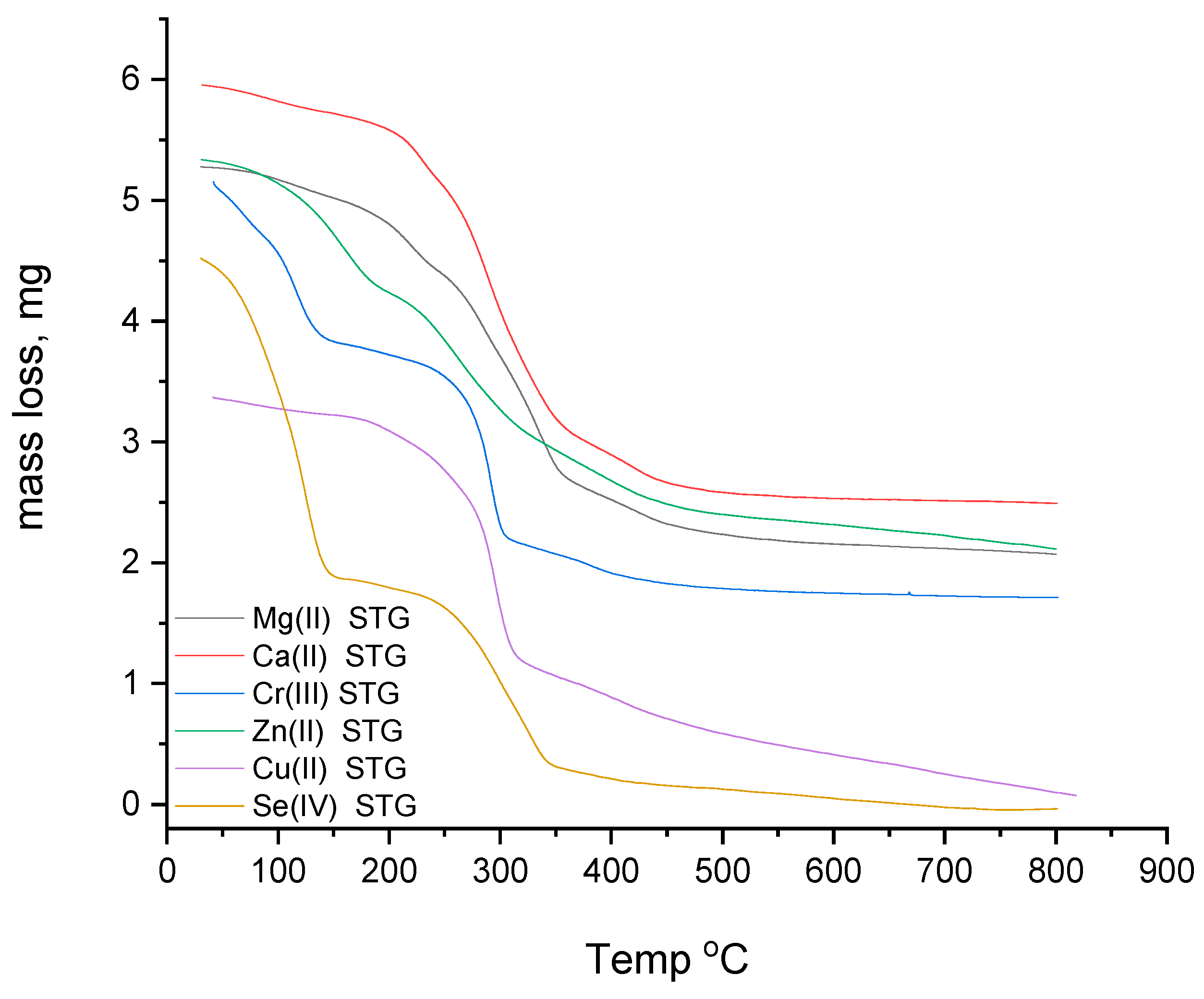
3.1.6. Thermogravimetric Study
3.2. Biology Part
3.2.1. Results
Oxidative Stress Enzymatic and Non-Enzymatic Biomarkers
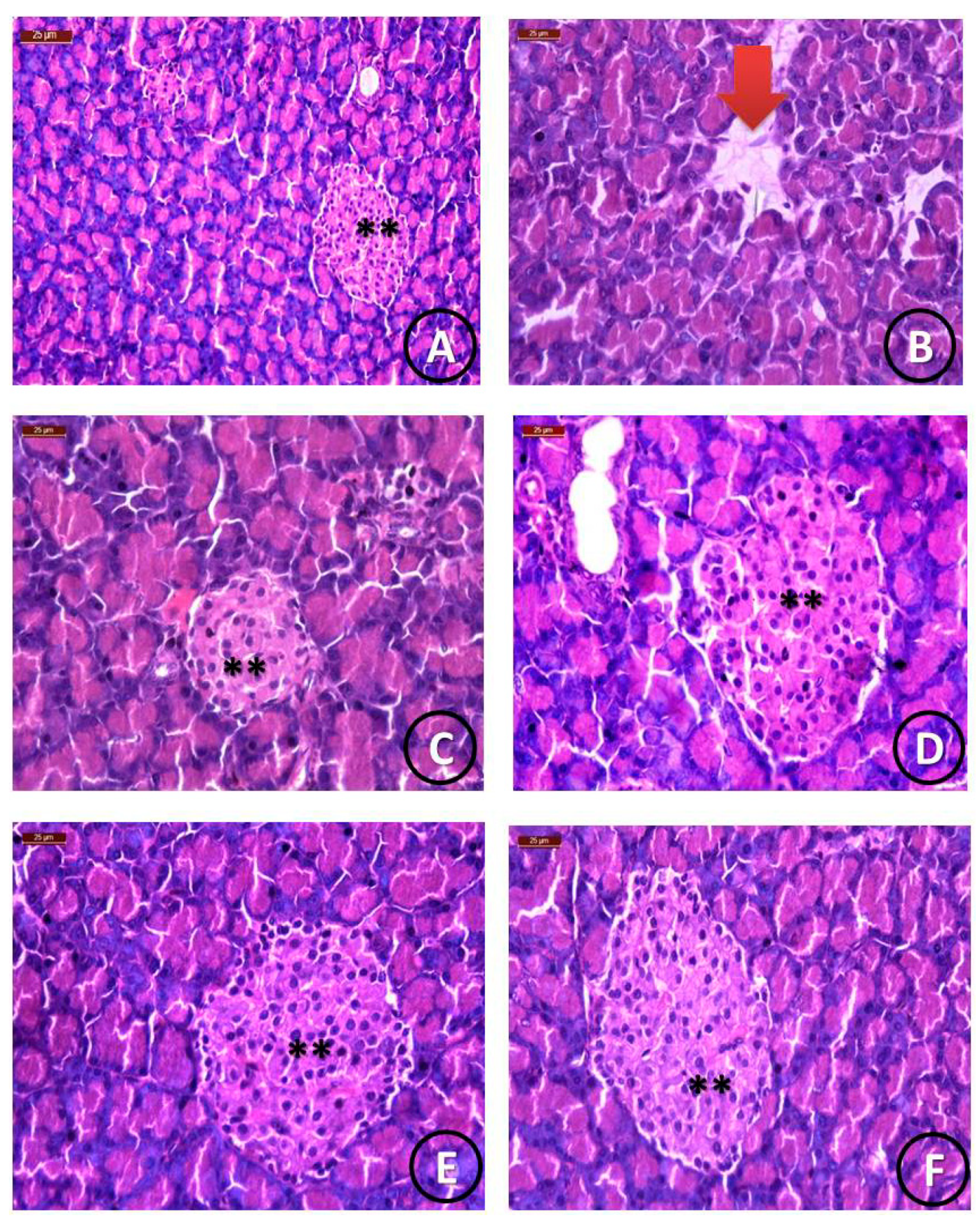
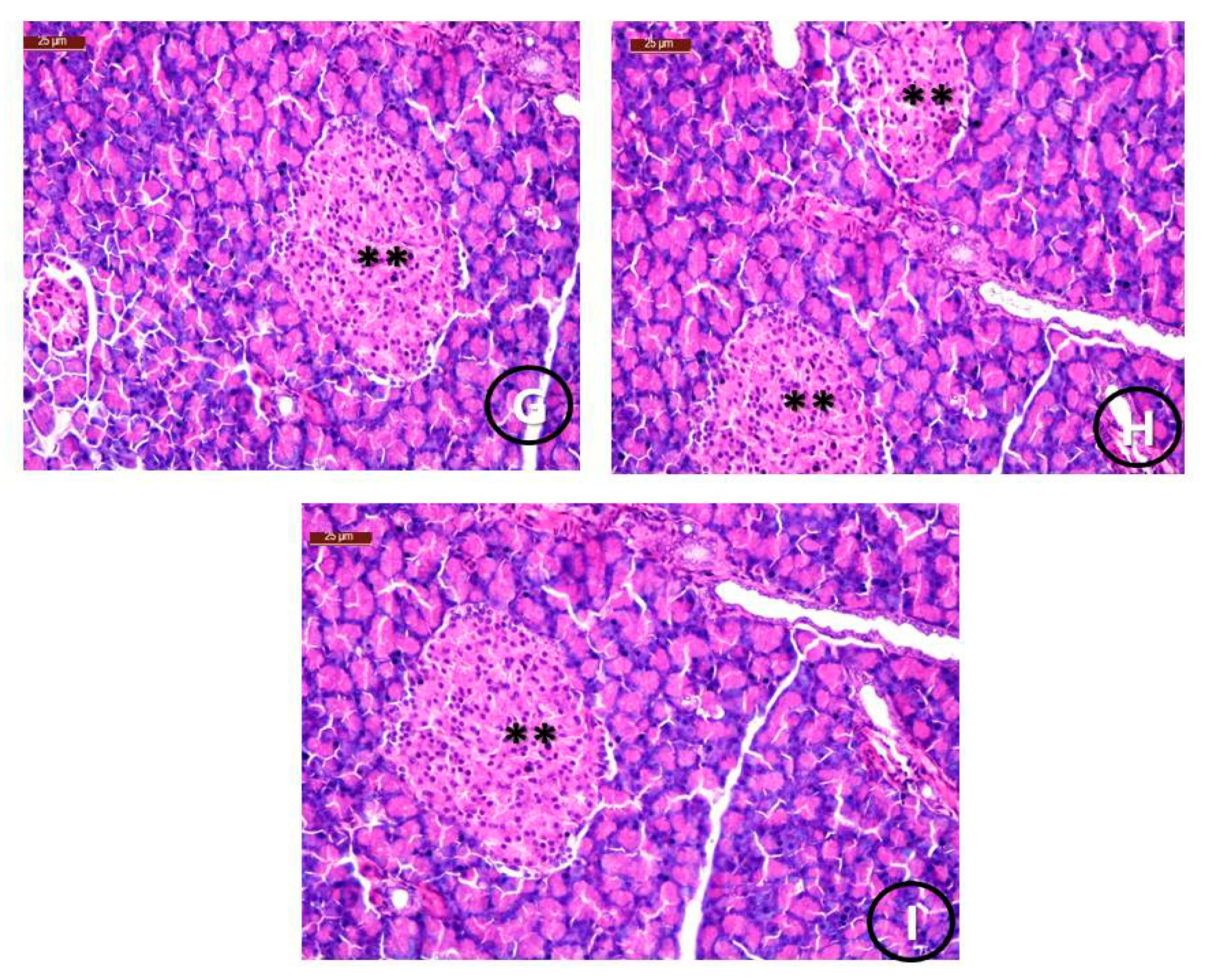
Histological Examination
3.2.2. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastassopoulou, J.; Theophanides, T. The Role of Metal Ions in Biological Systems and Medicine. In Bioinorganic Chemistry; Kessissoglou, D.P., Ed.; NATO ASI Series (Series C: Mathematical and Physical Sciences); Springer: Dordrecht, The Netherlands, 1995; Volume 459. [Google Scholar] [CrossRef]
- Refat, M.S.; El-Shazly, S.A. Identification of a new anti-diabetic agent by combining VOSO4 and vitamin E In a single molecule: Studies on its spectral, thermal and pharmacological properties. Eur. J. Med. Chem. 2010, 45, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Sharshar, T.; Elsabawy, K.M.; El-Sayed, M.Y.; Adam, A.M.A. Synthesis of new drug model has an effective antimicrobial and antitumors by combination of cephalosporin antibiotic drug with silver(I) ion in nano scale range: Chemical, physical and biological studies. J. Mol. Liq. 2017, 244C, 169–181. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z.; Almalki1, A.S.A.; Gobouri, A.A.; Alhadhrami, A.; Refat, M.S. Synthesis and speculated of new gold(III) melatonin nanometric complex structure: Drug model for testicular protection. Future Med. Chem. 2018, 10, 1693–1704. [Google Scholar] [CrossRef]
- Hamza, R.Z.; EL-Megharbel, S.M.; Altalhi, T.; Gobouri, A.A.; Alrogi, A.A. Hypolipidemic and hepatoprotective synergistic effects of selenium nanoparticles and vitamin. E against acrylamide-induced hepatic alterations in male albino mice. Appl. Organomet. Chem. 2020, 34, e5458. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z.; Refat, M.S. Preparation, spectroscopic, thermal, antihepatotoxicity, hematological parameters and liver antioxidant capacity characterizations of Cd(II), Hg(II), and Pb(II) mononuclear complexes of paracetamol anti-inflammatory drug. Spectrochim. Acta Part A 2014, 131C, 534–544. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z.; Refat, M.S. Synthesis, chemical identification and immunological evaluation studies of a novel silver(I) carbocysteine complex. Chem. Biol. Interact. 2014, 220, 169–180. [Google Scholar] [CrossRef] [PubMed]
