Detection of SARS-CoV-2 on Surfaces in Households of Persons with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Household Identification and Enrollment
2.2. Laboratory Testing
2.3. Statistical Analysis
2.4. Ethics Statement
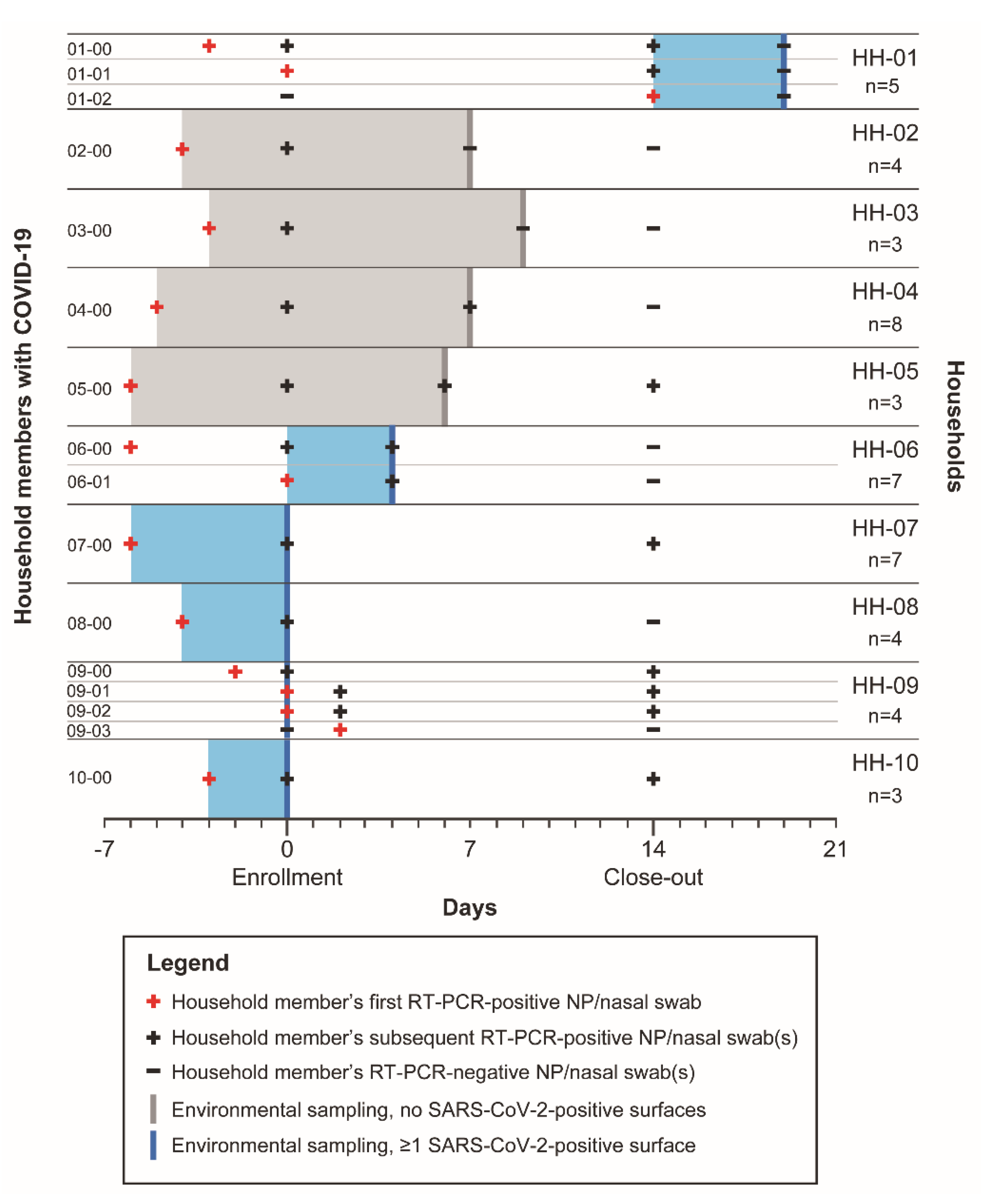
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Lim, X.F.; Lim, A.S.; Sutjipto, S.; Lee, P.H.; et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020, 11, 2800. [Google Scholar] [CrossRef]
- Zhou, J.; Otter, J.A.; Price, J.R.; Cimpeanu, C.; Gracia, D.M.; Kinross, J.; Boshier, P.R.; Mason, M.; Bolt, F.; Holmes, A.H.; et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin. Infect. Dis. 2020, ciaa905. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.L.; Morwitzer, J.; Creager, H.M.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.M.; Schnaubelt, E.R.; Broadhurst, M.J.; et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020, 10, 12732. [Google Scholar] [CrossRef]
- Vicente, V.A.; Lustosa, B.P.R.; Grisolia, M.E.; Beato, C.P.; Balsanelli, E.; De Souza, V.G.F.; Nogueira, M.B.; Raboni, S.M.; Carvalho, K.A.T.; Flor, I.C.; et al. Environmental Detection of SARS-CoV-2 Virus RNA in Health Facilities in Brazil and a Systematic Review on Contamination Sources. Int. J. Environ. Res. Public Health 2021, 18, 3824. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Lin, H.; Chen, S.; Wang, S.; Zheng, Z.; Wang, W.; Zhang, S.; Rebmann, T.; Li, Y.; Pan, Z.; et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J. Infect. 2020, 81, e1–e5. [Google Scholar] [CrossRef]
- Colaneri, M.; Seminari, E.; Novati, S.; Asperges, E.; Biscarini, S.; Piralla, A.; Percivalle, E.; Cassaniti, I.; Baldanti, F.; Bruno, R.; et al. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin. Microbiol. Infect. 2020, 26, 1094.e1–1094.e5. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Rickard, H.; Stevenson, D.; Arabega-Bou, P.; Pitman, J.; Crook, A.; Davies, K.; Spencer, A.; Burton, C.; Easterbrook, L.; et al. Detection of SARS-CoV-2 within the healthcare environment: A multi-centre study conducted during the first wave of the COVID-19 outbreak in England. J. Hosp. Infect. 2021, 108, 189–196. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.P.; Fuhrmeister, E.R.; Cantrell, M.E.; Pitol, A.K.; Swarthout, J.M.; Power, J.E.; Nadimpalli, M.L.; Julian, T.R.; Pickering, A.J. Longitudinal Monitoring of SARS-CoV-2 RNA on High-Touch Surfaces in a Community Setting. Environ. Sci. Technol. 2020, 8, 168–175. [Google Scholar] [CrossRef]
