Two-Photon Laser Ablation and In Vivo Wide-Field Imaging of Inferior Olive Neurons Revealed the Recovery of Olivocerebellar Circuits in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Transgenic Lines
2.2. Two-Photon Laser Ablation
2.3. Confocal Microscopy Imaging
2.4. Cell Counting
2.5. Whole-Mount Immunostaining
3. Results
3.1. Distribution of Inferior Olive Neurons in Zebrafish
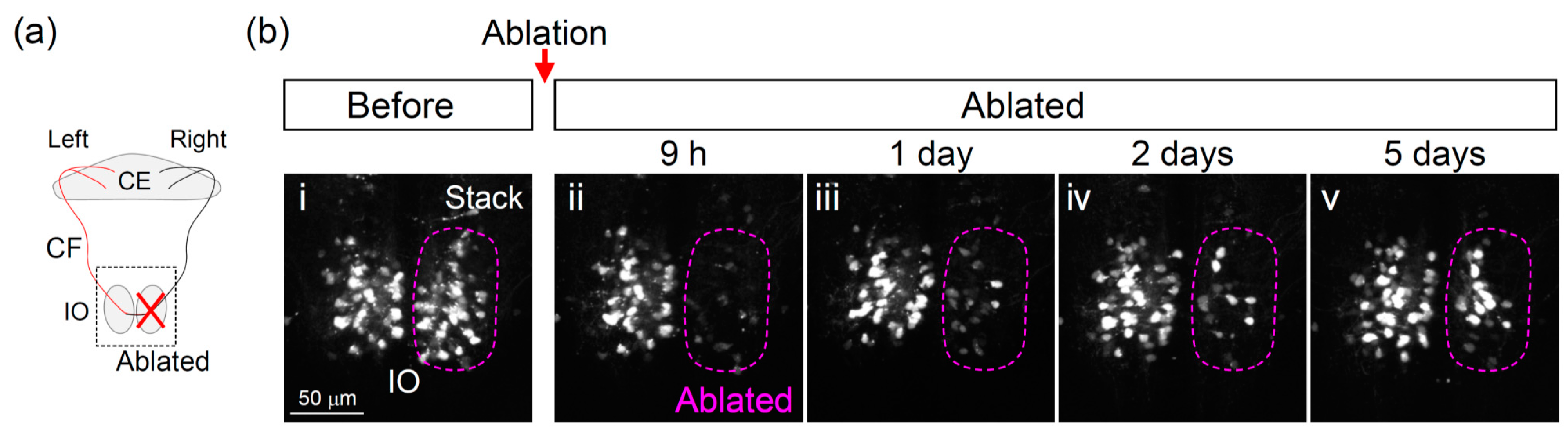
3.2. Two-Photon Laser Ablation of IO Neurons
3.3. Recovery of the Olivocerebellar Circuit after IO Ablation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolb, B.; Whishaw, I.Q. Brain plasticity and behavior. Annu. Rev. Psychol. 1998, 49, 43–64. [Google Scholar] [CrossRef]
- Holtmaat, A.; Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009, 10, 647–658. [Google Scholar] [CrossRef]
- Hummel, F.C.; Cohen, L.G. Drivers of brain plasticity. Curr. Opin. Neurol. 2005, 18, 667–674. [Google Scholar] [CrossRef]
- Espinosa, J.S.; Stryker, M.P. Development and plasticity of the primary visual cortex. Neuron 2012, 75, 230–249. [Google Scholar] [CrossRef]
- Nudo, R.J. Mechanisms for recovery of motor function following cortical damage. Curr. Opin. Neurobiol. 2006, 16, 638–644. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef]
- D’Angelo, E.; Casali, S. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front. Neural Circuits 2012, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006, 78, 272–303. [Google Scholar] [CrossRef]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-Garcia, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Marien, P.; et al. Consensus paper: Roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- Ito, M. The molecular organization of cerebellar long-term depression. Nat. Rev. Neurosci. 2002, 3, 896–902. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Itohara, S.; Ito, M. Reassessment of long-term depression in cerebellar Purkinje cells in mice carrying mutated GluA2 C terminus. Proc. Natl. Acad. Sci. USA 2016, 113, 10192–10197. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Lisberger, S.G.; Raymond, J.L. Diversity and dynamism in the cerebellum. Nat. Neurosci. 2021, 24, 160–167. [Google Scholar] [CrossRef]
- Honda, T.; Nagao, S.; Hashimoto, Y.; Ishikawa, K.; Yokota, T.; Mizusawa, H.; Ito, M. Tandem internal models execute motor learning in the cerebellum. Proc. Natl. Acad. Sci. USA 2018, 115, 7428–7433. [Google Scholar] [CrossRef]
- Mitoma, H.; Buffo, A.; Gelfo, F.; Guell, X.; Fuca, E.; Kakei, S.; Lee, J.; Manto, M.; Petrosini, L.; Shaikh, A.G.; et al. Consensus Paper. Cerebellar Reserve: From Cerebellar Physiology to Cerebellar Disorders. Cerebellum 2020, 19, 131–153. [Google Scholar] [CrossRef]
- Luciani, L. Il Cervelletto. Nuovi Studi di Fisiologia Normale e Patologica. Philos. Rev. 1893, 2, 475–477. [Google Scholar]
- Holmes, G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain 1917, 40, 461–535. [Google Scholar] [CrossRef]
- Molinari, M.; Petrosini, L. Hemicerebellectomy and motor behaviour in rats. III. Kinematics of recovered spontaneous locomotion after lesions at different developmental stages. Behav. Brain Res. 1993, 54, 43–55. [Google Scholar] [CrossRef]
