Effects of Acute Beta-Alanine Ingestion and Immersion-Plus-Exercise on Connectedness to Nature and Perceived Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
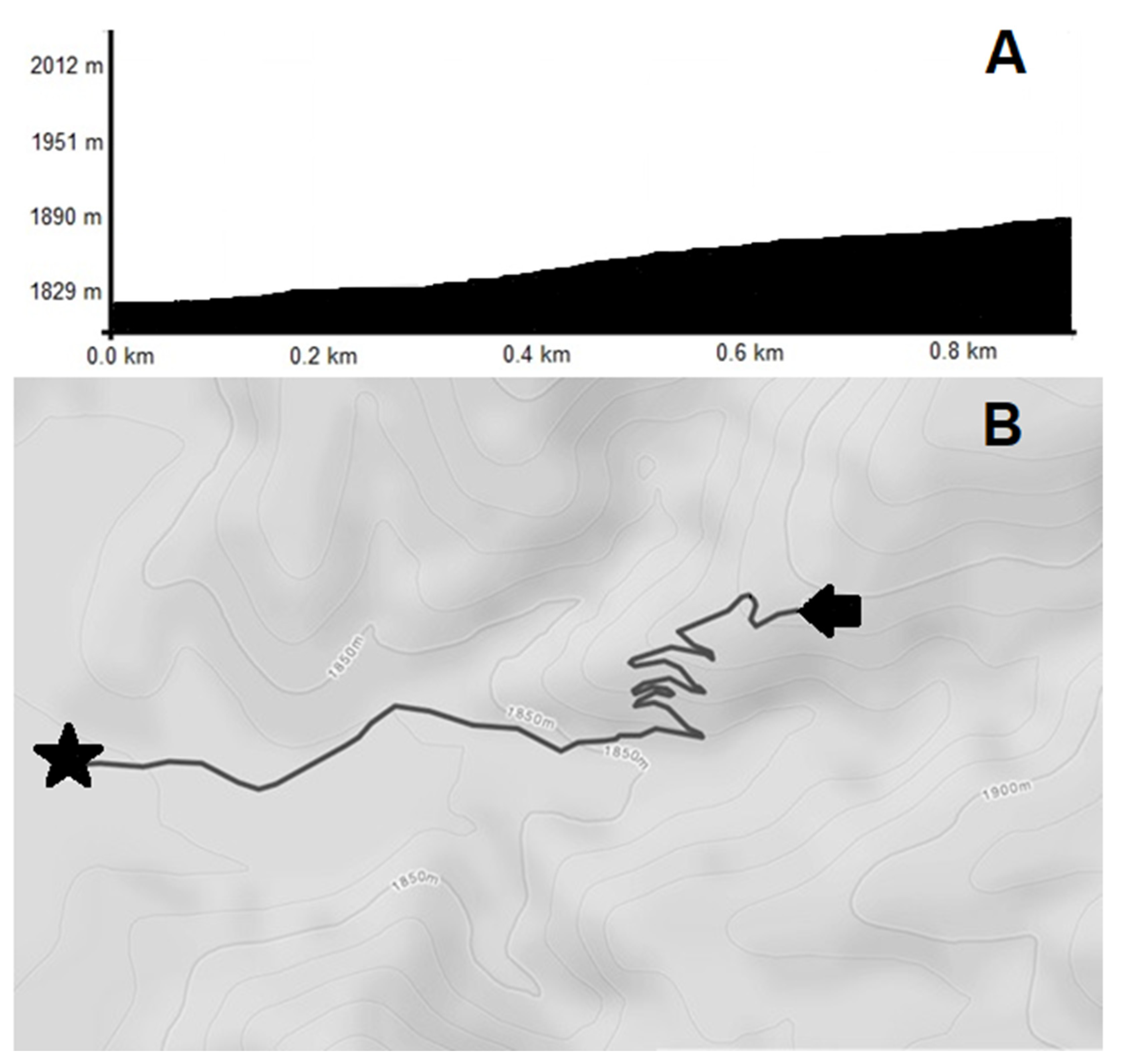
2.2. Protocol
2.3. Statistical Analysis
3. Results
3.1. Performance Variables
3.2. Love and Care of Nature
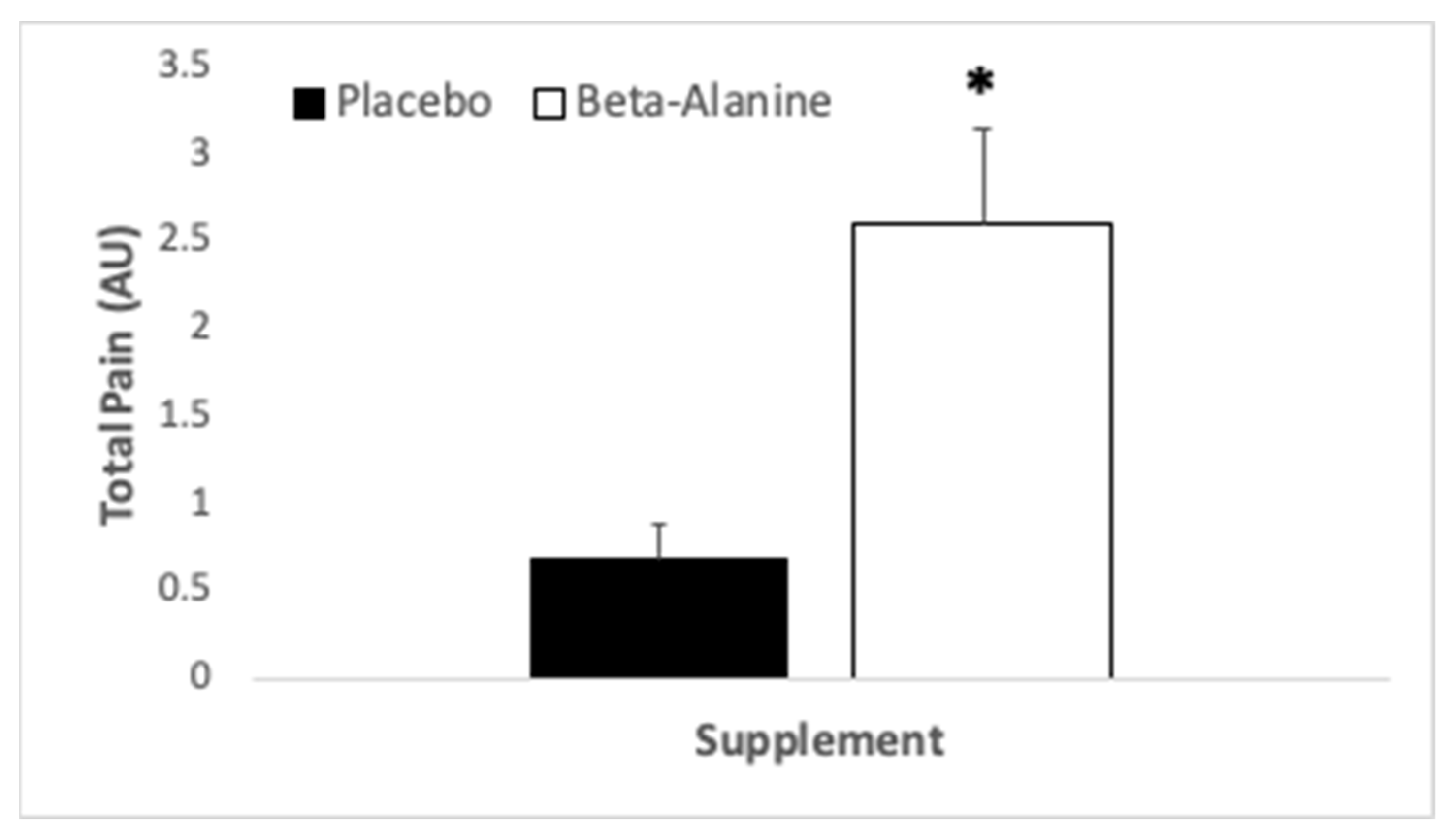
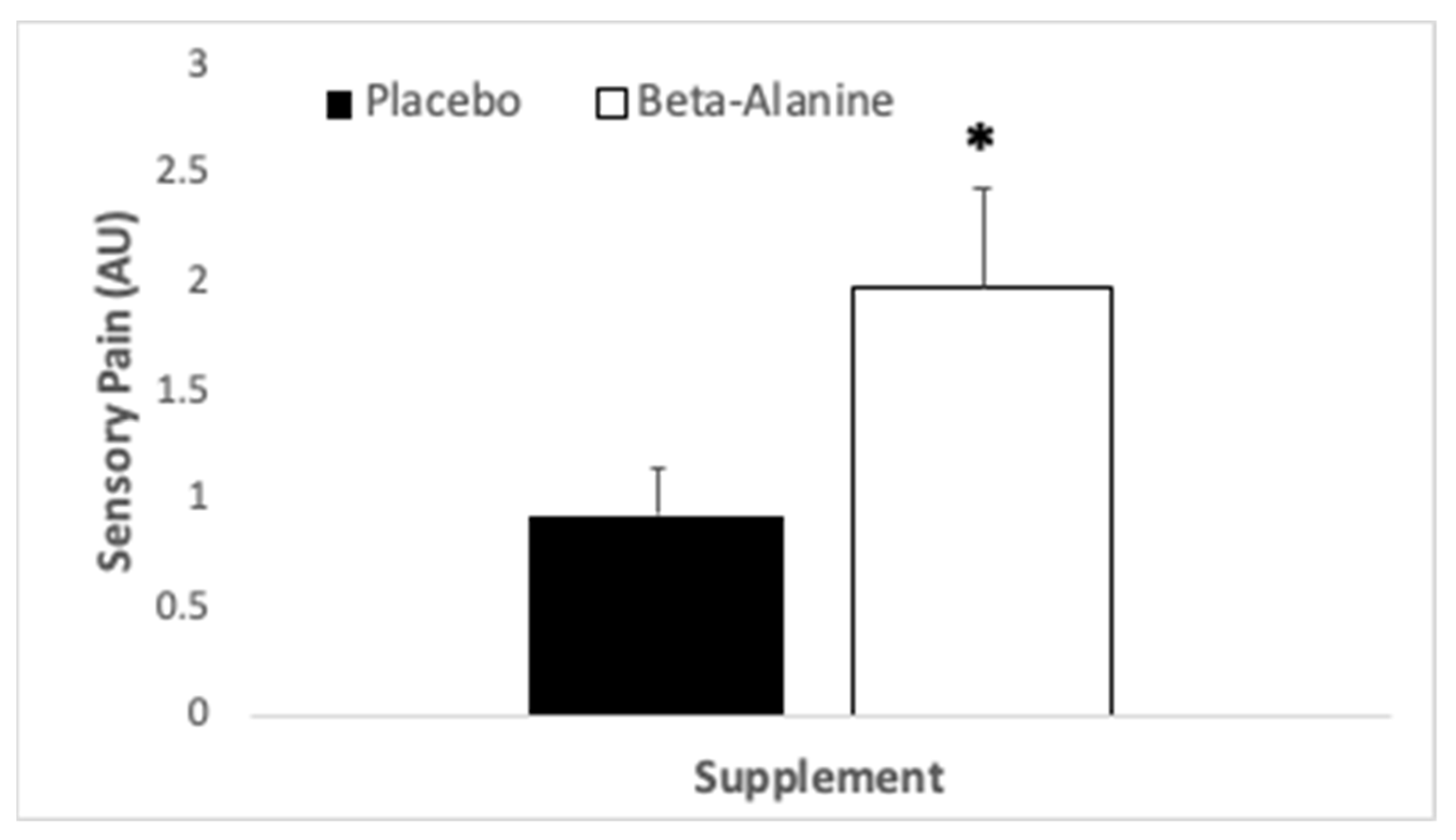
3.3. Perceptual Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, J.; Griffin, M.; Pretty, J. Exercise-, nature-and socially interactive-based initiatives improve mood and self-esteem in the clinical population. Perspect. Public Health 2012, 132, 89–96. [Google Scholar] [CrossRef]
- Mackay, G.J.; Neill, J.T. The effect of “green exercise” on state anxiety and the role of exercise duration, intensity, and greenness: A quasi-experimental study. Psychol. Sport Exerc. 2010, 11, 238–245. [Google Scholar] [CrossRef]
- Navalta, J.W.; Bodell, N.G.; Tanner, E.A.; Aguilar, C.D.; Radzak, K.N. Effect of exercise in a desert environment on physiological and subjective measures. Int. J. Environ. Health Res. 2021, 31, 121–131. [Google Scholar] [CrossRef]
- Han, J.-W.; Choi, H.; Jeon, Y.-H.; Yoon, C.-H.; Woo, J.-M.; Kim, W. The effects of forest therapy on coping with chronic widespread pain: Physiological and psychological differences between participants in a forest therapy program and a control group. Int. J. Environ. Res. Public Health 2016, 13, 255. [Google Scholar] [CrossRef]
- Rogerson, M.; Gladwell, V.; Gallagher, D.; Barton, J. Influences of green outdoors versus indoors environmental settings on psychological and social outcomes of controlled exercise. Int. J. Environ. Res. Public Health 2016, 13, 363. [Google Scholar] [CrossRef]
- Focht, B.C. Brief walks in outdoor and laboratory environments: Effects on affective responses, enjoyment, and intentions to walk for exercise. Res. Q. Exerc. Sport 2009, 80, 611–620. [Google Scholar] [CrossRef]