- El-Megharbel, S.M.; Hamza, R.Z.; Refat, M.S. Synthesis, spectroscopic and thermal characterizations of vanadyl (II) adenine complex prospective as antidiabetic drug agent. Spectrochim. Acta Part A 2015, 135, 850–864. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z.; Refat, M.S. Synthesis, spectroscopic and thermal studies of Mg(II), Ca(II), Sr(II) and Ba(II) diclofenac sodium complexes as anti-inflammatory drug and their protective effects on renal functions impairment and oxidative stress. Spectrochim. Acta Part A 2015, 135, 915–928. [Google Scholar] [CrossRef]
- Refat, M.S.; El-Megharbel, S.M.; Hussien, M.A.; Hamza, R.Z.; Al-Omar, M.A.; Naglah, A.M.; Afifi, W.M.; Kobeasy, M.I. Spectroscopic, structural characterizations and antioxidant capacity of the chromium(III) niacinamide compound as a diabetes mellitus drug mode. Spectrochim. Acta Part A 2017, 173C, 122–131. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Collery, P.; Etienne, J.-C.; Theophanides, T. Metal Ions in Biology and Medicine; John Libbey Eurotext: Paris, France; London, UK, 1992; Volume 2. [Google Scholar]
- Waker, W.E.C.; Vallee, B.L. Nucleic acids and metals I. Chromium, manganese, nickel, iron, and other metals in ribonucleic acid from diverse biological sources. J.Biol. Chem. 1959, 234, 3257. [Google Scholar]
- Vaghasiya, J.; Sheth, N.; Bhalodia, Y.; Manek, R. Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul. Pept. 2011, 166, 48–54. [Google Scholar] [CrossRef]
- Makdissi, A.; Ghanim, H.; Vora, M.; Green, K.; Abuaysheh, S.; Chaudhuri, A.; Dhindsa, S. Paresh Dandona Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 2012, 97, 3333–3341. [Google Scholar] [CrossRef]
- Gumieniczeka, A.; Berecka, A.; Mroczek, T.; Wojtanowski, K.; Dąbrowska, K.; Stępień, K. Determination of chemical stability of sitagliptin by LC-UV, LC-MS and FT-IR methods. Analysis 2019, 164, 789–807. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Nunes, S.; Rolo, A.P.; Reis, F.; Palmeira, C.M. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front. Physiol. 2018, 9, 1857. [Google Scholar] [CrossRef] [PubMed]
- Sakura, H.; Hashimoto, N.; Sasamoto, K.; Ohashi, H.; Hasumi, S.; Ujihara, N.; Kasahara, T.; Tomonaga, O.; Nunome, H.; Honda, M.; et al. E_ect of sitagliptin on blood glucose control in patients with type 2 diabetes mellitus who are treatment naive or poorly responsive to existing antidiabetic drugs: The JAMP study. BMC Endocr. Disord. 2016, 16, 70. [Google Scholar] [CrossRef] [Green Version]
