Odiel River (SW Spain), a Singular Scenario Affected by Acid Mine Drainage (AMD): Graphical and Statistical Models to Assess Diatoms and Water Hydrogeochemistry Interactions
Abstract
:1. Introduction
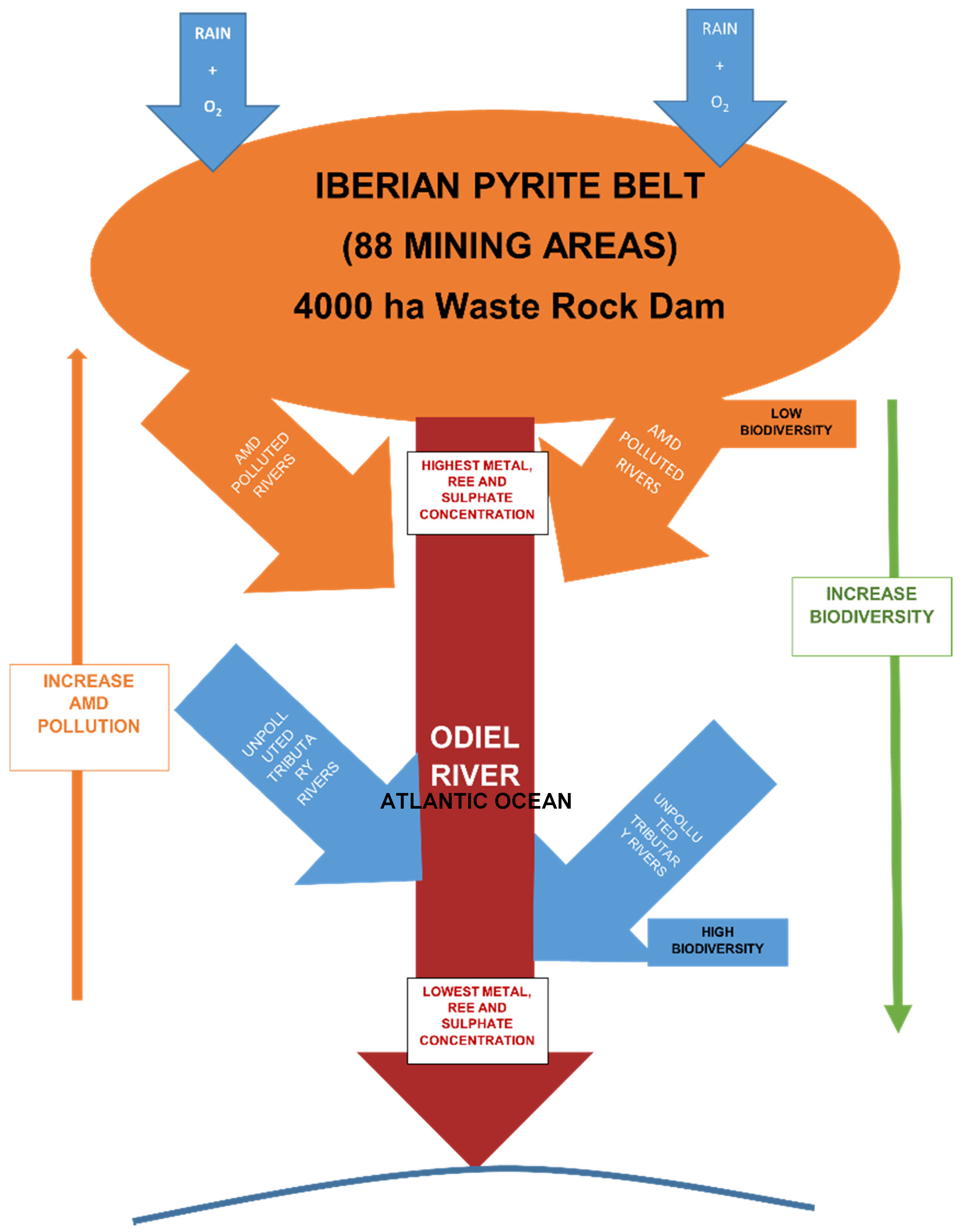
2. Location Setting
2.1. Hydrological and Mining Framework
2.2. Climatic Framework
