CD19 Cell Count at Baseline Predicts B Cell Repopulation at 6 and 12 Months in Multiple Sclerosis Patients Treated with Ocrelizumab
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Ocrelizumab Effectiveness in the Whole Sample, Secondary Progressive, Primary Progressive and Relapsing Remitting Patients
3.2. Ocrelizumab Effectiveness in Patients with Fast and Slow Repopulation Rate
3.3. Predictors of B Cell Repopulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. OPERA I and OPERA II Clinical Investigators. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Hauser, S.L.; Belachew, S.; Kappos, L. Ocrelizumab in primary progressive and relapsing multiple sclerosis. N. Engl. J. Med. 2017, 376, 1694. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Ocrelizumab: First global approval. Drugs 2017, 77, 1035–1041. [Google Scholar] [CrossRef]
- Bigaut, K.; De Seze, J.; Collongues, N. Ocrelizumab for the treatment of multiple sclerosis. Expert Rev. Neurother. 2019, 19, 97–108. [Google Scholar] [CrossRef]
- Disanto, G.; Morahan, J.M.; Barnett, M.H.; Giovannoni, G.; Ramagopalan, S.V. The evidence for a role of B cells in multiple sclerosis. Neurology 2012, 78, 823–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barra, M.E.; Soni, D.; Huy Vo, K.; Chitnis, T.; Stankiewicz, J.M. Experience with long-term rituximab use in a multiple sclerosis clinic. Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 2055217316672100. [Google Scholar] [CrossRef] [Green Version]
- Signoriello, E.; Bonavita, S.; Di Pietro, A.; Abbadessa, G.; Rossi, F.; Miele, G.; Casertano, S.; Lus, G. BMI influences CD20 kinetics in multiple sclerosis patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2020, 43, 102186. [Google Scholar] [CrossRef]
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72, 1–5. [Google Scholar] [CrossRef]
- Giovannoni, G.; Tomic, D.; Bright, J.R.; Havrdova, E. No evident disease activity: The use of combined assessments in the management of patients with multiple sclerosis. Mult. Scler. 2017, 23, 1179–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkes, C.H.; Giovannoni, G. The McDonald Criteria for multiple sclerosis: Time for clarification. Mult. Scler. 2010, 16, 566–575. [Google Scholar] [CrossRef]
