Occurrence of Extended Spectrum Beta Lactamase (ESBL) Producers, Quinolone and Carbapenem Resistant Enterobacteriaceae Isolated from Environmental Samples along Msimbazi River Basin Ecosystem in Tanzania
Abstract
:1. Introduction
2. Materials and Methods
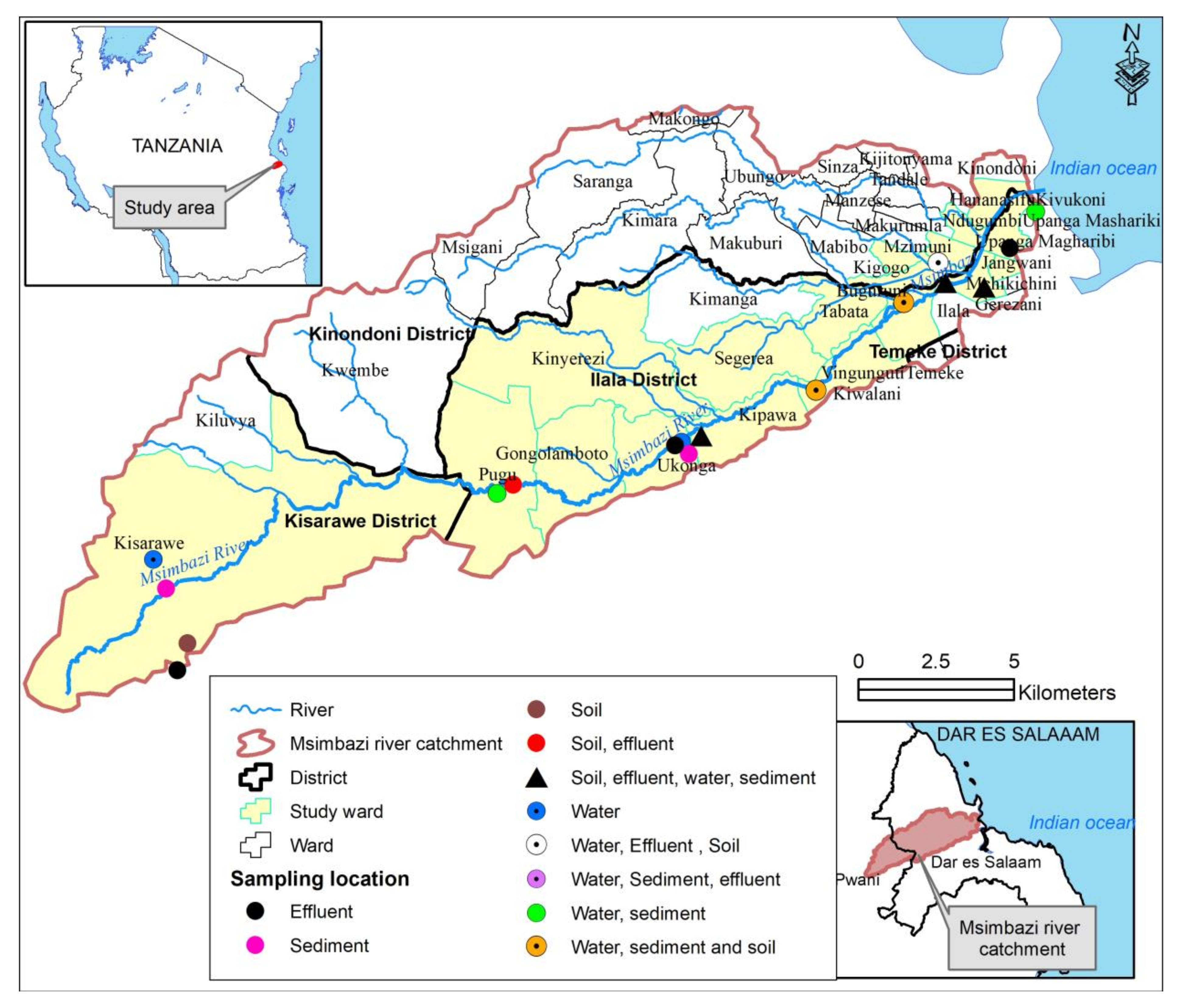
2.1. Study Area
2.2. Sampling Frame
2.3. Sampling Strategy and Sample Sites
2.4. Sample Collection and Processing
2.5. Isolation and Identification of Bacterial Strains
2.6. Antimicrobial Susceptibility Testing
2.7. Screening and Confirmation of ESBL Production
3. Results