3. Materials and Methods
3.1. Sampling
3.2. Field Measurements
3.3. Water Chemical Analysis
3.4. Diatom Analysis
3.5. Statistical Analysis
4. Results and Discussion
4.1. Grouping of Sites
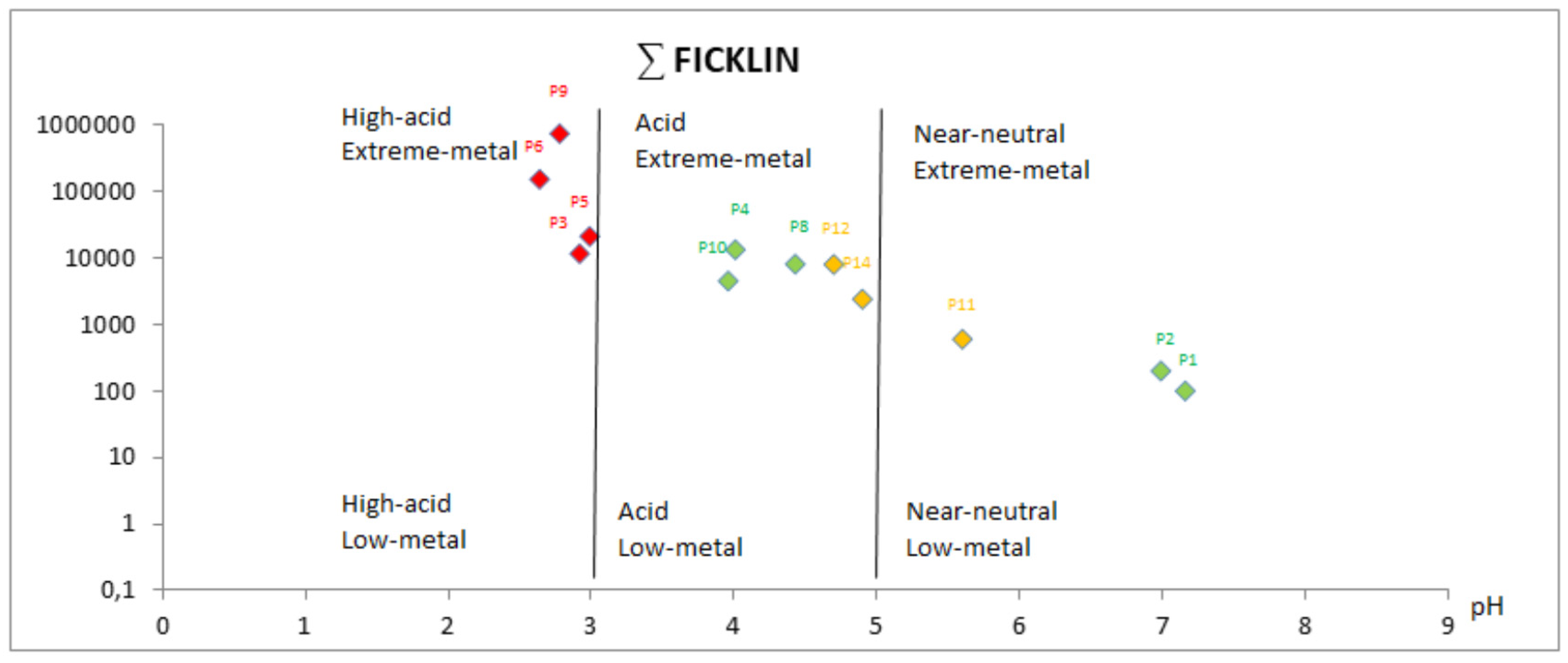
- Group 1 (Green): P1, P2, P4, P8 and 10, located in the Odiel riverbed.