- Montagna, M.; De Giglio, O.; Calia, C.; Pousis, C.; Apollonio, F.; Campanale, C.; Diella, G.; Lopuzzo, M.; Marzella, A.; Triggiano, F.; et al. First Detection of Severe Acute Respiratory Syndrome Coronavirus 2 on the Surfaces of Tourist-Recreational Facilities in Italy. Int. J. Environ. Res. Public Health 2021, 18, 3252. [Google Scholar] [CrossRef]
- Luo, L.; Liu, D.; Zhang, H.; Li, Z.; Zhen, R.; Zhang, X.; Xie, H.; Song, W.; Liu, J.; Huang, Q.; et al. Air and surface contamination in non-health care settings among 641 environmental specimens of 39 COVID-19 cases. PLoS Negl. Trop. Dis. 2020, 14, e0008570. [Google Scholar] [CrossRef]
- Jiang, F.-C.; Jiang, X.-L.; Wang, Z.-G.; Meng, Z.-H.; Shao, S.-F.; Anderson, B.D.; Ma, M.-J. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 RNA on Surfaces in Quarantine Rooms. Emerg. Infect. Dis. 2020, 26, 2162. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Ohnishi, M.; Matsunaga, N.; Kakimoto, K.; Kamiya, H.; Okamoto, K.; Suzuki, M.; Gu, Y.; Sakaguchi, M.; Tajima, T.; et al. Environmental Sampling for Severe Acute Respiratory Syndrome Coronavirus 2 During a COVID-19 Outbreak on the Diamond Princess Cruise Ship. J. Infect. Dis. 2020, 222, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Madewell, Z.J.; Yang, Y.; Longini, I.M., Jr.; Halloran, M.E.; Dean, N.E. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2031756. [Google Scholar] [CrossRef]
- Thompson, H.A.; Mousa, A.; Dighe, A.; Fu, H.; Arnedo-Pena, A.; Barrett, P.; Bellido-Blasco, J.; Bi, Q.; Caputi, A.; Chaw, L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Setting-specific Transmission Rates: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2021, ciab100. [Google Scholar] [CrossRef]
- Lewis, N.M.; Chu, V.T.; Ye, D.; Conners, E.E.; Gharpure, R.; Laws, R.L.; Reses, E.H.; Freeman, B.D.; Fajans, M.; Rabold, E.; et al. Household Transmission of SARS-CoV-2 in the United States. Clin. Infect. Dis. 2020, ciaa1166. [Google Scholar] [CrossRef]
- Park, G.W.; Chhabra, P.; Vinjé, J. Swab Sampling Method for the Detection of Human Norovirus on Surfaces. J. Vis. Exp. 2017, 120, e55205. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Sakthivel, S.K.; Whitaker, B.; Murray, J.; Kamili, S.; Lynch, B.; Malapati, L.; Burke, S.A.; Harcourt, J.; et al. US CDC Real-Time Reverse Transcription PCR Panel for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.; Lester, S.; Mills, L.; Ur Rashhed, M.A.; Moye, S.; Abiona, O.; Hutchinson, G.B.; Morales-Betoulle, M.; Krapinunaya, I.; Gibbons, A.; et al. Validation of a SARS-CoV-2 spike protein ELISA for use in contact investigations and serosurveillance. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Harcourt, J.; Tamin, A.; Lu, X.; Kamili, S.; Sakthivel, S.K.; Murray, J.; Queen, K.; Tao, Y.; Paden, C.R.; Zhang, J.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States. Emerg. Infect. Dis. 2020, 26, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 7 December 2020).
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.M.; Duca, L.M.; Marcenac, P.; Dietrich, E.A.; Gregory, C.J.; Fields, V.L.; Banks, M.M.; Rispens, J.R.; Hall, A.; Harcourt, J.L.; et al. Characteristics and Timing of Initial Virus Shedding in Severe Acute Respiratory Syndrome Coronavirus 2, Utah, USA. Emerg. Infect. Dis. 2021, 27, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, Division of Viral Diseases. Isolate If You Are Sick. Available online: https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/isolation.html (accessed on 5 April 2021).
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, Division of Viral Diseases. How to Protect Yourself & Others. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 5 April 2021).
| Household ID | Number (% b) of Sampled Surfaces with Detectable SARS-CoV-2 RNA | HH Surfaces with Detectable SARS-CoV-2 RNA (Ct) |
|---|---|---|
| HH-01 | 2 (13) | Pillow of secondary HH case (36.4); phone (37.0) |
| HH-02 | 0 (0) | .. |
| HH-03 | 0 (0) | .. |
| HH-04 | 0 (0) | .. |
| HH-05 | 0 (0) | .. |
| HH-06 | 3 (20) | Light switch (37.2); pillow of index case (35.0); trash can lid (32.1) |
| HH-07 | 2 (13) | Nightstand of index case (34.1); computer (36.6) |
| HH-08 | 1 (7) | Pillow of other HH member (36.2) |
| HH-09 | 13 (87) | 2 light switches (29.6, 33.4); refrigerator handle (29.3); nightstand of index case (26.4), nightstands of 2 secondary HH cases (33.8, 35.7); 2 doorknobs (29.8, 30.6); kitchen counter (33.2); microwave handle (31.8); kitchen sink handle (34.8); furniture (34.7); TV remote control (28.8) |
| HH-10 | 2 (13) | Pillow of index case (32.8); bathroom sink handle (34.8) |
| Environmental Surface | Number of Surfaces Sampled b | Number of Surfaces Positive for SARS-CoV-2 (%) | Number of HHs Sampled for Surface (%) | Number of Sampled HHs with SARS-CoV-2-Positive Surface (%) | Median Ct [Range] |
|---|---|---|---|---|---|
| Light switch | 21 | 3 (14) | 10 (100) | 2 (20) | 33.4 [29.6–37.2] |
| Refrigerator handle | 11 | 1 (9) | 10 (100) | 1 (10) | 29.3 |