3.1. Detection of Enterobacteriaceae Isolates and the Prevalence of Resistance from Effluent, River Water, River Sediment and Crop Soil
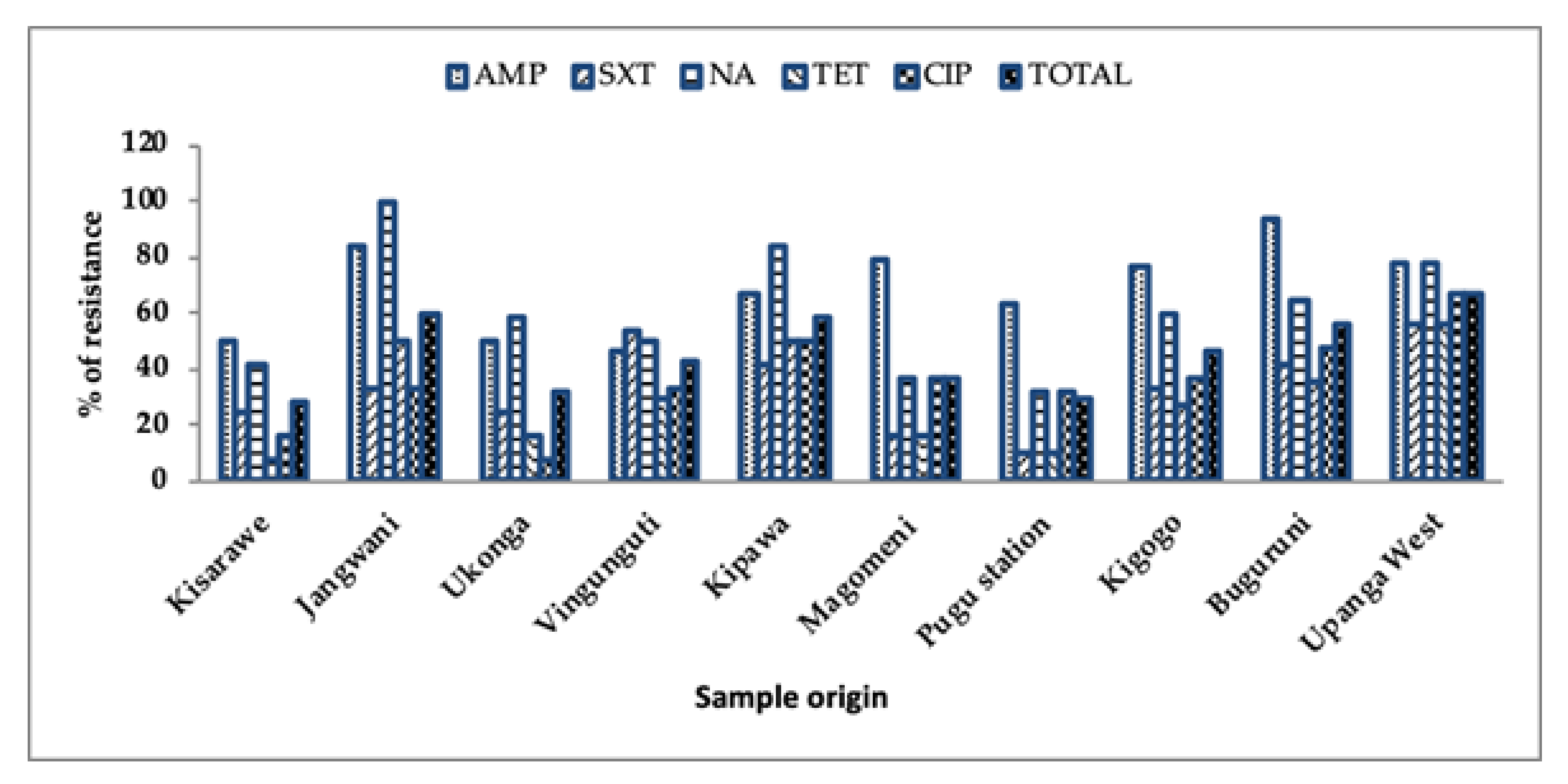
3.2. Prevalence of Antibiotic Resistance from Different Sample Locations
3.3. Multidrug Resistant E. coli and K. pneumoniae Isolates in Effluent, River Water, River Sediment and Crop Soil
3.4. Prevalence of Quinolone Resistance, ESBL Producers and Carbapenem Resistant E. coli and K. pneumoniae from the Effluent, River Water, Sediment and Crop Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; AMR Review: London, UK, 2016; p. 84. Available online: https://amr-review.org (accessed on 9 January 2018).