- Group 2 (Red): P3, P5, P6 and PP9 located in highly contaminated tributaries (pH < 3).
- Group 3 (Orange): P11, P12 and P14, slightly affected by AMD (pH ~ 5), located in the Odiel River tributaries.
- Group 4 (Blue): P7, P13 and P15, uncontaminated (pH ~ 7).
4.2. Graphical Treatment
4.3. Statistical Treatment
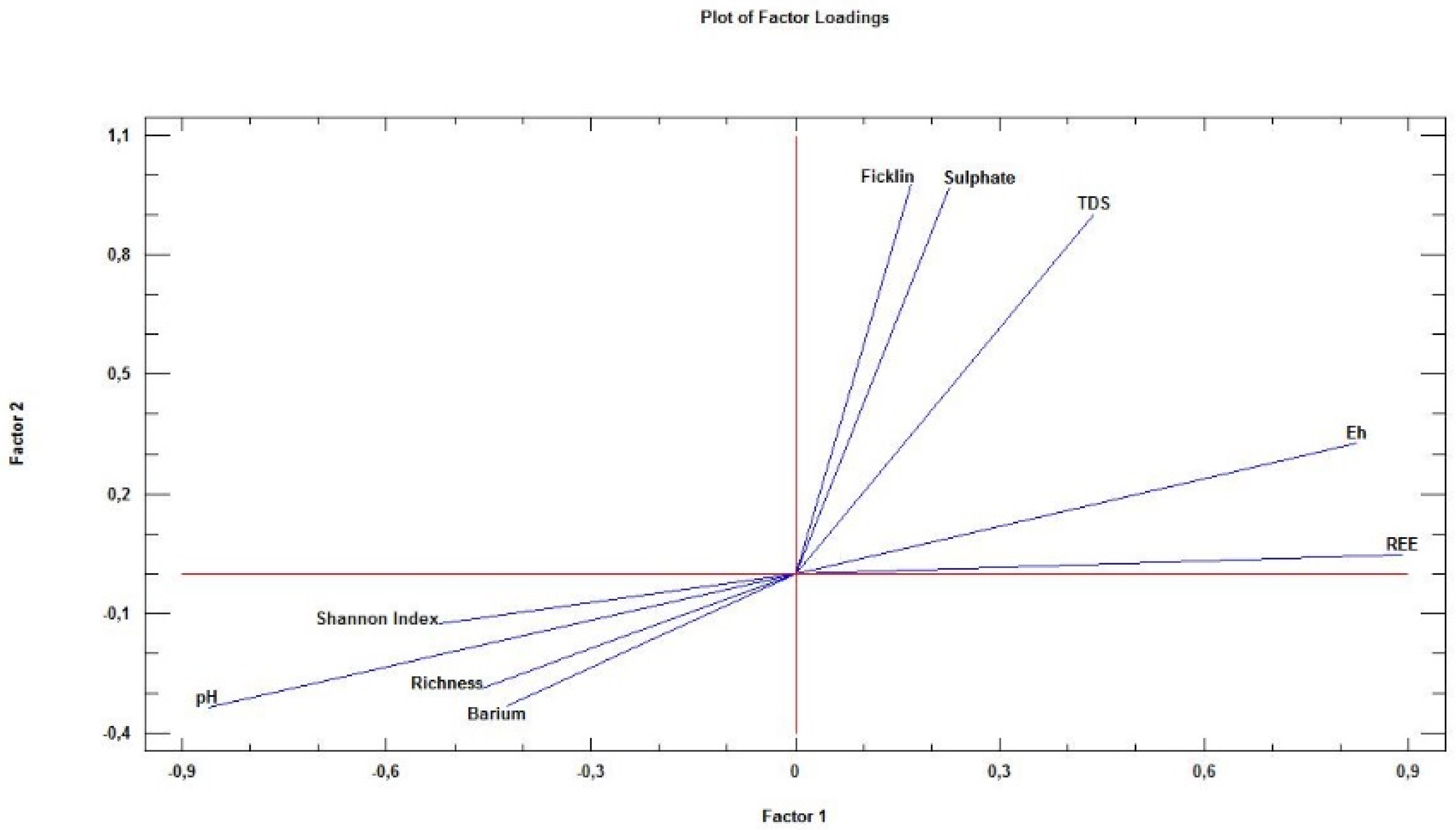
4.4. Factor Analysis
- Factor 1: Water balance. The most (and positively) affected variables are ∑REE and, at a short distance, Eh. The meaning of this is that the greater the water balance, the greater the values of these two variables. This phenomenon has already been described by [76]. At the opposite extreme, the variable pH is found; this fact is the response to the logarithmic character of pH and also because at lower pH, the water acidity is greater, and thus, the dissolution capacity is greater. Close to pH are the Shannon–Wiener and richness variables, as expected, as for extreme/low pH, extreme/low richness and diversity were found, a fact already described by [6,8,50,78,79]. It should be remembered that this region has a negative hydric balance, so in some of the sampling points, water will be found just in the rainy season. This fact has a significant impact on the survival capacities of different diatom species; thus, both richness and Shannon–Wiener are negatively affected by Factor 2. The same happens with barium, spatially associated with the previous ones in Figure 5, as it is an external element of the primary paragenesis of polymetallic sulphides and its presence is due to the anthropic activity when used as a waterproofing agent.
- Factor 2: AMD Processes. AMD processes are a determinant factor for the values taken by the variables under study. In fact, the sulphides’ oxidation into sulphates causes a release of hydrogenions and, therefore, the pH is directly and negatively affected by AMD processes. At the opposite extreme of Figure 5, three variables are found, most closely linked to AMD, ∑Ficklin, sulphates and TDS, because these variables are very highly and positively influenced by Factor 2. Factor 2 also exerts a positive influence, but much more discreet, on the dissolved REE and Eh. The reasons are different: while REE solubility product conditions its position in Factor 2 lower “weights”, as many of them are more soluble than heavy metals, the Eh is somehow more affected than the previous ones, but clearly lower than the typical AMD indicators—note how this variable appears diametrically opposite to pH, giving almost a specular line at the origin. This fact was already described by [73,76].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sampling Point | pH | Eh (mV) /100 | TDS (mg/L) /1000 | Sulphate (mg/L) /10,000 | Barium (µg/L)/10 | ∑Ficklin (µg/L)/100 | ∑REE (µg/L) /10,000 | Shannon-Wiener Index · 2 | Richness /10 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.16 | 2.37 | 0.18 | <0.01 | 4.40 | <0.01 | 0.01 | 6.22 | 4.20 |