- Duncan, M.; Clarke, N.; Birch, S.; Tallis, J.; Hankey, J.; Bryant, E.; Eyre, E. The effect of green exercise on blood pressure, heart rate and mood state in primary school children. Int. J. Environ. Res. Public Health 2014, 11, 3678–3688. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Yabunaka, N.; Takayama, S. Shinrin-yoku (forest-air bathing and walking) effectively decreases blood glucose levels in diabetic patients. Int. J. Biometeorol. 1998, 41, 125–127. [Google Scholar] [CrossRef]
- Mao, G.X.; Lan, X.G.; Cao, Y.B.; Chen, Z.M.; He, Z.H.; Lv, Y.D.; Wang, Y.Z.; Hu, X.L.; Wang, G.F.; Jing, Y. Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province, China. Biomed. Environ. Sci. 2012, 25, 317–324. [Google Scholar]
- Mayer, F.S.; Frantz, C.M. The connectedness to nature scale: A measure of individuals’ feeling in community with nature. J. Environ. Psychol. 2004, 24, 503–515. [Google Scholar] [CrossRef]
- Martin, C.; Czellar, S. The extended inclusion of nature in self scale. J. Environ. Psychol. 2016, 47, 181–194. [Google Scholar] [CrossRef]
- Perkins, H.E. Measuring love and care for nature. J. Environ. Psychol. 2010, 30, 455–463. [Google Scholar] [CrossRef]
- Gladwell, V.F.; Brown, D.K.; Wood, C.; Sandercock, G.R.; Barton, J.L. The great outdoors: How a green exercise environment can benefit all. Extrem. Physiol. Med. 2013, 2, 3. [Google Scholar] [CrossRef]
- Niedermeier, M.; Grafetstätter, C.; Hartl, A.; Kopp, M. A randomized crossover trial on acute stress-related physiological responses to mountain hiking. Int. J. Environ. Res. Public Health 2017, 14, 905. [Google Scholar] [CrossRef]
- Byrka, K.; Ryczko, N. Positive effects of dancing in natural versus indoor settings: The mediating role of engagement in physical activity. J. Environ. Psychol. 2018, 57, 25–33. [Google Scholar] [CrossRef]
- Bemben, M.; Witten, M.; Carter, J.; Eliot, K.; Knehans, A.; Bemben, D. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J. Nutr. Health Aging 2010, 14, 155–159. [Google Scholar] [CrossRef]