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2017. Available online: http://www.who.int/drugresistance/global_action_plan/en/ (accessed on 12 May 2019).
- World Health Organization. Report on Surveillance of Antibiotic Consumption. 2018. Available online: http://apps.who.int/iris%0Ahttps://apps.who.int/iris/bitstream/handle/10665/277359/9789241514880-eng.pdf (accessed on 3 April 2021).
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef]
- Tamhankar, A.J.; Lundborg, C.S. Antimicrobials and antimicrobial resistance in the environment and its remediation: A global one health perspective. Int. J. Environ. Res. Public Health 2019, 16, 4614. [Google Scholar] [CrossRef] [Green Version]
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial use and resistance in food-producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Huijbers, P.M.; Flach, C.F.; Larsson, D.J. A conceptual framework for the environmental surveillance of antibiotics and antibiotic resistance. Environ. Int. 2019, 130, 104880. [Google Scholar] [CrossRef]
- Moremi, N.; Manda, E.V.; Falgenhauer, L.; Ghosh, H.; Imirzalioglu, C.; Matee, M.; Chakraborty, T.; Mshana, S.E. Predominance of CTX-M-15 among ESBL producers from environment and fish gut from the shores of Lake Victoria in Mwanza, Tanzania. Front. Microbiol. 2016, 7, 1862. [Google Scholar] [CrossRef] [Green Version]
- Nonga, H.E.; Simon, C.; Karimuribo, E.D.; Mdegela, R.H. Assessment of antimicrobial usage and residues in commercial chicken eggs from smallholder poultry keepers in Morogoro municipality, Tanzania. Zoonoses Public Health 2010, 57, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Caudell, M.A.; Quinlan, M.B.; Subbiah, M.; Call, D.R.; Roulette, C.J.; Roulette, J.W.; Roth, A.; Matthews, L.; Quinlan, R.J. Antimicrobial use and veterinary care among agro-pastoralists in Northern Tanzania. PLoS ONE 2017, 12, e0170328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afema, J.A.; Byarugaba, D.K.; Shah, D.H.; Atukwase, E.; Nambi, M.; Sischo, W.M. Potential sources and transmission of Salmonella and antimicrobial resistance in Kampala, Uganda. PLoS ONE 2016, 11, e0152130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainda, G.; Bessell, P.B.; Muma, J.B.; McAteer, S.P.; Chase-Topping, M.E.; Gibbons, J.; Stevens, M.P.; Gally, D.L.; Bronsvoort, B.M. Prevalence and patterns of antimicrobial resistance among Escherichia coli isolated from Zambian dairy cattle across different production systems. Sci. Rep. 2015, 5, 12439. [Google Scholar] [CrossRef] [Green Version]
- Katakweba, A.A.S.; Mtambo, M.M.A.; Olsen, J.E.; Muhairwe, A.P. Awareness of human health risks associated with the use of antimicrobials among livestock keepers and factors that contribute to selection of antibiotic resistance bacteria within livestock in Tanzania. Livestock Res. Rural Dev. 2012, 24, 170. [Google Scholar]
- Sindato, C.; Mboera, L.E.; Katale, B.Z.; Frumence, G.; Kimera, S.; Clark, T.G.; Legido-Quigley, H.; Mshana, S.E.; Rweyemamu, M.M.; Matee, M. Knowledge, attitudes and practices regarding antimicrobial use and resistance among communities of Ilala, Kilosa and Kibaha districts of Tanzania. Antimicrob. Resist. Infect. Control 2020, 9, 194. [Google Scholar] [CrossRef]
- Tacconelli, E.; Magrini, N.; World Health Organisation. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://www.aidsdatahub.org/sites/default/files/resource/who-global-priority-list-antibiotic-resistant-bacteria.pdf (accessed on 13 May 2021).