| 2 | 6.99 | 1.41 | 0.17 | 0.04 | 4.16 | <0.01 | 0.04 | 5.14 | 3.40 |
| 3 | 2.92 | 4.36 | 4.08 | 0.51 | 1.22 | 1.23 | 1.77 | 2.68 | 1.30 |
| 4 | 4.01 | 3.65 | 0.82 | 0.09 | 3.07 | 0.13 | 1.87 | 5.14 | 3.70 |
| 5 | 2.99 | 4.77 | 2.71 | 0.35 | 0.79 | 0.21 | 2.76 | 0.88 | 0.50 |
| 6 | 2.64 | 4.52 | 4.12 | 0.80 | 0.65 | 1.53 | 0.78 | 3.24 | 1.50 |
| 7 | 6.90 | 1.95 | 0.16 | 0.01 | 7.82 | <0.01 | 0.09 | 2.22 | 0.80 |
| 8 | 4.43 | 2.13 | 0.68 | 0.06 | 2.29 | 0.08 | 1.25 | 5.64 | 3.50 |
| 9 | 2.78 | 4.39 | 9.95 | 3.07 | 0.36 | 7.42 | 1.29 | 2.98 | 0.60 |
| 10 | 3.96 | 3.08 | 0.47 | 0.04 | 1.95 | 0.05 | 0.78 | 5.08 | 4.30 |
| 11 | 5.60 | 1.80 | 0.83 | 0.03 | 2.47 | 0.01 | 0.52 | 1.78 | 1.40 |
| 12 | 4.70 | 3.03 | 1.29 | 0.01 | 2.04 | 0.08 | 1.41 | 1.82 | 0.70 |
| 13 | 7.70 | 0.42 | 0.31 | <0.01 | 1.32 | <0.01 | 0.02 | 5.82 | 3.60 |
| 14 | 4.90 | 2.19 | 0.60 | 0.03 | 1.96 | 0.02 | 0.52 | 4.58 | 1.60 |
| 15 | 7.10 | 0.60 | 0.44 | 0.01 | 1.85 | <0.01 | 0.03 | 3.06 | 1.60 |
References
- Grande, J.A. Drenaje Ácido de Mina en la Faja Pirítica Ibérica: Técnicas de Estudio e Inventario de Explotaciones; Servicio de Publicaciones de la Universidad de Huelva: Huelva, Spain, 2016; p. 345. [Google Scholar]
- Grande, J.A.; Santisteban, M.; de la Torre, M.L.; Fortes, J.C.; de Miguel, E.; Curiel, J.; Dávila, J.M.; Biosca, B. The paradigm of Circular Mining in the world: The Iberian Pyrite Belt as a potential scenario of interaction. Environ. Earth Sci. 2018, 77, 391. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Towsend, C.R. Ecología. Individuos, Poblaciones y Comunidades, 1st ed.; Omega: Barcelona, Spain, 1986; p. 886. [Google Scholar]
- Odum, W.E. The Ecology of Tidal Freshwater Marshes of the United States East Coast: A Community Profile; US.Fish Wildl. Serv, 1984; p. 177.
- Valente, T.; Rivera, M.J.; Almeida, S.F.P.; Delgado, C.; Gomes, P.; Grande, J.A.; de la Torre, M.L.; Santisteban, M. Characterization of water reservoirs affected by acid mine drainage: Geochemical, mineralogical, and biological (diatoms) properties of the water. Environ. Sci. Pollut. Res. 2016, 23, 6002–6011. [Google Scholar] [CrossRef] [PubMed]
- Luís, A.T.; Grande, J.A.; Dávila, J.M.; Aroba, J.; Durães, N.; Almeida, S.F.P.; de la Torre, M.L.; Sarmiento, A.M.; Fortes, J.C.; Ferreira da Silva, E.; et al. Application of fuzzy logic tools for the biogeochemical characterisation of (un) contaminated waters from Aljustrel mining area (South Portugal). Chemosphere 2018, 211, 736–744. [Google Scholar] [CrossRef]