- Salatto, R.W.; Arevalo, J.A.; Brown, L.E.; Wiersma, L.D.; Coburn, J.W. Caffeine’s Effects on an Upper-Body Resistance Exercise Workout. J. Strength Cond. Res. 2018, 34, 1643–1648. [Google Scholar] [CrossRef]
- Kendrick, I.P.; Harris, R.C.; Kim, H.J.; Kim, C.K.; Dang, V.H.; Lam, T.Q.; Bui, T.T.; Smith, M.; Wise, J.A. The effects of 10 weeks of resistance training combined with β-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 2008, 34, 547–554. [Google Scholar] [CrossRef]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 2007, 32, 381–386. [Google Scholar] [CrossRef]
- Stout, J.R.; Cramer, J.T.; Mielke, M.; O’Kroy, J.; Torok, D.J.; Zoeller, R.F. Effects of twenty-eight days of beta-alanine and creatine monohydrate supplementation on the physical working capacity at neuromuscular fatigue threshold. J. Strength Cond. Res. 2006, 20, 928–931. [Google Scholar] [CrossRef]
- Glenn, J.M.; Smith, K.; Moyen, N.E.; Binns, A.; Gray, M. Effects of Acute Beta-Alanine Supplementation on Anaerobic Performance in Trained Female Cyclists. J. Nutr. Sci. Vitaminol. 2015, 61, 161–166. [Google Scholar] [CrossRef]
- Huerta-Ojeda, A.; Contreras-Montilla, O.; Galdames-Maliqueo, S.; Jorquera-Aguilera, C.; Fuentes-Kloss, R.; Guisado-Barrilao, R. Effects of acute supplementation with beta-alanine on a limited time test at maximum aerobic speed on endurance athletes. Nutr. Hosp. 2019, 36, 698–705. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef]
- Talbot, K.; Madden, V.J.; Jones, S.L.; Moseley, G.L. The sensory and affective components of pain: Are they differentially modifiable dimensions or inseparable aspects of a unitary experience? A systematic review. Br. J. Anaesthiol. 2019, 123, e263–e272. [Google Scholar] [CrossRef]
- Auvray, M.; Myin, E.; Spence, C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci. Biobehav. Rev. 2010, 34, 214–223. [Google Scholar] [CrossRef]
- Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef]
- Melzack, R.; Katz, J. The McGill Pain Questionnaire: Appraisal and current status. In Handbook of Pain Assessment, 2nd ed.; The Guilford Press: New York, NY, USA, 2001; pp. 35–52. [Google Scholar]