- Petrosini, L.; Molinari, M.; Gremoli, T. Hemicerebellectomy and motor behaviour in rats. I. Development of motor function after neonatal lesion. Exp. Brain Res. 1990, 82, 472–482. [Google Scholar] [CrossRef]
- Molinari, M.; Petrosini, L.; Gremoli, T. Hemicerebellectomy and motor behaviour in rats. II. Effects of cerebellar lesion performed at different developmental stages. Exp. Brain Res. 1990, 82, 483–492. [Google Scholar]
- Mitoma, H.; Manto, M.; Hampe, C.S. Time Is Cerebellum. Cerebellum 2018, 17, 387–391. [Google Scholar] [CrossRef]
- Paulson, H.L. The spinocerebellar ataxias. J. Neuroophthalmol. 2009, 29, 227–237. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M. The physiological basis of therapies for cerebellar ataxias. Ther. Adv. Neurol. Disord. 2016, 9, 396–413. [Google Scholar] [CrossRef]
- Cendelin, J.; Buffo, A.; Hirai, H.; Magrassi, L.; Mitoma, H.; Sherrard, R.; Vozeh, F.; Manto, M. Task Force Paper On Cerebellar Transplantation: Are We Ready to Treat Cerebellar Disorders with Cell Therapy? Cerebellum 2019, 18, 575–592. [Google Scholar] [CrossRef]
- Cutuli, D.; Rossi, S.; Burello, L.; Laricchiuta, D.; De Chiara, V.; Foti, F.; De Bartolo, P.; Musella, A.; Gelfo, F.; Centonze, D.; et al. Before or after does it matter? Different protocols of environmental enrichment differently influence motor, synaptic and structural deficits of cerebellar origin. Neurobiol. Dis. 2011, 42, 9–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.F.; Shi, Q.L.; Li, M.Y.; Wang, C.J.; Wang, X.; Wang, W.Y.; Wu, Y. The Neuronal Activation of Deep Cerebellar Nuclei Is Essential for Environmental Enrichment-Induced Post-Stroke Motor Recovery. Aging Dis. 2019, 10, 530–543. [Google Scholar] [CrossRef]
- Eshra, A.; Hirrlinger, P.; Hallermann, S. Enriched Environment Shortens the Duration of Action Potentials in Cerebellar Granule Cells. Front. Cell. Neurosci. 2019, 13, 289. [Google Scholar] [CrossRef]
- Mancuso, J.J.; Kim, J.; Lee, S.; Tsuda, S.; Chow, N.B.; Augustine, G.J. Optogenetic probing of functional brain circuitry. Exp. Physiol. 2011, 96, 26–33. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Denk, W. The Big and the Small: Challenges of Imaging the Brain’s Circuits. Science 2011, 334, 618–623. [Google Scholar] [CrossRef]
- Asakawa, K.; Suster, M.L.; Mizusawa, K.; Nagayoshi, S.; Kotani, T.; Urasaki, A.; Kishimoto, Y.; Hibi, M.; Kawakami, K. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 2008, 105, 1255–1260. [Google Scholar] [CrossRef]
- Scott, E.K.; Mason, L.; Arrenberg, A.B.; Ziv, L.; Gosse, N.J.; Xiao, T.; Chi, N.C.; Asakawa, K.; Kawakami, K.; Baier, H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 2007, 4, 323–326. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef]
- Ahrens, M.B.; Li, J.M.; Orger, M.B.; Robson, D.N.; Schier, A.F.; Engert, F.; Portugues, R. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 2012, 485, 471–477. [Google Scholar] [CrossRef]
- Portugues, R.; Severi, K.E.; Wyart, C.; Ahrens, M.B. Optogenetics in a transparent animal: Circuit function in the larval zebrafish. Curr. Opin. Neurobiol. 2013, 23, 119–126. [Google Scholar] [CrossRef]
- Tsuda, S. Optogenetics. In Behavioral and Neural Genetics of Zebrafish; Elsevier Academic Press: Cambridge, MA, USA, 2020; pp. 272–292. [Google Scholar]