- Blinn, D.; Herbst, D. Use of diatoms and soft algae as indicators of environmental determinants in the Lahontan Basin, USA. In Annual Report for California State Water Resources Board; Contract Agreement 704558.CT766; 2003; p. 35. [Google Scholar]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Ector, L.; Matos, J.X.; Ferreira da Silva, E. Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut. 2009, 200, 147–167. [Google Scholar] [CrossRef]
- Gensemer, R.W. Role of aluminium and growth rate on changes in cell size and silica content of silica-limited populations of Asterionella ralfsii var. americana (Bacillariophyceae). J. Phycol. 1990, 26, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Matos, J.X.; Ferreira da Silva, E. Environmental impact of mining activities in the Lousal area (Portugal): Chemical and diatom characterization of metal-contaminated stream sediments and surface water of Corona stream. Sci. Total Environ. 2011, 409, 4312–4325. [Google Scholar] [CrossRef]
- Lessmann, D.; Fyson, A.; Nixdorf, B. Phytoplankton of the extremely acidic mining lakes of Lusatia (Germany) with pH < 3. Hydrobiologia 2000, 433, 123–128. [Google Scholar]
- Luís, A.T.; Bonet, B.; Corcoll, N.; Almeida, S.F.P.; Ferreira da Silva, E.; Figueira, E.; Guasch, H. Experimental evaluation of the contribution of acidic pH and Fe concentration to the structure, function and tolerance to metals (Cu and Zn) in fluvial biofilms. Ecotoxicology 2014, 23, 1270–1282. [Google Scholar] [CrossRef]
- Davis, R.A.; Welty, A.T.; Borrego, J.; Morales, J.A.; Pendon, J.G.; Ryan, J.G. Rio Tinto estuary (Spain): 5000 years of pollution. Environ. Geol. 2000, 39, 1107–1116. [Google Scholar] [CrossRef]
- Grande, J.A.; Borrego, J.; de La Torre, M.L.; Sainz, A. Application of cluster analysis to the geochemistry zonation of the estuary waters in the Tinto and Odiel Rivers (Huelva, Spain). Environ. Geochem. Health 2003, 25, 233–246. [Google Scholar] [CrossRef]
- Grande, J.A.; Santisteban, M.; De la Torre, M.L.; Valente, T.; Pérez-Ostalé, E. Characterisation of AMD pollution in the reservoirs of the Iberian Pyrite Belt. Mine Water Environ. 2013, 32, 321–330. [Google Scholar] [CrossRef]
- Pinedo Vara, I. Piritas de Huelva. Su historia, Minería y Aprovechamiento; Summa: Madrid, Spain, 1963; p. 1003. [Google Scholar]
- MOPU. Medio Ambiente en España 1986; Dirección General del Medio Ambiente: Madrid, Spain, 1987; p. 352. [Google Scholar]
- Cabrera, F.; Conde, B.; Flores, V. Heavy metals in the surface sediments of the tidal river Tinto (SW Spain). Fresen. Env. Bull 1992, 1, 400–405. [Google Scholar]