- Borg, G.A. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 90–98. [Google Scholar]
- Watt, B.; Grove, R. Perceived exertion. Antecedents and applications. Sports Med. 1993, 15, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Calogiuri, G.; Litleskare, S.; Fagerheim, K.A.; Rydgren, T.L.; Brambilla, E.; Thurston, M. Experiencing Nature through Immersive Virtual Environments: Environmental Perceptions, Physical Engagement, and Affective Responses during a Simulated Nature Walk. Front. Psychol. 2017, 8, 2321. [Google Scholar] [CrossRef]
- Akers, A.; Barton, J.; Cossey, R.; Gainsford, P.; Griffin, M.; Micklewright, D. Visual color perception in green exercise: Positive effects on mood and perceived exertion. Environ. Sci. Technol. 2012, 46, 8661–8666. [Google Scholar] [CrossRef]
- Smith, A.E.; Stout, J.R.; Kendall, K.L.; Fukuda, D.H.; Cramer, J.T. Exercise-induced oxidative stress: The effects of beta-alanine supplementation in women. Amino Acids 2012, 43, 77–90. [Google Scholar] [CrossRef]
- Medicine, American College of Sports. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Baltimore, MA. USA, 2013. [Google Scholar]
- Salatto, R.W.; Davis, D.W.; Carrier, B.; Barrios, B.; Sertic, J.V.L.; Cater, P.C.; Navalta, J.W. Efficient method of delivery for powdered supplement or placebo for an outdoor exercise investigation. Top. Exerc. Sci. Kinesiol. 2020, 1, 5. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Melzack, R. The short-form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Pretty, J.N.; Griffin, M.; Peacock, J.; Hine, R.; Sellens, M.; South, N. A Countryside for Health and Wellbeing: The Physical and Mental Health Benefits of Green Exercise; Countryside Recreation Network Sheffield: Sheffield, UK, 2005. [Google Scholar]