- Buse, J.B.; Bethel, M.A.; Green, J.B.; Stevens, S.R.; Lokhnygina, Y.; Aschner, P.; Grado, C.R.; Tankova, T.; Wainstein, J.; Josse, R.; et al. Pancreatic safety of sitagliptin in the TECOS study. Diabetes Care 2017, 40, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Vrang, N.; Jelsing, J.; Simonsen, L.; Jensen, A.E.; Thorup, I.; Soeborg, H.; Knudsen, L.B. The e_ects of 13 wk of liraglutide treatment on endocrine and exocrine pancreas in male and female ZDF rats: A quantitative and qualitative analysis revealing no evidence of drug-induced pancreatitis. AJP Endocrinol. Metab. 2012, 303, E253–E264. [Google Scholar] [CrossRef] [Green Version]
- El-Megharbel, S.M.; Hamza, R.Z.; Gobouri, A.A.; Refat, M.S. Synthesis of new antidiabetic agent by complexity between vanadyl (II) sulfate and vitamin B1: Structural, characterization, anti-DNA damage, structural alterations and antioxidative damage studies. Appl. Organomet. Chem. 2019, 33, e4892. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Shawky, L.M.; Morsi, A.A.; el Bana, E.; Hanafy, S.M. The Biological Impacts of Sitagliptin on the Pancreas of a Rat Model of Type 2 Diabetes Mellitus: Drug Interactions with Metformin. Biology 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. J. Anal. Biochem. 1968, 25, 192. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351. [Google Scholar] [CrossRef]
- Beers, J.R.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133. [Google Scholar] [CrossRef]
- Refat, M.S. Complexes of uranyl (II), vanadyl (II) and zirconyl (II) with orotic acid “vitamin B13”: Synthesis, spectroscopic, thermal studies and antibacterial activity. J. Mol. Struct. 2007, 842, 24–37. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Lever, A.B.P.; Mantovani, E. Far-infrared and electronic spectra of some bis (ethylenediamine) and related complexes of copper (II) and the relevance of these data to tetragonal distortion and bond strengths. Inorg. Chem. 1971, 10, 817–826. [Google Scholar] [CrossRef]
- Drago, R.S.; Meek, D.W.; Joosten, M.S.; Laroche, L. Spectrochemical studies of a series of amides as ligands with nickel (II) and chromium (III). Inorg. Chem. 1963, 2, 124. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiely: New York, NY, USA, 1978. [Google Scholar]
- Vein, D.L.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Silverstein, R.M.; Besslor, G.C.; Morrni, D.T.C. Spectrometric Identification of Organic Compounds, 4th ed.; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addision-Wesley Publishing Company: New York, NY, USA, 1978. [Google Scholar]