- Nelson, C.H.; Lamothe, P.J. Heavy metals anomalies in the Tinto and Odiel River and Estuary System, Spain. Estuaries 1993, 16, 496–511. [Google Scholar] [CrossRef]
- Azcue, J.M. Environmental Impacts of Mining Activities; Springer: Berlin/Heidelberg, Germany, 1999; p. 300. [Google Scholar]
- Elbaz-Poulichet, F.; Morley, N.H.; Cruzado, A.; Velasquez, Z.; Achterberg, E.P.; Braungardt, C.B. Trace metal and nutrient distribution in an extremely low pH (2.5) river-estuarine system, the Ria of Huelva (south-west Spain). Sci. Total Environ. 1999, 227, 73–83. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Braungardt, C.; Achterberg, E.; Morley, N.; Cossa, D.; Beckers, J.; Nomérange, P.; Cruzado, A.; Leblanc, M. Metal biogeochemistry In the Tinto- Odiel rivers (Southern Spain) and in the Gulf of Cadiz: A synthesis of results of TOROS project. Cont. Shelf Res. 2001, 21, 1961–1973. [Google Scholar] [CrossRef]
- Grande, J.A.; Sáinz, A.; de la Torre, M.L.; López, N. Caracterización de procesos AMD y ARD en la cuenca del río Odiel mediante aplicación de análisis univariante. Ingeopres 2000, 88, 52–60. [Google Scholar]
- Grande, J.A.; Borrego, J.; Morales, J.A.; de la Torre, M.L. A description of how metal pollution occurs in the Tinto-Odiel rias (Huelva-Spain) through the application of cluster analysis. Mar. Pollut. Bull. 2003, 46, 475–480. [Google Scholar] [CrossRef]
- Luís, A.T.; Durães, N.; Almeida, S.F.P.; Ferreira da Silva, E. Integrating geochemical (surface waters, stream sediments) and biological (diatoms) approaches to assess environmental impact in a pyritic mining area: Aljustrel (Alentejo, Portugal). J. Environ. Sci. 2016, 42, 215–226. [Google Scholar] [CrossRef]
- Grande, J.A.; Andújar, J.M.; Aroba, J.; de la Torre, M.L.; Beltrán, R. Precipitation, pH and metal load in AMD river basins: An application of fuzzy clustering algorithms to the process characterization. J. Environ. Monitor. 2005, 7, 325–334. [Google Scholar] [CrossRef]
- Grande, J.A.; Beltrán, R.; Sáinz, A.; Santos, J.C.; de la Torre, M.L.; Borrego, J. Acid mine drainage and acid rock drainage processes in the environment of Herrerías Mine (Iberian Pyrite Belt, Huelva-Spain) and impact on the Andevalo Dam. Environ. Geol. 2005, 47, 185–196. [Google Scholar] [CrossRef]
- Grande, J.A.; Aroba, J.; Andujar, J.M.; Gómez, T.; de la Torre, M.L.; Borrego, J.; Romero, S.; Barranco, C.; Santisteban, M. Tinto versus Odiel: Two AMD polluted rivers and an unresolved issue. An artificial intelligence approach. Water Resour. Manag. 2011, 25, 3575–3594. [Google Scholar]