- Pietrzkowski, Z.; Mercado-Sesma, A.R.; Argumedo, R.; Cervantes, M.; Nemzer, B.; Reyes-Izquierdo, T. Effects of once-daily versus twice daily dosing of calcium fructoborate on knee discomfort. A 90 day, double-blind, placebo controlled randomized clinical study. J. Aging Res. Clin. Pract. 2018, 7, 31–36. [Google Scholar]
- Raskin, J.; Pritchett, Y.L.; Wang, F.; D’Souza, D.N.; Waninger, A.L.; Iyengar, S.; Wernicke, J.F. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005, 6, 346–356. [Google Scholar] [CrossRef]
- Leighton, R.F.; Gordon, N.F.; Small, G.S.; Davis, W.J.; Ward, E.S., Jr. Dental and gingival pain as side effects of niacin therapy. Chest 1998, 114, 1472–1474. [Google Scholar] [CrossRef]
- Costa, A.; Ravaglia, S.; Sances, G.; Antonaci, F.; Pucci, E.; Nappi, G. Nitric oxide pathway and response to nitroglycerin in cluster headache patients: Plasma nitrite and citrulline levels. Cephalalgia 2003, 23, 407–413. [Google Scholar] [CrossRef]
- Decombaz, J.; Beaumont, M.; Vuichoud, J.; Bouisset, F.; Stellingwerff, T. Effect of slow-release beta-alanine tablets on absorption kinetics and paresthesia. Amino Acids 2012, 43, 67–76. [Google Scholar] [CrossRef]
- Liu, Q.; Sikand, P.; Ma, C.; Tang, Z.; Han, L.; Li, Z.; Sun, S.; LaMotte, R.H.; Dong, X. Mechanisms of itch evoked by beta-alanine. J. Neurosci. 2012, 32, 14532–14537. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; Opperhuizen, A.; Jansen, E.; Hageman, G.J.; Bast, A.; Haenen, G. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol Vitr. 2017, 44, 206–212. [Google Scholar] [CrossRef]
- Sawaengsri, H.; Bergethon, P.R.; Qiu, W.Q.; Scott, T.M.; Jacques, P.F.; Selhub, J.; Paul, L. Transcobalamin 776C-->G polymorphism is associated with peripheral neuropathy in elderly individuals with high folate intake. Am. J. Clin. Nutr. 2016, 104, 1665–1670. [Google Scholar] [CrossRef]
- Stein, C.; Hassan, A.H.; Lehrberger, K.; Giefing, J.; Yassouridis, A. Local analgesic effect of endogenous opioid peptides. Lancet 1993, 342, 321–324. [Google Scholar] [CrossRef]
- Carlsson, K.; Andersson, J.; Petrovic, P.; Petersson, K.M.; Ohman, A.; Ingvar, M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage 2006, 32, 1804–1814. [Google Scholar] [CrossRef]