- Nelson, M.; Bhandari, N.; Wener, J. Sitagliptin-induced pancreatitis—A longer road than expected.Clin. Case Rep. 2014, 2, 149–152. [Google Scholar]
- Kelany, M.E.; Hakami, T.M.; Omar, A.H.; Abdallah, M.A. Combination of Sitagliptin and Insulin against Type 2 Diabetes Mellitus with Neuropathy in Rats: Neuroprotection and Role of Oxidative and Inflammation Stress. Pharmacology 2016, 98, 242–250. [Google Scholar] [CrossRef]
- Yang, V.L. Zinc and insulin in pancreatic beta-cells. Endocrine 2014, 45, 178–189. [Google Scholar]
- Rouse, R.; Xu, L.; Stewart, S.; Zhang, J. High fat diet and GLP-1 drugs induce pancreatic injury in mice. Toxicol. Appl. Pharmacol. 2014, 276, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Motaani, S.E.; Al-Talhi, T. Therapeutic and Ameliorative Effects of Active Compounds of Combretum Molle in the Treatment and Relief from Wounds in a Diabetes Mellitus Experimental Model. Coatings 2021, 11, 324. [Google Scholar] [CrossRef]
- Bao, W.; Morimoto, K.; Hasegawa, T.; Sasaki, N.; Yamashita, T.; Hirata, K.; Okita, Y.; Okada, K. Orally administered dipeptidyl peptidase-4 inhibitor (alogliptin) prevents abdominal aortic aneurysm formation through an antioxidant effect in rats. J. Vasc. Surg. 2014, 59, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shao, M.; Yang, H.; Chen, L.; Yu, L.; Cong, W.; Tian, H.; Zhang, F.; Cheng, P.; Jin, L.; et al. Attenuation of Hyperlipidemia- and Diabetes-Induced Early-Stage Apoptosis and Late-Stage Renal Dysfunction via Administration of Fibroblast Growth Factor-21 Is Associated with Suppression of Renal Inflammation. PLoS ONE 2013, 8, e82275. [Google Scholar] [CrossRef] [Green Version]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Wortham, M.; Sander, M. Mechanisms of β-cell functional adaptation to changes in workload. Diabetes Obes. Metab. 2016, 18, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. In Diabetes Epidemiology, Genetics, Pathogenesis, Diagnosis, Prevention, and Treatment; Bonora, E., DeFronzo, R.A., Eds.; Springer: Cham, Switzerland, 2018; pp. 181–253. [Google Scholar]
- Skovsø, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, M.S.; Hamza, R.Z. Potential Ameliorative Effects of Selenium and Chromium Supplementation Against Toxicity and Oxidative Stress in Streptozotocin Diabetic Rats. Int. J. Pharm. 2016, 12, 483–495. [Google Scholar] [CrossRef]