- Grande, J.A.; Valente, T.; de la Torre, M.L.; Santisteban, M.; Cerón, J.C.; Pérez-Ostalé, E. Characterization of acid mine drainage sources in the Iberian Pyrite Belt: Base methodology for quantifying affected areas and for environmental management. Environ. Earth Sci. 2014, 71, 2729–2738. [Google Scholar] [CrossRef]
- Grande, J.A.; Santisteban, M.; Valente, T.; de la Torre, M.L.; Gomes, P. Hydrochemical characterization of a river affected by acid mine drainage in the Iberian Pyrite Belt. Water Sci. Technol. 2017, 75, 2499–2507. [Google Scholar] [CrossRef]
- Leblanc, M.; Morales, J.M.; Borrego, J.; Elbaz-Poulichet, F. 4.500 year-old mining pollution in Southwestern Spain: Long-term implications for modern mining pollution. Econ. Geol. 2000, 95, 655–662. [Google Scholar]
- Borrego, J.; Morales, J.A.; de la Torre, M.L.; Grande, J.A. Geochemical characteristics of heavy metal pollution in surface sediments of the Tinto and Odiel river estuary (southwestern Spain). Environ. Geol. 2002, 41, 785–796. [Google Scholar] [CrossRef]
- Sáinz, A.; Grande, J.A.; de la Torre, M.L.; Sánchez-Rodas, D. Characterisation of sequential leachate discharges of mining waste rock dumps in the Tinto and Odiel rivers. J. Environ. Manag. 2002, 64, 345–353. [Google Scholar] [CrossRef]
- Sáinz, A.; Grande, J.A.; De la Torre, M.L. Analysis of the impact of local corrective measures on the input of contaminants from the Odiel river to the ria of Huelva (Spain). Water Air Soil Pollut. 2003, 144, 375–389. [Google Scholar] [CrossRef]
- Sáinz, A.; Grande, J.A.; de la Torre, M.L. Odiel river, acid mine drainage and current characterisation by means of univariate analysis. Environ. Int. 2003, 29, 51–59. [Google Scholar] [CrossRef]
- Sáinz, A.; Grande, J.A.; de la Torre, M.L. Characterization of heavy metal discharge into the ria of Huelva. Environ. Int. 2004, 30, 557–566. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Pamo, E.L.; Santofimia, E.; Aduvire, O.; Reyes, J.; Barettino, D. Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): Geochemistry, mineralogy and environmental implications. Appl. Geochem. 2005, 20, 1320–1356. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water—Hydrology, Pollution, Remediation. Environmental Pollution; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; p. 442. [Google Scholar]
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Cánovas, C.; Riba, I.; Kalman, J.; Delvalls, T.A. Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva estuary. Environ. Int. 2007, 33, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, C.R.; Hubbard, C.G.; Olías, M.; Nieto, J.M.; Black, S.; Coleman, M.L. Hydrochemical variations and contaminant load in the Río Tinto (Spain) during flood events. J. Hydrol. 2008, 350, 25–40. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Olías, M.; Nieto, J.M.; Sarmiento, A.M.; Cerón, J.C. Hydrogeochemical characteristics of the Tinto and Odiel rivers (SW Spain). Factor controlling metal contents. Sci. Total Environ. 2007, 373, 363–382. [Google Scholar]