- Montes, J.; Stone, T.M.; Manning, J.W.; McCune, D.; Tacad, D.K.; Young, J.C.; Debeliso, M.; Navalta, J.W. Using Hexoskin Wearable Technology to Obtain Body Metrics During Trail Hiking. Int. J. Exerc. Sci. 2015, 8, 425–430. [Google Scholar] [PubMed]
- Tobias, G.; Benatti, F.B.; de Salles Painelli, V.; Roschel, H.; Gualano, B.; Sale, C.; Harris, R.C.; Lancha, A.H., Jr.; Artioli, G.G. Additive effects of beta-alanine and sodium bicarbonate on upper-body intermittent performance. Amino Acids 2013, 45, 309–317. [Google Scholar] [CrossRef]
- Ducker, K.J.; Dawson, B.; Wallman, K.E. Effect of beta-alanine supplementation on 800-m running performance. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 554–561. [Google Scholar] [CrossRef]
- Roveratti, M.C.; Jacinto, J.L.; Oliveira, D.B.; da Silva, R.A.; Andraus, R.A.C.; de Oliveira, E.P.; Ribeiro, A.S.; Aguiar, A.F. Effects of beta-alanine supplementation on muscle function during recovery from resistance exercise in young adults. Amino Acids 2019, 51, 589–597. [Google Scholar] [CrossRef]
- Borg, G.; Ljunggren, G.; Ceci, R. The increase of perceived exertion, aches and pain in the legs, heart rate and blood lactate during exercise on a bicycle ergometer. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 343–349. [Google Scholar] [CrossRef]
- Cook, D.B.; O’Connor, P.J.; Eubanks, S.A.; Smith, J.C.; Lee, M. Naturally occurring muscle pain during exercise: Assessment and experimental evidence. Med. Sci. Sports Exerc. 1997, 29, 999–1012. [Google Scholar] [CrossRef]
- Invernizzi, P.L.; Benedini, S.; Saronni, S.; Merati, G.; Bosio, A. The acute administration of Carnosine and beta-Alanine does not improve running anaerobic performance and has no effect on the metabolic response to exercise. Adv. Phys. Educ. 2013, 3, 169–174. [Google Scholar] [CrossRef][Green Version]