- Hashimoto, M.; Hibi, M. Development and evolution of cerebellar neural circuits. Dev. Growth Differ. 2012, 54, 373–389. [Google Scholar] [CrossRef]
- Bae, Y.K.; Kani, S.; Shimizu, T.; Tanabe, K.; Nojima, H.; Kimura, Y.; Higashijima, S.; Hibi, M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev. Biol. 2009, 330, 406–426. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuda, K.; Yamaguchi, S.; Asakawa, K.; Miyasaka, N.; Lal, P.; Yoshihara, Y.; Koga, A.; Kawakami, K.; Shimizu, T.; et al. Establishment of Gal4 transgenic zebrafish lines for analysis of development of cerebellar neural circuitry. Dev. Biol. 2015, 397, 1–17. [Google Scholar] [CrossRef]
- Matsui, H.; Namikawa, K.; Babaryka, A.; Koster, R.W. Functional regionalization of the teleost cerebellum analyzed in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 11846–11851. [Google Scholar] [CrossRef]
- Knogler, L.D.; Markov, D.A.; Dragomir, E.I.; Stih, V.; Portugues, R. Sensorimotor Representations in Cerebellar Granule Cells in Larval Zebrafish Are Dense, Spatially Organized, and Non-temporally Patterned. Curr. Biol. 2017, 27, 1288–1302. [Google Scholar] [CrossRef]
- Knogler, L.D.; Kist, A.M.; Portugues, R. Motor context dominates output from purkinje cell functional regions during reflexive visuomotor behaviours. eLife 2019, 8, e42138. [Google Scholar] [CrossRef]
- Miyazawa, H.; Okumura, K.; Hiyoshi, K.; Maruyama, K.; Kakinuma, H.; Amo, R.; Okamoto, H.; Yamasu, K.; Tsuda, S. Optical interrogation of neuronal circuitry in zebrafish using genetically encoded voltage indicators. Sci. Rep. 2018, 8, 6048. [Google Scholar] [CrossRef]
- Matsuda, K.; Yoshida, M.; Kawakami, K.; Hibi, M.; Shimizu, T. Granule cells control recovery from classical conditioned fear responses in the zebrafish cerebellum. Sci. Rep. 2017, 7, 11865. [Google Scholar] [CrossRef]
- Aspatwar, A.; Tolvanen, M.E.; Jokitalo, E.; Parikka, M.; Ortutay, C.; Harjula, S.K.; Ramet, M.; Vihinen, M.; Parkkila, S. Abnormal cerebellar development and ataxia in CARP VIII morphant zebrafish. Hum. Mol. Genet. 2013, 22, 417–432. [Google Scholar] [CrossRef]
- Namikawa, K.; Dorigo, A.; Zagrebelsky, M.; Russo, G.; Kirmann, T.; Fahr, W.; Dubel, S.; Korte, M.; Koster, R.W. Modeling Neurodegenerative Spinocerebellar Ataxia Type 13 in Zebrafish Using a Purkinje Neuron Specific Tunable Coexpression System. J. Neurosci. 2019, 39, 3948–3969. [Google Scholar] [CrossRef]
- Harmon, T.C.; Magaram, U.; McLean, D.L.; Raman, I.M. Distinct responses of Purkinje neurons and roles of simple spikes during associative motor learning in larval zebrafish. eLife 2017, 6, e22537. [Google Scholar] [CrossRef]
- Llinas, R.R. The olivo-cerebellar system: A key to understanding the functional significance of intrinsic oscillatory brain properties. Front. Neural Circuits 2013, 7, 96. [Google Scholar] [CrossRef]
- Llinas, R.R. Cerebellar motor learning versus cerebellar motor timing: The climbing fibre story. J. Physiol. 2011, 589, 3423–3432. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kano, M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci. Res. 2005, 53, 221–228. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, Y.; Watson, C. The inferior olive of the C57BL/6J mouse: A chemoarchitectonic study. Anat. Rec. 2014, 297, 289–300. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef]