- Giovannoni, G.; Turner, B.; Gnanapavan, S.; Offiah, C.; Schmierer, K.; Marta, M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult. Scler. Relat. Disord. 2015, 4, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Pellkofer, H.L.; Krumbholz, M.; Berthele, A.; Hemmer, B.; Gerdes, L.A.; Havla, J.; Bittner, R.; Canis, M.; Meinl, E.; Hohlfeld, R.; et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 2011, 76, 1310–1315. [Google Scholar] [CrossRef]
- Yang, C.S.; Yang, L.; Li, T.; Zhang, D.Q.; Jin, W.N.; Li, M.S.; Su, N.; Zhangning, N.; Liu, Q.; Shao, Z.H.; et al. Responsiveness to reduced dosage of rituximab in Chinese patients with neuromyelitis optica. Neurology 2013, 81, 710–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comi, G.; Dalla Costa, G.; Moiola, L. Newly approved agents for relapsing remitting multiple sclerosis: How real-world evidence compares with randomized clinical trials? Expert Rev. Neurother. 2021, 21, 21–34. [Google Scholar] [CrossRef]
- Trojano, M.; Tintore, M.; Montalban, X.; Hillert, J.; Kalincik, T.; Iaffaldano, P.; Spelman, T.; Sormani, M.P.; Butzkueven, H. Treatment decisions in multiple sclerosis—insights from real-world observational studies. Nat. Rev. Neurol. 2017, 13, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Gingele, S.; Jacobus, T.L.; Konen, F.F.; Hümmert, M.W.; Sühs, K.W.; Schwenkenbecher, P.; Ahlbrecht, J.; Möhn, N.; Müschen, L.H.; Bönig, L.; et al. Ocrelizumab Depletes CD20+ T Cells in Multiple Sclerosis Patients. Cells 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Velasco, J.I.; Kuhle, J.; Monreal, E.; Meca-Lallana, V.; Meca-Lallana, J.; Izquierdo, G.; Gascón-Giménez, F.; Sainz de la Maza, S.; Walo-Delgado, P.E.; Maceski, A.; et al. Effect of Ocrelizumab in Blood Leukocytes of Patients with Primary Progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e940. [Google Scholar] [CrossRef] [PubMed]
- Gibiansky, E.; Petry, C.; Mercier, F.; Günther, A.; Herman, A.; Kappos, L.; Hauser, S.; Yamamoto, Y.; Wang, Q.; Model, F.; et al. Pharmacokinetics, pharmacodynamics and exposure-response analyses of ocrelizumab in patients with multiple sclerosis. Neurology 2019, 92, N4.001. [Google Scholar] [CrossRef]
- Kim, S.H.; Hyun, J.W.; Kim, H.J. Individualized B cell-targeting therapy for neuromyelitis optica spectrum disorder. Neurochem. Int. 2019, 130, 104347. [Google Scholar] [CrossRef]
- Barun, B.; Gabelić, T.; Adamec, I.; Babić, A.; Lalić, H.; Batinić, D.; KrbotSkorić, M.; Habek, M. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult. Scler. Relat. Disord. 2021, 48, 102704. [Google Scholar] [CrossRef]