- Taylor, P.; Reeder, R. Antibiotic use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- Wuijts, S.; van den Berg, H.H.; Miller, J.; Abebe, L.; Sobsey, M.; Andremont, A.; Medlicott, K.O.; van Passel, M.W.; de Roda Husman, A.M. Towards a research agenda for water, sanitation and antimicrobial resistance. J. Water Health 2017, 15, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrutu, A.; Nkotagu, H.; Luilo, G. Spatial distribution of heavy metals in Msimbazi River mangrove sediments in Dar es Salaam coastal zone, Tanzania. Int. J. Environ. Sci. 2013, 3, 1641–1655. [Google Scholar]
- World Bank. The Msimbazi Opportunity: Transforming the Msimbazi Basin into a Beacon of Urban Resilience, Strategy and Management Framework. 2019. Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/694491555396781552/volume-a-strategy-and-management-framework (accessed on 30 March 2020).
- Taggar, G.; Rheman, M.A.; Boerlin, P.; Diarra, M.S. Molecular epidemiology of carbapenemases in enterobacteriales from humans, animals, food and the environment. Antibiotics 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, S.M.; Fawzi, M.A.; Ali, F.M.; Abd, E.L.; Galil, K.H. Prevalence and characterization of extended-spectrum beta-lactamases producing Enterobacteriaceae in healthy children and associated risk factors. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manaia, C.M. Assessing the Risk of Antibiotic Resistance Transmission from the Environment to Humans: Non-Direct Proportionality between Abundance and Risk. Trends Microbiol. 2017, 25, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Kimera, Z.I.; Frumence, G.; Mboera, L.E.G.; Rweyemamu, M.M.; Mshana, S.E.; Matee, M.I.N. Assessment of drivers of antimicrobial use and resistance in pig and poultry farming in the Msimbazi River Basin in Tanzania. Antibiotics 2020, 9, 838. [Google Scholar] [CrossRef]
- Lupindu, A.M.; Dalsgaard, A.; Msoffe, P.L.M.; Ngowi, H.A.; Mtambo, M.M.; Olsen, J.E. Transmission of antibiotic-resistant Escherichia coli between cattle, humans and the environment in peri-urban livestock keeping communities in Morogoro, Tanzania. Prev. Vet. Med. 2015, 118, 477–482. [Google Scholar] [CrossRef]
- Moffat, J.; Chalmers, G.; Reid-Smith, R.; Mulvey, M.R.; Agunos, A.; Calvert, J.; Cormier, A.; Ricker, N.; Scott-Weese, J.; Boerlin, P. Resistance to extended-spectrum cephalosporins in Escherichia coli and other Enterobacterales from Canadian turkeys. PLoS ONE 2020, 15, e0236442. [Google Scholar] [CrossRef]
- Hamza, E.; Dorgham, S.M.; Hamza, D.A. Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. Integr. Med. Res. 2016, 7, 8–10. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute—CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 2019. Available online: http://www.emeraldinsight.com/doi/10.1108/08876049410065598 (accessed on 18 May 2020).
- Magiorakos, A.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbath, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An internatiojnal expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Dellgren, L.; Claesson, C.; Högdahl, M.; Forsberg, J.; Hanberger, H.; Nilsson, L.E.; Hällgren, A. Phenotypic screening for quinolone resistance in Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1765–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwegoha, W.J.S.; Leonard, L.S.; Kihampa, C. Heavy metal pollutions and urban agriculture in Msimbazi River valley: Health risk and public awareness. Int. J. Plants Anim. Environ. Stud. 2012, 2, 107–118. [Google Scholar]
- Kayombo, M.C.; Mayo, A.W. Assessment of Microbial Quality of Vegetables Irrigated with Polluted Waters in Dar es Salaam City, Tanzania. Environ. Ecol. Res. 2018, 6, 229–239. [Google Scholar] [CrossRef]
- Tanzania URT of the 2012 Population and Housing Census. Population Distribution by Administrative Areas. 2013. Available online: http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/water/WSDP/Background_information/2012_Census_General_Report.pdf (accessed on 18 April 2021).
- Tanzania URT of the National Sample Census of Agriculture: Small holder Agriculture. Ministry of Livestock and Fisheries Development. 2012. Available online: https://www.instepp.umn.edu/sites/instepp.umn.edu/files/product/downloadable/Tanzania_2007-8_Vol_3.pdf (accessed on 12 June 2018).