- Riley, D.P. Functional mimics of superoxide dismutase enzymes as therapeutic agents. Chem. Rev. 1999, 99, 2573–2588. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Diab, A.A. Testicular protective and antioxidant effects of selenium nanoparticles on Monosodium glutamate-induced testicular structure alterations in male mice. Toxicol. Rep. 2020, 7, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Al-Baqami, N.M.; Hamza, R.Z. Protective Effect of Resveratrol against Hepatotoxicity of Cadmium in Male Rats: Antioxidant and Histopathological Approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- Kramer, R. The Pharmaceutical Potential of Manganese-Based Superoxide Dismutase Mimics. Angew Chem. Int. Ed. 2000, 39, 4469–4470. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, J.; Jin, Y.; Gao, L.; Li, L.; Yang, J.; Lu, C.; Zhao, Q.H.; Xie, M. A manganese-salen complex as dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Int. J. Biol. Macromol. 2018, 120, 1232–1239. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef]
- Hocher, B.; Reichetzeder, C.; Alter, M.L. Renal and cardiac effects of DPP4 inhibitors—From preclinical development to clinical research. Kidney Blood Press Res. 2012, 36, 65–84. [Google Scholar] [CrossRef]
- Salcedo, I.; Tweedie, D.; Li, Y.; Greig, N.H. Neuroprotective and neurotrophic actions of glucagon- like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012, 166, 1586–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeel, M. Recent advances in understanding the role of oxidative stress in diabetic neuropathy. Diabetes Metab. Syndr. 2015, 9, 373–378. [Google Scholar] [CrossRef]
- Al-Baqami, N.M.; Hamza, R.Z. Synergistic antioxidant capacities of vanillin and chitosan nanoparticles against reactive oxygen species, hepatotoxicity, and genotoxicity induced by aging in male Wistar rats. Hum. Exp. Toxicol. 2021, 40, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Dawson-Hughes, B.; Li, T.; van Dam, R.M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Vitamin D and Calcium Intake in Relation to Type 2 Diabetes in Women. Diabetes Care 2006, 29, 650–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.O.; Forloines-Lynn, S.; Cross, R.E.; Heizer, W.D. Chromium deficiency after long-term total parenteral nutrition. Dig. Dis. Sci. 1986, 31, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.A.; Abtar, A.N.; Othman, H.H.; Aziz, T.A. Effects of quercetin, sitagliptin alone or in combination in testicular toxicity induced by doxorubicin in rats. Drug Des. Dev. Ther. 2019, 13, 3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