- Link, J.; Ramanujam, R.; Auer, M.; Ryner, M.; Hässler, S.; Bachelet, D.; Mbogning, C.; Warnke, C.; Buck, D.; Hyldgaard Jensen, P.E.; et al. Clinical practice of analysis of anti-drug antibodies against interferon beta and natalizumab in multiple sclerosis patients in Europe: A descriptive study of test results. PLoS ONE 2017, 12, e0170395. [Google Scholar] [CrossRef] [PubMed]
- Dunn, N.; Juto, A.; Ryner, M.; Manouchehrinia, A.; Piccoli, L.; Fink, K.; Piehl, F.; Fogdell-Hahn, A. Rituximab in multiple sclerosis: Frequency and clinical relevance of anti-drug antibodies. Mult. Scler. J. 2018, 24, 1224–1233. [Google Scholar] [CrossRef]
- Song, A.; Hendricks, R.; Chung, S.; Wang, O.; Chin, P.; Garren, H. Immunogenicity with Repeated Dosing of Ocrelizumab in Patients with Multiple Sclerosis. Neurology 2016, 86, P2.087. [Google Scholar]
- Traboulsee, A.; Simon, J.H.; Stone, L.; Fisher, E.; Jones, D.E.; Malhotra, A.; Newsome, S.D.; Oh, J.; Reich, D.S.; Richert, N.; et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. Am. J. Neuroradiol. 2016, 37, 394–401. [Google Scholar] [CrossRef] [PubMed]
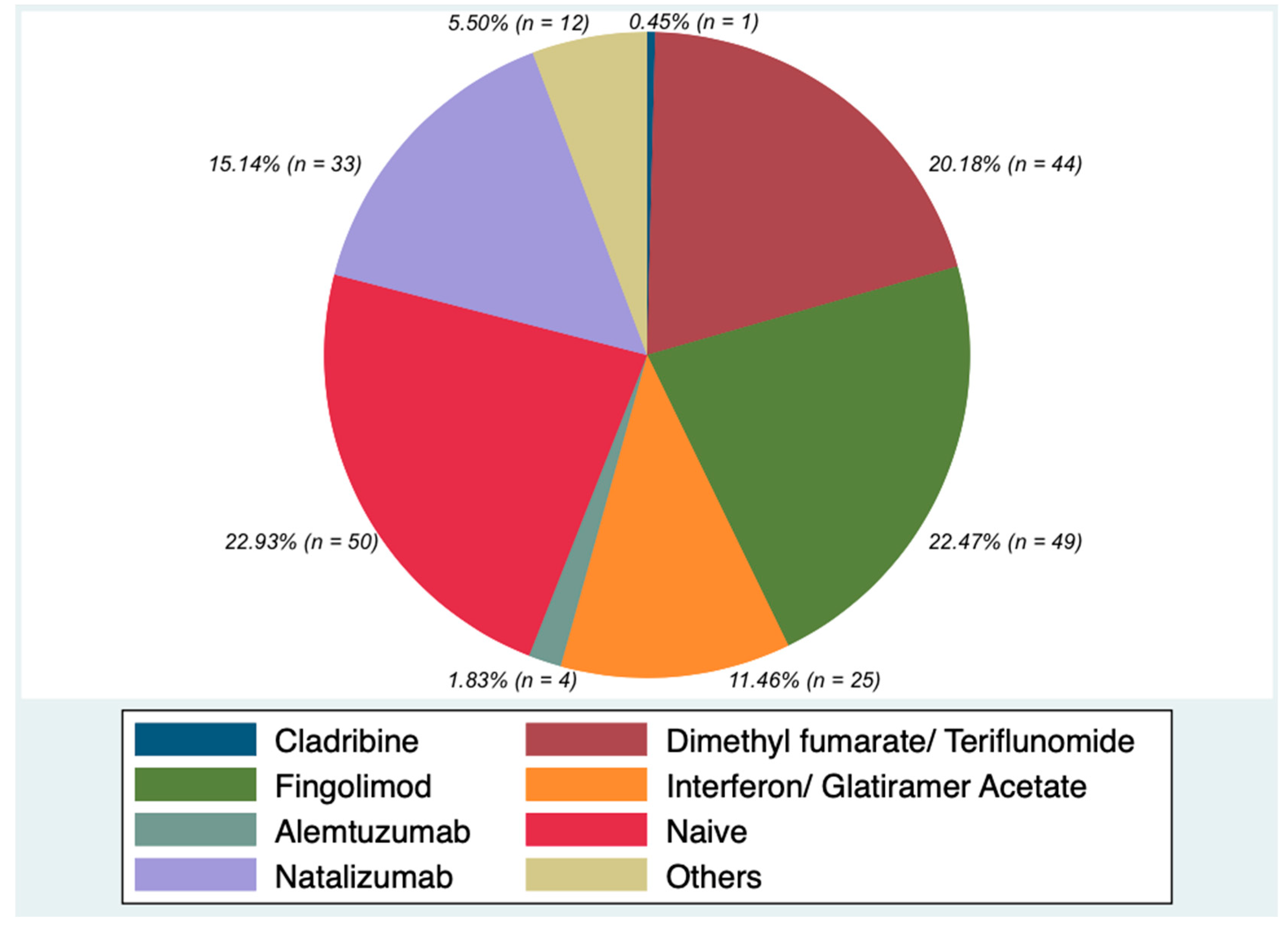

| Demographic and Clinical Characteristics |
Descriptive Statistics |
Overall (218) |
SP (43) |
PP (48) |
RR (127) | p Value |
|---|---|---|---|---|---|---|
| Age (y) | Mean (SD) | 42.01 (13.45) | 50.18 (8.62) | 48.68 (8.59) | 40.87 (12.43) | <0.001 |
| Sex (female) | Number (%) | 124 (56.88) | 46 (37.09) | 67 (54.03) | 79 (64.70) | 0.0042 |
| Age at disease onset (y) | Mean (SD) | 31.39 (12) | 35.89 (13.65) | 32.12 (13.36) | 36.92 (19.15) | 0.2074 |
| Disease duration (y) | Mean (SD) | 18.75 (27.19) | 25.33 (33.48) | 21.10 (25.79) | 25.81 (39.11) | 0.6969 |
| ARR previous year | Mean (SD) | 0.56 (0.78) | 0.13 (0.34) | 0.19 (0.39) | 0.82 (0.87) | <0.001 |
| Baseline EDSS | Mean (SD) | 4.59 (2.05) | 5.58 (1.47) | 5.83 (1.44) | 3.41 (1.62) | <0.001 |
| Wash out (months) | Mean (SD) | 10.06 (21.31) | 4.77 (7.59) | 10.41 (16.72) | 8.13 (15.05) | 0.312 |
| Treatment duration (y) | Mean (SD) | 1.30 (0.87) | 1.93 (0.88) | 1.98 (0.80) | 1.81 (0.80) | 0.351 |
| Age at Ocrelizumab start (y) | Mean (SD) | 40.70 (13.64) | 48.25 (8.89) | 46.70 (8.72) | 39.14 (12.49) | <0.001 |
|
Clinical and Radiological Features | Overall Population | p Value | SP | p Value | PP | p Value | RR | p Value |
|---|---|---|---|---|---|---|---|---|