- Alouache, S.; Estepa, V.; Messai, Y.; Ruiz, E.; Torres, C.; Bakour, R. Characterization of ESBLs and Associated Quinolone Resistance in Escherichia coli and Klebsiella pneumoniae Isolates from an Urban Wastewater Treatment Plant in Algeria. Microb. Drug Resist. 2013, 20, 30–38. [Google Scholar] [CrossRef]
- Moges, F.; Endris, M.; Belyhun, Y.; Worku, W. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, Northwest Ethiopia. BMC Res. Notes 2014, 7, 2–7. [Google Scholar] [CrossRef]
- De Boeck, H.; Lunguya, O.; Muyembe, J.J.; Glupczynski, Y.; Jacobs, J. Presence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in waste waters, Kinshasa, the Democratic Republic of the Congo. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3085–3088. [Google Scholar] [CrossRef]
- Chah, K.F.; Ugwu, I.C.; Okpala, A.; Adamu, K.Y.; Alonso, C.A.; Ceballos, S.; Nwanta, J.N.; Torres, C. Detection and molecular characterisation of extended-spectrum Beta—lactamase-producing enteric bacteria from pigs and chickens in Nsukka, Nigeria. Glob. Antimicrob. Res. 2018, 15, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, X.; Liu, C. Occurrence and distribution of antibiotic resistance genes in various rural environmental media. Environ. Sci. Pollut. Res. 2020, 27, 29191–29203. [Google Scholar] [CrossRef]
- Mervat, A.A.; Hesham, M.M.; Safaa, M.E.; Essam, H.A.; Mostafa, A.E. Antimicrobial Resistance Profiles of Enterobacteriaceae Isolated from Rosetta Branch of River Nile, Egypt. World Appl. Sci. J. 2012, 19, 1234–1243. [Google Scholar]
- Said, L.B.; Jouini, A.; Klibi, N.; Dziri, R.; Alonso, C.A.; Boudabous, A.; Slama, K.B.; Torres, C. Detection of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in vegetables, soil and water of the farm environment in Tunisia. Int. J. Food Microbiol. 2015, 203, 86–92. [Google Scholar] [CrossRef]
- Mwaikono, K.S.; Maina, S.; Gwakisa, P. Prevalence and Antimicrobial Resistance Phenotype of Enteric Bacteria from a Municipal Dumpsite. J. Appl. Environ. Microbiol. 2015, 3, 82–94. [Google Scholar]
- Ma, Y.; Chen, J.; Fong, K.; Nadya, S.; Allen, K.; Laing, C.; Ziebell, K.; Topp, E.; Carroll, L.M.; Wiedmann, M.; et al. Antibiotic resistance in shiga toxigenic Escherichia coli isolates from surface waters and sediments in a mixed use urban agricultural landscape. Antibiotics 2021, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Salah, F.D.; Soubeiga, S.T.; Ouattara, A.K.; Sadji, A.Y.; Metuor-Dabire, A.; Obiri-Yeboah, D.; Banla-Kere, A.; Karou, S.; Simpore, J. Distribution of quinolone resistance gene (qnr) in ESBL-producing Escherichia coli and Klebsiella spp. in Lomé, Togo. Antimicrob. Resist. Infect. Control 2019, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Abgottspon, H.; Haċhler, H.; Nuësch-Inderbinen, M.; Stephan, R. Quinolone resistance mechanisms among Extended- Spectrum Beta-Lactamase (ESBL) producing Escherichia coli isolated from rivers and lakes in Switzerland. PLoS ONE 2014, 9, e95864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimera, Z.I.; Mgaya, F.X.; Misinzo, G.; Mshana, S.E.; Moremi, N.; Matee, M.I.N. Multidrug-resistant, including extended-spectrum beta lactamase-producing and quinolone-resistant, Escherichia coli isolated from poultry and domestic pigs in dar es salaam, Tanzania. Antibiotics 2021, 10, 406. [Google Scholar] [CrossRef]