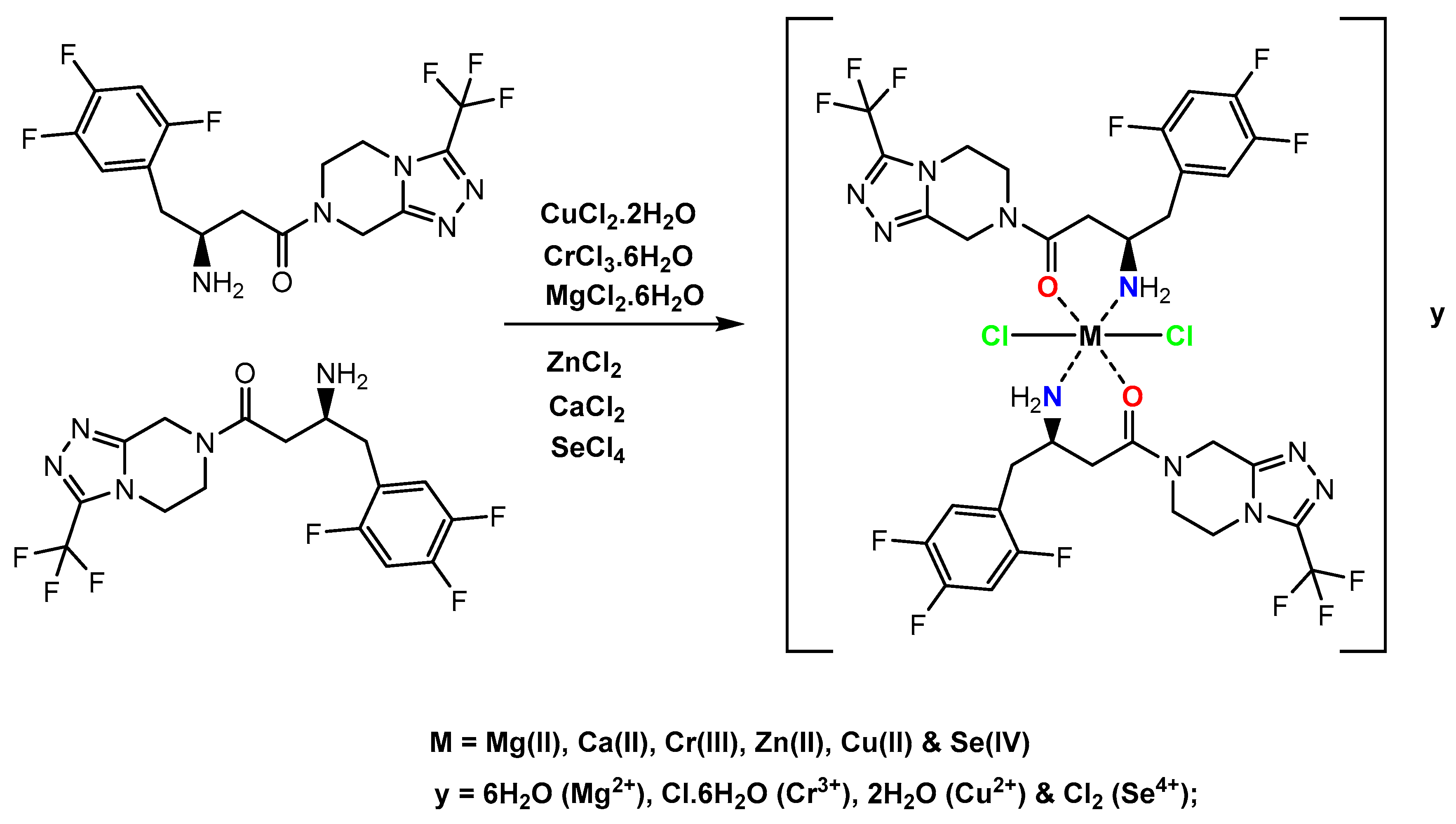



| Complexes | Molecular Weight | Elemental Analysis % Found (Calcd.) | |||
|---|---|---|---|---|---|
| C | H | N | M | ||
| [Mg(STG)2(Cl)2]·6H2O | 1017.94 | 37.46 (37.76) | 4.11 (4.16) | 13.29 (13.76) | 2.30 (2.39) |
| [Ca(STG)2(Cl)2] | 925.62 | 41.50 (41.52) | 3.20 (3.27) | 15.10 (15.13) | 4.21 (4.33) |
| [Cr(STG)2(Cl)2]Cl·6H2O | 1081.08 | 35.48 (35.55) | 3.90 (3.92) | 12.93 (12.96) | 4.80 (4.81) |
| [Zn(STG)2(Cl)2] | 950.92 | 40.39 (40.42) | 3.08 (3.18) | 14.66 (14.73) | 6.83 (6.88) |
| [Cu(STG)2(Cl)2]2·H2O | 985.12 | 39.00 (39.02) | 3.44 (3.48) | 14.20 (14.22) | 6.42 (6.45) |
| [Se(STG)2(Cl)2]Cl2 | 1035.41 | 37.09 (37.12) | 2.90 (2.92) | 13.50 (13.53) | 7.61 (7.63) |
| Assignments | Compounds | ||||||
|---|---|---|---|---|---|---|---|
| STG | Mg(II) | Ca(II) | Cr(III) | Zn(II) | Cu(II) | Se(IV) | |
| N–H stretching | 3357 | 3300 | 3316 | 3340 | 3341 | 3305 | 3340 |
| C–H stretching | 3059 2917 2850 | 2918 2850 | 2917 2849 | 2919 | 2918 2850 | 2923 2854 | 2918 2851 |
| C=O stretching | 1669 | - | - | - | - | - | - |
| C=N stretching | 1634 | 1645 | 1634 | 1652 | 1635 | 1644 | 1646 |
| NH2 bending | 1609 | - | - | - | - | - | - |
| C–C and C–N stretching | 1556 1514 1426 | 1516 | 1518 1426 | 1515 1427 | 1579 1514 1424 | 1518 1426 | 1517 1426 |
| CH in plane bending | 1324 1274 | 1335 1311 1275 | 1336 1277 | 1315 | 1335 1274 | 1372 1335 1275 | 1315 |
| C–F stretching | 1146 978 | 1138 1015 | 1056 | 1159 1013 | 1107 1013 | 1140 1048 | 1107 1013 |
| CH out-of-plane bending | 913 881 844 725 | 889 848 665 | 891 | 950 897 664 | 894 667 | 845 621 | 951 897 664 |
| M–O stretching | - | 504 | 515 | 556 | 557 | 558 515 | 555 519 |
| M–N stretching | - | 446 | 476 | 445 | 477 | 466 445 | 440 |
| Complexes | Steps | Temp. Range (°C) | DTA Peak (°C) | TGA Weight Loss (%) | Assignments | |
|---|---|---|---|---|---|---|
| Calc. | Found | |||||
| Mg(II) STG | 1st | 30–250 250–800 | 84, 222 662 | 17.58 | 17.50 | 6H2O and Cl2 |
| 2nd | 50.50 | 50.28 | 2STG molecule | |||
| Residual | 31.92 | 32.22 | MgO and polluted carbon | |||
| Ca(II) STG | 1st | 30–250 250–800 | 80, 225 425, 674 | 7.67 | 7.45 | Cl2 |
| 2nd | 56.33 | 56.47 | 2STG molecule | |||