| Toilet handle in bathroom | 13 | 0 (0) | 9 (90) | 0 (0) | .. |
| Bathroom sink handle | 13 | 1 (8) | 10 (100) | 1 (10) | 34.8 |
| Pillowcof: | 23 | 4 (17) | 8 (80) | 4 (50) | 35.6 [32.8–36.4] |
| Index case | 8 | 2 (25) | 8 (80) | 2 (25) | 33.9 [32.8–35.0] |
| Secondary HH case | 2 | 1 (50) | 1 (10) | 1 (100) | 36.4 |
| Other HH member | 13 | 1 (8) | 7 (70) | 1 (14) | 36.2 |
| Nightstand c of: | 6 | 4 (67) | 2 (20) | 2 (100) | 34.0 [26.4–35.7] |
| Index case | 2 | 2 (100) d | 2 (20) | 2 (100) | 30.2 [26.4–34.1] |
| Secondary HH case | 2 | 2 (100) | 1 (10) | 1 (100) | 34.8 [33.8–35.7] |
| Other HH member | 2 | 0 (0) | 1 (10) | 0 (0) | .. |
| Doorknob/handle | 17 | 2 (12) | 10 (100) | 1 (10) | 30.2 [29.8–30.6] |
| Kitchen countertop | 9 | 1 (11) | 9 (90) | 1 (11) | 33.2 |
| Microwave handle/button | 7 | 1 (14) | 7 (70) | 1 (14) | 31.8 |
| Kitchen sink handle | 5 | 1 (20) | 5 (50) | 1 (20) | 34.8 |
| Trash can lid | 1 | 1 (100) | 1 (10) | 1 (100) | 32.1 |
| Furniture e | 4 | 1 (25) | 4 (40) | 1 (25) | 34.7 |
| Phone (mobile, landline) | 3 | 1 (33) | 3 (30) | 1 (33) | 37.0 |
| Computer (mouse, keyboard) | 4 | 1 (25) | 3 (30) | 1 (33) | 36.6 |
| TV remote control | 7 | 1 (14) | 7 (70) | 1 (14) | 28.8 |
| Miscellaneous electronics f | 4 | 0 (0) | 4 (40) | 0 (0) | .. |
| Banister | 1 | 0 (0) | 1 (10) | 0 (0) | .. |
| Cat litter box | 1 | 0 (0) | 1 (10) | 0 (0) | .. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcenac, P.; Park, G.W.; Duca, L.M.; Lewis, N.M.; Dietrich, E.A.; Barclay, L.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Rispens, J.; et al. Detection of SARS-CoV-2 on Surfaces in Households of Persons with COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 8184. https://doi.org/10.3390/ijerph18158184
Marcenac P, Park GW, Duca LM, Lewis NM, Dietrich EA, Barclay L, Tamin A, Harcourt JL, Thornburg NJ, Rispens J, et al. Detection of SARS-CoV-2 on Surfaces in Households of Persons with COVID-19. International Journal of Environmental Research and Public Health. 2021; 18(15):8184. https://doi.org/10.3390/ijerph18158184
Chicago/Turabian StyleMarcenac, Perrine, Geun Woo Park, Lindsey M. Duca, Nathaniel M. Lewis, Elizabeth A. Dietrich, Leslie Barclay, Azaibi Tamin, Jennifer L. Harcourt, Natalie J. Thornburg, Jared Rispens, and et al. 2021. "Detection of SARS-CoV-2 on Surfaces in Households of Persons with COVID-19" International Journal of Environmental Research and Public Health 18, no. 15: 8184. https://doi.org/10.3390/ijerph18158184