| ARR pre therapy | 0.56 (0.78) | <0.001 | 0.13 (0.34) | 0.414 | 0.19 (0.39) | 0.008 | 0.82 (0.87) | <0.001 |
| ARR 12 months post therapy | 0.10 (0.32) | 0.07 (0.27) | 0.02 (0.14) | 0.14 (0.37) | ||||
| EDSS baseline | 4.37 (1.92) | 0.736 | 5.58 (1.47) | 0.176 | 5.83 (1.44) | 0.490 | 3.41 (1.62) | 0.177 |
| EDSS six months | 4.33 (1.97) | 5.7 (1.54 | 5.81 (1.47) | 3.42 (1.68) | ||||
| EDSS baseline | 4.37 (1.92) | 0,929 | 5.58 (1.47) | 0.113 | 5.83 (1.44) | 0.919 | 3.41 (1.62) | 0.340 |
| EDSS twelve months | 4.16 (1.99) | 5.6 (1.67) | 5.40 (1.79) | 3.47 (1.65) | ||||
| Active lesions baseline | 0.28 (0.49) | <0.001 | 0.16 (0.37) | 0.0253 | 0.35 (0.59) | 0.0028 | 0.29 (0.47) | <0.001 |
| Active lesions 6 months | 0.04 (0.26) | 0 (0) | 0.03 (0.17) | 0.06 (0.30) | ||||
| Active lesions baseline | 0.28 (0.49) | <0.001 | 0.16 (0.37) | 0.0833 | 0.35 (0.59) | 0.0458 | 0.29 (0.47) | <0.001 |
| Active lesions 12 months | 0.009 (0.09) | 0 (0) | 0 (0) | 0.01 (0.11) | ||||
| NEDA-3 | 69.49% | |||||||
|
Clinical And Radiological Features | FR (n = 41) | p Value | SR (n = 114) | p Value |
|---|---|---|---|---|
| ARR pre therapy | 0.40 (0.72) | 0.0012 | 0.61 (0.80) | <0.001 |
| ARR 12 months post therapy | 0.09 (0.35) | 0.12 (0.33) | ||
| EDSS baseline | 4.59 (1.74) | 0.705 | 4.23 (1.95) | 0.990 |
| EDSS 6 months | 4.51 (1.74) | 4.23(2.00) | ||
| EDSS baseline | 4.59 (1.74) | 0.9127 | 4.23 (1.95) | 0.6454 |
| EDSS 12 months | 4.21 (1.80) | 4.18 (2.03) | ||
| Active lesions baseline | 0.31 (0.54) | 0.0195 | 0.25 (0.45) | <0.001 |
| Active lesions 6 months | 0.06 (0.33) | 0.04 (0.23) | ||
| Active lesions baseline | 0.31 (0.54) | 0.0143 | 0.25 (0.45) | <0.001 |
| Active lesions 12 months | 0.04 (0.20) | 0 (0) |
| Immunological Asset | FR (n = 41) | SR (n = 114) | p Value |
|---|---|---|---|
| Lymphocytes (mean, sd) | 2027.2 (1066.63) | 2104.441 (1032.16) | 0.60 |
| CD4 cells (mean, sd) | 937.02 (617.97) | 918.23 (498.34) | 0.61 |
| %CD4 cells (mean, sd) | 42.32 (10.66) | 43.56 (10.82) | |
| CD8 cells (mean, sd) | 468.18 (286.19) | 522.40 (356.25) | 0.92 |
| %CD8 cells (mean, sd) | 24.44 (8.35) | 24.49 (7.39) | |
| CD19 cells (mean, sd) | 349.73 (373.37) | 256.42 (225.27) | 0.02 |
| %CD19 cells (mean, sd) | 15.76 (9.23) | 12.45 (7.29) | |
| CD16CD56 cells (mean, sd) | 241.33 (117.76) | 274.70 (216.06) | 0.61 |
| %CD16CD56 cells (mean, sd) | 16.26 (15.52) | 16.82 (18.22) |