- Centonze, D.; Rossi, S.; De Bartolo, P.; De Chiara, V.; Foti, F.; Musella, A.; Mataluni, G.; Rossi, S.; Bernardi, G.; Koch, G.; et al. Adaptations of glutamatergic synapses in the striatum contribute to recovery from cerebellar damage. Eur. J. Neurosci. 2008, 27, 2188–2196. [Google Scholar] [CrossRef]
- D’Agata, V.; Drago, F.; Serapide, F.; Cicirata, F. Effects of cerebellectomy on motivation-related behavior: A time-course study. Physiol. Behav. 1993, 53, 173–176. [Google Scholar] [CrossRef]
- Anderson, W.A.; Flumerfelt, B.A. A light and electron microscopic study of the effects of 3-acetylpyridine intoxication on the inferior olivary complex and cerebellar cortex. J. Comp. Neurol. 1980, 190, 157–174. [Google Scholar] [CrossRef]
- Mackel, R. The role of the monkey sensory cortex in the recovery from cerebellar injury. Exp. Brain Res. 1987, 66, 638–652. [Google Scholar] [CrossRef]
- Muto, A.; Kawakami, K. Prey capture in zebrafish larvae serves as a model to study cognitive functions. Front. Neural Circuits 2013, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Yamamoto, T.; Nakajima, Y.; Hatta, K. Multistepped optogenetics connects neurons and behavior. Curr. Biol. 2014, 24, R1155–R1156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lee, H.; Henle, S.J.; Cheever, T.R.; Ekker, S.C.; Henley, J.R. Primary neuron culture for nerve growth and axon guidance studies in zebrafish (Danio rerio). PLoS ONE 2013, 8, e57539. [Google Scholar] [CrossRef]
- Gramsbergen, A. Normal and abnormal development of motor behavior: Lessons from experiments in rats. Neural Plast. 2001, 8, 17–29. [Google Scholar] [CrossRef]
- Jones, N.; Stelz, T.; Batini, C.; Caston, J. Effects of lesion of the inferior olivary complex in learning of the equilibrium behavior in the young rat during ontogenesis. I. Total lesion of the inferior olive by 3-acetylpyridine. Brain Res. 1995, 697, 216–224. [Google Scholar] [CrossRef]
- Pham, N.C.; Kim, Y.G.; Kim, S.J.; Kim, C.H. Differential effects of inferior olive lesion on vestibulo-ocular and optokinetic motor learning. Neuroreport 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Deuschl, G.; Toro, C.; Valls-Sole, J.; Hallett, M. Symptomatic and essential palatal tremor. 3. Abnormal motor learning. J. Neurol. Neurosurg. Psychiatry 1996, 60, 520–525. [Google Scholar] [CrossRef][Green Version]
- Louis, E.D.; Lenka, A. The Olivary Hypothesis of Essential Tremor: Time to Lay this Model to Rest? Tremor Other Hyperkinetic Mov. 2017, 7, 473. [Google Scholar] [CrossRef]
- Wang, S.S.; Kloth, A.D.; Badura, A. The cerebellum, sensitive periods, and autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef]
- Walter, J.T.; Alvina, K.; Womack, M.D.; Chevez, C.; Khodakhah, K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat. Neurosci. 2006, 9, 389–397. [Google Scholar] [CrossRef]
- Cerminara, N.L.; Lang, E.J.; Sillitoe, R.V.; Apps, R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat. Rev. Neurosci. 2015, 16, 79–93. [Google Scholar] [CrossRef]