| Residual | 36.00 | 36.08 | CaO and polluted carbon | |||
| Cr(III) STG | 1st | 30–300 300–800 | 74, 294 686 | 19.84 | 19.60 | 6H2O and ³/²Cl2 |
| 2nd | 47.10 | 47.14 | 2STG molecule | |||
| Residual | 33.06 | 33.26 | ½Cr2O3 and polluted carbon | |||
| Zn(II) STG | 1st | 30–120 120–800 | 78 565 | 7.47 | 7.35 | Cl2 |
| 2nd | 58.20 | 58.44 | 2STG molecule | |||
| Residual | 34.06 | 34.21 | ZnO and polluted carbon | |||
| Cu(II) STG | 1st | 30–350 350–800 | 184, 294 654 | 10.86 | 10.60 | 2H2O and Cl2 |
| 2nd | 55.11 | 55.30 | 2STG molecule | |||
| Residual | 34.03 | 34.10 | CuO and polluted carbon | |||
| Se(IV) STG | 1st | 30–800 | 90, 330 | 100 | 100 | 2Cl2 and 2STG |
| Residual | 0.0 | 0.0 | Selenium metal sublimated | |||
| Groups | Blood Glucose (mg/dL) | Insulin (uIU/mL) | HbA1C (mmol/mol) | Fasting Serum C-Peptide (ng/mL) |
|---|---|---|---|---|
| Group 1 (Control) | 87.4 ± 1.9 | 25.1 ± 0.6 | 3.2 ± 0.3 | 4.2 ± 0.1 |
| Group 2 (STZ) | 377.3 ± 4.0 * | 4.2 ± 0.3 * | 9.2 ± 0.2 * | 0.5 ± 0.06 * |
| Group 3 (STZ and STG) | 141.6 ± 4.0 *# | 18.9 ± 1.3 *# | 5.0 ± 0.5 *# | 2.6 ± 0.3 *# |
| Group 4 (STZ and STG/Cu) | 130.8 ± 3.8 *# | 19.8 ± 1.5 *# | 3.0 ± 0.2# | 3.3 ± 0.3 *# |
| Group 5 (STZ and STG/Mg) | 128.98 ± 2.58 # | 20.58 ± 1.08 # | 3.1 ± 0.08 # | 3.80 ± 0.6 # |
| Group 6 (STZ and STG/Zn) | 95.4 ± 2.9 # | 23.5 ± 0.6 # | 3.3 ± 0.5 # | 4.1 ± 0.4 # |
| Group 7 (STZ and STG/Ca) | 110 ± 3.58 # | 22.39 ± 2.01 # | 4.05 ± 0.36 # | 2.50 ± 0.63 # |
| Group 8 (STZ + STG/Cr) | 90.24 ± 3.68 # | 23.68 ± 1.88 # | 3.01 ± 0.69 # | 3.98 ± 0.87 # |
| Group 9 (STZ and STG/Se) | 100.69 ± 2.69 # | 21.02 ± 1.69 # | 3.68 ± 0.39 # | 3.21 ± 0.63 # |
| p-value | <0.001 (HS) | <0.001 (HS) | <0.001 (HS) | <0.001 (HS) |
| Groups | GPX (U/g) | CAT (U/g) | MDA (U/g) | SOD (U/g) |
|---|---|---|---|---|
| Group 1 (Control) | 34.1 ± 3.0 | 1.7 ± 0.2 | 3.1 ± 0.2 | 21.5 ± 1.2 |
| Group 2 (STZ) | 7.4 ± 0.2 * | 0.24 ± 0.05 * | 80.3 ± 1.2 * | 5.4 ± 0.3 * |
| Group 3 (STZ and STG) | 20.9 ± 1.5 *# | 1.0 ± 0.2 *# | 20.0 ± 0.8 *# | 14.0 ± 0.8 *# |
| Group 4 (STZ and STG/Cu) | 22.06 ± 0.68 *# | 1.03 ± 0.01 *# | 17.68 ± 0.85 *# | 17.29 ± 1.25 # |
| Group 5 (STZ and STG/Mg) | 24.88 ± 0.45 # | 1.45 ± 0.1 # | 10.02 ± 1.36 # | 18.05 ± 1.59 # |
| Group 6 (STZ and STG/Zn) | 31.0 ± 1.6 # | 1.60 ± 0.3 # | 7.9 ± 0.7 # | 19.9 ± 1.3 # |
| Group 7 (STZ and STG/Ca) | 29.58 ± 1.78 # | 1.54 ± 0.02 # | 8.05 ± 1.58 # | 19.05 ± 1.36 # |
| Group 8 (STZ and STG/Cr) | 32.17 ± 2.36 # | 1.68 ± 0.05 # | 4.98 ± 1.02 # | 20.54 ± 1.98 # |