| Immunological Asset | FR (n = 10) | SR (n = 145) | p Value |
|---|---|---|---|
| Lymphocytes (mean, sd) | 2344.28 (1274.75) | 2092.61 (1112.69) | 0.38 |
| CD4 cells (mean, sd) | 1130.57 (630.84) | 897.44 (537.58) | 0.07 |
| %CD4 cells (mean, sd) | 44.58 (8.81) | 42.01(10.84) | |
| CD8 cells (mean, sd) | 517.89 (329.51) | 517.59 (383.66) | 0.75 |
| %CD8 cells (mean, sd) | 20.81(8.56) | 24.70 (7.62) | |
| CD19 cells (mean, sd) | 510.69 (502.71) | 255.72 (230.40) | 0.0025 |
| %CD19 cells (mean, sd) | 18.51 (9.68) | 12.20 (6.35) | |
| CD16CD56 cells (mean, sd) | 236 (149.26) | 284.80 (219.60) | 0.29 |
| %CD16CD56 cells (mean, sd) | 16.06 (20.70) | 15.89 (10.13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbadessa, G.; Miele, G.; Cavalla, P.; Valentino, P.; Marfia, G.A.; Signoriello, E.; Landi, D.; Bosa, C.; Vercellino, M.; De Martino, A.; et al. CD19 Cell Count at Baseline Predicts B Cell Repopulation at 6 and 12 Months in Multiple Sclerosis Patients Treated with Ocrelizumab. Int. J. Environ. Res. Public Health 2021, 18, 8163. https://doi.org/10.3390/ijerph18158163
Abbadessa G, Miele G, Cavalla P, Valentino P, Marfia GA, Signoriello E, Landi D, Bosa C, Vercellino M, De Martino A, et al. CD19 Cell Count at Baseline Predicts B Cell Repopulation at 6 and 12 Months in Multiple Sclerosis Patients Treated with Ocrelizumab. International Journal of Environmental Research and Public Health. 2021; 18(15):8163. https://doi.org/10.3390/ijerph18158163
Chicago/Turabian StyleAbbadessa, Gianmarco, Giuseppina Miele, Paola Cavalla, Paola Valentino, Girolama Alessandra Marfia, Elisabetta Signoriello, Doriana Landi, Chiara Bosa, Marco Vercellino, Antonio De Martino, and et al. 2021. "CD19 Cell Count at Baseline Predicts B Cell Repopulation at 6 and 12 Months in Multiple Sclerosis Patients Treated with Ocrelizumab" International Journal of Environmental Research and Public Health 18, no. 15: 8163. https://doi.org/10.3390/ijerph18158163
APA StyleAbbadessa, G., Miele, G., Cavalla, P., Valentino, P., Marfia, G. A., Signoriello, E., Landi, D., Bosa, C., Vercellino, M., De Martino, A., Missione, R., Sparaco, M., Lavorgna, L., Lus, G., & Bonavita, S. (2021). CD19 Cell Count at Baseline Predicts B Cell Repopulation at 6 and 12 Months in Multiple Sclerosis Patients Treated with Ocrelizumab. International Journal of Environmental Research and Public Health, 18(15), 8163. https://doi.org/10.3390/ijerph18158163