- Hounmanou, Y.M.G.; Mdegela, R.H. Current situation for antimicrobial use, antimicrobial resistance and antimicrobial residues in the food and agriculture sectors in Tanzania: A review. Tanzania Vet. J. 2017, 35, 58–62. [Google Scholar]
- Mshana, S.E.; Matee, M.; Rweyemamu, M. Antimicrobial resistance in human and animal pathogens in Zambia, Democratic Republic of Congo, Mozambique and Tanzania: An urgent need of a sustainable surveillance system. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebomah, K.E.; Adefisoye, M.A.; Okoh, A.I. Pathogenic Escherichia coli strains recovered from selected aquatic resources in the eastern cape, South Africa, and its significance to public health. Int. J. Environ. Res. Public Health 2018, 15, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial resistance genes in ESBL-producing Escherichia coli isolates from animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Bonardi, S.; Pitino, R. Carbapenemase-producing bacteria in food-producing animals, wildlife and environment: A challenge for human health. Ital. J. Food Saf. 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Mgaya, F.X.; Matee, M.I.; Muhairwa, A.P.; Hoza, A.S. Occurrence of multidrug resistant Escherichia coli in raw meat and cloaca swabs in poultry processed in slaughter slabs in Dar es Salaam, Tanzania. Antibiotics 2021, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.G.; Novais, A.; Peixe, L.; Machado, E. Atypical epidemiology of CTX-M-15 among Enterobacteriaceae from a high diversity of non-clinical niches in Angola. J. Antimicrob. Chemother. 2016, 71, 1169–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef] [Green Version]
- Tanzania UR of the Environmental Management Act No 20. 2004. Available online: https://www.vpo.go.tz/publications/legislations (accessed on 14 April 2020).
- Tanzania UR of the Animal Welfare Act No. 19. 2008. Available online: https://www.mifugouvuvi.go.tz/publications/45 (accessed on 1 April 2020).
- Tanzania UR of the Veterinary Act No.16. Tanzania. 2003. Available online: https://www.mifugouvuvi.go.tz/publications/45 (accessed on 1 April 2020).
- Tanzania UR of the Tanzania Food, Drugs and Cosmetics Act No. 1 of 2003. 2003. Available online: https://www.tanzania.go.tz/egov_uploads/documents/The_Tanzania_Food,_Drugs_and_Cosmetics_Act,_1-2003_en.pdf (accessed on 1 April 2020).

| Organism | Effluent (n = 176) | Water (n = 22) | Sediment (n = 10) | Soil (n = 5) | Total |
|---|---|---|---|---|---|
| No (%) | No (%) | No (%) | No (%) | No (%) | |
| Klebsiella pneumoniae | 83 (85.6) | 8 (8.3) | 3 (3.1) | 3 (3.1) | 97 (100.0) |
| Escherichia coli | 53 (84.1) | 8 (12.7) | 1 (1.6) | 1 (1.6) | 63 (100.0) |
| Other organisms | 40 (75.5) | 6 (11.3) | 6 (11.3) | 1 (1.9) | 53 (100.0) |
| No of Antibiotics Classes | Resistant Pattern | No. of Isolates | Prevalence (%) |
|---|---|---|---|
| 3 | CEP/QNL/PEN | 9 | 5.63 |
| SUL/PHE/PEN | 3 | 1.88 | |
| CEP/QNL/CAR | 2 | 1.25 | |
| PHE/TET/PEN | 3 | 1.88 | |
| QNL/PEN/SUL | 15 | 9.34 | |
| TET/CAR/QNL | 3 | 1.88 | |
| QNL/PEN/TET | 11 | 6.88 | |
| PEN/AMN/SUL | 2 | 1.25 | |
| CAR/CEP/PHE | 3 | 1.88 | |
| SUL/PHE/AMN | 1 | 0.63 | |
| CEP/QNL/AMN | 2 | 1.25 | |