| Group 9 (STZ and STG/Se) | 30.25 ± 2.58 # | 1.34 ± 0.04 # | 5.25 ± 1.32 # | 19.85 ± 2.05 # |
| p-value | <0.001 (HS) | <0.001 (HS) | <0.001 (HS) | <0.001 (HS) |
| Groups | Pancreatic Parenchyma | Islets of Langerhans Size | ||||
|---|---|---|---|---|---|---|
| Normal | Detached | Normal | Reduced | Mild | Enlarged | |
| Group 1 (Control) | 90% | 0% | 90% | 0% | 0% | 0% |
| Group 2 (STZ) | 0% | 90% | 0% | 90% | 25% | 0% |
| Group 3 (STZ +STG) | 80% | 25% | 80% | 0% | 80% | 80% |
| Group 4 (STZ+ STG/Cu) | 90% | 0% | 90% | 0% | 25% | 90% |
| Group 5 (STZ+ STG/Mg) | 90% | 0% | 90% | 0% | 25% | 90% |
| Group 6 (STZ+ STG/Zn) | 90% | 0% | 90% | 0% | 25% | 90% |
| Group 7 (STZ+ STG/Ca) | 90% | 0% | 90% | 0% | 25% | 90% |
| Group 8 (STZ+ STG/Cr) | 90% | 0% | 90% | 0% | 25% | 90% |
| Group 9 (STZ+ STG/Se) | 90% | 0% | 90% | 0% | 25% | 90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Megharbel, S.M.; Refat, M.S.; Al-Salmi, F.A.; Hamza, R.Z. In Situ Neutral System Synthesis, Spectroscopic, and Biological Interpretations of Magnesium(II), Calcium(II), Chromium(III), Zinc(II), Copper(II) and Selenium(IV) Sitagliptin Complexes. Int. J. Environ. Res. Public Health 2021, 18, 8030. https://doi.org/10.3390/ijerph18158030
El-Megharbel SM, Refat MS, Al-Salmi FA, Hamza RZ. In Situ Neutral System Synthesis, Spectroscopic, and Biological Interpretations of Magnesium(II), Calcium(II), Chromium(III), Zinc(II), Copper(II) and Selenium(IV) Sitagliptin Complexes. International Journal of Environmental Research and Public Health. 2021; 18(15):8030. https://doi.org/10.3390/ijerph18158030
Chicago/Turabian StyleEl-Megharbel, Samy M., Moamen S. Refat, Fawziah A. Al-Salmi, and Reham Z. Hamza. 2021. "In Situ Neutral System Synthesis, Spectroscopic, and Biological Interpretations of Magnesium(II), Calcium(II), Chromium(III), Zinc(II), Copper(II) and Selenium(IV) Sitagliptin Complexes" International Journal of Environmental Research and Public Health 18, no. 15: 8030. https://doi.org/10.3390/ijerph18158030
APA StyleEl-Megharbel, S. M., Refat, M. S., Al-Salmi, F. A., & Hamza, R. Z. (2021). In Situ Neutral System Synthesis, Spectroscopic, and Biological Interpretations of Magnesium(II), Calcium(II), Chromium(III), Zinc(II), Copper(II) and Selenium(IV) Sitagliptin Complexes. International Journal of Environmental Research and Public Health, 18(15), 8030. https://doi.org/10.3390/ijerph18158030