- Sugihara, I.; Shinoda, Y. Molecular, topographic, and functional organization of the cerebellar cortex: A study with combined aldolase C and olivocerebellar labeling. J. Neurosci. 2004, 24, 8771–8785. [Google Scholar] [CrossRef]
- Dhar, M.; Brenner, J.M.; Sakimura, K.; Kano, M.; Nishiyama, H. Spatiotemporal dynamics of lesion-induced axonal sprouting and its relation to functional architecture of the cerebellum. Nat. Commun. 2016, 7, 12938. [Google Scholar] [CrossRef]
- Ausim Azizi, S. …And the olive said to the cerebellum: Organization and functional significance of the olivo-cerebellar system. Neuroscientist 2007, 13, 616–625. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Tropepe, V. Changes in the social environment induce neurogenic plasticity predominantly in niches residing in sensory structures of the zebrafish brain independently of cortisol levels. Dev. Neurobiol. 2014, 74, 1053–1077. [Google Scholar] [CrossRef]
- Giacomini, A.C.; Abreu, M.S.; Zanandrea, R.; Saibt, N.; Friedrich, M.T.; Koakoski, G.; Gusso, D.; Piato, A.L.; Barcellos, L.J. Environmental and Pharmacological Manipulations Blunt the Stress Response of Zebrafish in a Similar Manner. Sci. Rep. 2016, 6, 28986. [Google Scholar] [CrossRef]
- Marcon, M.; Mocelin, R.; Benvenutti, R.; Costa, T.; Herrmann, A.P.; de Oliveira, D.L.; Koakoski, G.; Barcellos, L.J.G.; Piato, A. Environmental enrichment modulates the response to chronic stress in zebrafish. J. Exp. Biol. 2018, 221, jeb176735. [Google Scholar] [CrossRef]
- Dos Santos, T.G.; Mussulini, B.H.M.; Frangipani, L.A.; de Oliveira, D.L. Differential impact of shorter and longer periods of environmental enrichment on adult zebrafish exploratory activity (Danio rerio) in the novel tank paradigm. Behav. Process. 2020, 181, 104278. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiyoshi, K.; Saito, K.; Fukuda, N.; Matsuzaki, T.; Yoshikawa, H.Y.; Tsuda, S. Two-Photon Laser Ablation and In Vivo Wide-Field Imaging of Inferior Olive Neurons Revealed the Recovery of Olivocerebellar Circuits in Zebrafish. Int. J. Environ. Res. Public Health 2021, 18, 8357. https://doi.org/10.3390/ijerph18168357
Hiyoshi K, Saito K, Fukuda N, Matsuzaki T, Yoshikawa HY, Tsuda S. Two-Photon Laser Ablation and In Vivo Wide-Field Imaging of Inferior Olive Neurons Revealed the Recovery of Olivocerebellar Circuits in Zebrafish. International Journal of Environmental Research and Public Health. 2021; 18(16):8357. https://doi.org/10.3390/ijerph18168357
Chicago/Turabian StyleHiyoshi, Kanae, Kaito Saito, Narumi Fukuda, Takahisa Matsuzaki, Hiroshi Y. Yoshikawa, and Sachiko Tsuda. 2021. "Two-Photon Laser Ablation and In Vivo Wide-Field Imaging of Inferior Olive Neurons Revealed the Recovery of Olivocerebellar Circuits in Zebrafish" International Journal of Environmental Research and Public Health 18, no. 16: 8357. https://doi.org/10.3390/ijerph18168357
APA StyleHiyoshi, K., Saito, K., Fukuda, N., Matsuzaki, T., Yoshikawa, H. Y., & Tsuda, S. (2021). Two-Photon Laser Ablation and In Vivo Wide-Field Imaging of Inferior Olive Neurons Revealed the Recovery of Olivocerebellar Circuits in Zebrafish. International Journal of Environmental Research and Public Health, 18(16), 8357. https://doi.org/10.3390/ijerph18168357