| 4 | PHE/TET/CAR/PEN | 2 | 1.25 |
| PHE/TET/CAR/SUL | 4 | 2.5 | |
| CEP/QNL/PEN/TET | 2 | 1.25 | |
| CEP/QNL/PEN/CAR | 3 | 1.88 | |
| TET/CAR/CEP/PEN | 1 | 0.65 | |
| SUL/PHE/TET/QNL | 3 | 1.88 | |
| PEN/AMN/SUL/TET | 3 | 1.88 | |
| 5 | SUL/PHE/TET/CAR/CEP | 2 | 1.25 |
| TET/CAR/CEP/QNL/SUL | 3 | 1.88 | |
| CAR/CEP/QNL/PEN/SUL | 1 | 0.65 | |
| 6 | CEP/QNL/PEN/AMN/SUL/TET | 2 | 1.25 |
| SUL/PHE/TET/CAR/CEP/QNL | 1 | 0.63 | |
| TET/CAR/CEP/QNL/PEN/SUL | 3 | 1.88 | |
| 7 | SUL/PHE/TET/CAR/CEP/QNL/PEN | 1 | 0.63 |
| Total | 85 | 53.2 |
| Antibiotic | % of Resistance E. coli and K. pneumoniae Isolates | p-Value | % of Resistance E. coli and K. pneumoniae Isolates | p-Value | % of Resistance E. coli and K. pneumoniae Isolates | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| ESBL Producers (n = 23) | Non-ESBL Producers (n = 137) | Quinolone Resistant (n = 57) | Non-Quinolone Resistant (n = 103) | Carbapenem Resistant (n = 14) | Non-Carbapenem Resistant (n = 146) | ||||
| AMP | NA | NA | NA | 41 (71.9) | 71 (68.9) | 0.692 | NA | NA | NA |
| GEN | 14 (60.9) | 12 (8.7) | 0.992 | 14 (24.6) | 9 (8.7) | 0.994 | 13 (92.9) | 13 (8.9) | 0.824 |
| SXT | 17 (73.9) | 41 (29.7) | 0.036 | 31 (54.4) | 22 (21.4) | 0.000 | 8 (57.1) | 47 (32.2) | 0.419 |
| CHL | 12 (52.2) | 11 (8.0) | 0.432 | 13 (22.8) | 8 (7.8) | 0.555 | 12 (85.7) | 12 (8.2) | 0.444 |
| TET | 13 (56.5) | 33 (24.1) | 0.053 | 31 (54.4) | 12 (11.7) | 0.000 | 9 (64.3) | 38 (26) | 0.436 |
| Antibiotic | ESBL Producers (n = 23) | Quinolone Resistant (n = 57) | Carbapenem Resistant (n = 14) | |||
|---|---|---|---|---|---|---|
| R | S | R | S | R | S | |
| AMP | NA | NA | 41 (71.9) | 16 (28.3) | 14 (100) | 0 (0.0) |
| GEN | 14 (60.9) | 9 (39.1) | 14 (24.6) | 43 (75.4) | 13 (92.9) | 1 (7.1) |
| SXT | 17 (73.9) | 6 (26.1) | 31 (54.4) | 26 (45.6) | 8 (57.1) | 6 (42.9) |
| CHL | 12 (52.2) | 11 (47.8) | 13 (22.8) | 44 (77.2) | 12 (85.7) | 2 (14.3) |
| TET | 13 (56.5) | 10 (43.5) | 31 (54.4) | 26 (45.6) | 9 (64.3) | 5 (35.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimera, Z.I.; Mgaya, F.X.; Mshana, S.E.; Karimuribo, E.D.; Matee, M.I.N. Occurrence of Extended Spectrum Beta Lactamase (ESBL) Producers, Quinolone and Carbapenem Resistant Enterobacteriaceae Isolated from Environmental Samples along Msimbazi River Basin Ecosystem in Tanzania. Int. J. Environ. Res. Public Health 2021, 18, 8264. https://doi.org/10.3390/ijerph18168264
Kimera ZI, Mgaya FX, Mshana SE, Karimuribo ED, Matee MIN. Occurrence of Extended Spectrum Beta Lactamase (ESBL) Producers, Quinolone and Carbapenem Resistant Enterobacteriaceae Isolated from Environmental Samples along Msimbazi River Basin Ecosystem in Tanzania. International Journal of Environmental Research and Public Health. 2021; 18(16):8264. https://doi.org/10.3390/ijerph18168264
Chicago/Turabian StyleKimera, Zuhura I., Fauster X. Mgaya, Stephen E. Mshana, Esron D. Karimuribo, and Mecky I. N. Matee. 2021. "Occurrence of Extended Spectrum Beta Lactamase (ESBL) Producers, Quinolone and Carbapenem Resistant Enterobacteriaceae Isolated from Environmental Samples along Msimbazi River Basin Ecosystem in Tanzania" International Journal of Environmental Research and Public Health 18, no. 16: 8264. https://doi.org/10.3390/ijerph18168264