- Gross, M.; Boesch, C.; Bolliger, C.S.; Norman, B.; Gustafsson, T.; Hoppeler, H.; Vogt, M. Effects of beta-alanine supplementation and interval training on physiological determinants of severe exercise performance. Eur. J. Appl. Physiol. 2014, 114, 221–234. [Google Scholar] [CrossRef]
- Macphee, S.; Weaver, I.N.; Weaver, D.F. An Evaluation of Interindividual Responses to the Orally Administered Neurotransmitter beta -Alanine. J. Amino Acids 2013, 2013, 429847. [Google Scholar] [CrossRef]
- Baguet, A.; Reyngoudt, H.; Pottier, A.; Everaert, I.; Callens, S.; Achten, E.; Derave, W. Carnosine loading and washout in human skeletal muscles. J. Appl. Physiol. 2009, 106, 837–842. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salatto, R.W.; McGinnis, G.R.; Davis, D.W.; Carrier, B.; Manning, J.W.; DeBeliso, M.; Navalta, J.W. Effects of Acute Beta-Alanine Ingestion and Immersion-Plus-Exercise on Connectedness to Nature and Perceived Pain. Int. J. Environ. Res. Public Health 2021, 18, 8134. https://doi.org/10.3390/ijerph18158134
Salatto RW, McGinnis GR, Davis DW, Carrier B, Manning JW, DeBeliso M, Navalta JW. Effects of Acute Beta-Alanine Ingestion and Immersion-Plus-Exercise on Connectedness to Nature and Perceived Pain. International Journal of Environmental Research and Public Health. 2021; 18(15):8134. https://doi.org/10.3390/ijerph18158134
Chicago/Turabian StyleSalatto, R. W., Graham R. McGinnis, Dustin W. Davis, Bryson Carrier, Jacob W. Manning, Mark DeBeliso, and James W. Navalta. 2021. "Effects of Acute Beta-Alanine Ingestion and Immersion-Plus-Exercise on Connectedness to Nature and Perceived Pain" International Journal of Environmental Research and Public Health 18, no. 15: 8134. https://doi.org/10.3390/ijerph18158134
APA StyleSalatto, R. W., McGinnis, G. R., Davis, D. W., Carrier, B., Manning, J. W., DeBeliso, M., & Navalta, J. W. (2021). Effects of Acute Beta-Alanine Ingestion and Immersion-Plus-Exercise on Connectedness to Nature and Perceived Pain. International Journal of Environmental Research and Public Health, 18(15), 8134. https://doi.org/10.3390/ijerph18158134