- Jiménez, A.; Aroba, J.; de la Torre, M.L.; Andújar, J.M.; Grande, J.A. Model of behaviour of conductivity versus pH in acid mine drainage water, based on fuzzy logic and data mining techniques. J. Hydroinform. 2009, 11, 147–153. [Google Scholar] [CrossRef]
- Sarmiento, A.M.; Nieto, J.; Casiot, C.; Elbaz-Poulichet, F.; Ega, M. Inorganic arsenic speciation at river basin scales: The Tinto and Odiel rivers in the Iberian Pyrite Belt, SW Spain. Environ. Pollut. 2009, 157, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, M.; Nieto, J.M.; Olías, M.; Cánovas, C. Hydrochemical characteristics and seasonal influence on the pollution by acid mine drainage in the Odiel river Basin (SW Spain). Appl. Geochem. 2009, 24, 697–714. [Google Scholar] [CrossRef]
- Sarmiento, A.M.; Grande, J.A.; Luís, A.T.; Dávila, J.M.; Fortes, J.C.; Santisteban, M.; Curiel, J.; de la Torre, M.L.; Ferreira da Silva, E.F. Negative pH values in an open-air radical environment affected by acid mine drainage. Characterization and proposal of a hydrogeochemical model. Sci. Total Environ. 2018, 644, 1244–1253. [Google Scholar] [CrossRef]
- Viers, J.; Grande, J.A.; Zouiten, C.; Freydier, R.; Masbou, J.; Valente, T.; de la Torre, M.L.; Destrigneville, C.; Pokrovsky, O.S. Are Cu isotopes a useful tool to trace metal sources and processes in acid mine drainage (AMD) context? Chemosphere 2018, 193, 1071–1079. [Google Scholar] [CrossRef]
- González-Toril, E.; Llobet-Brossa, E.; Casamayor, E.O.; Amann, R.; Amils, R. Microbial ecology of an extreme acidic environment, the Tinto River. Appl. Environ. Microbiol. 2003, 69, 4853–4865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, A.; Souza-Egipsy, V.; Gomez, F.; Amils, R. Development and structure of eukaryotic biofilms in an extreme acidic environment, Río Tinto (SW, Spain). Microb. Ecol. 2007, 53, 294–305. [Google Scholar] [CrossRef]
- Amils, R.; González-Toril, E.; Fernández-Remolar, D.; Gómez, F.; Aguilera, Á.; Rodríguez, N.; Malki, M.; García-Moyano, A.; Fairén, G.A.; de la Fuente, V.; et al. Extreme environments as Mars terrestrial analogs: The Rio Tinto case. Planet. Space. Sci. 2007, 55, 370–381. [Google Scholar] [CrossRef]
- Rivera, M.J.; Luís, A.T.; Grande, J.A.; Sarmiento, A.M.; Dávila, J.M.; Fortes, J.C.; Córdoba, F.; Díaz-Curiel, J.; Santisteban, M. Physico-Chemical Influence of Surface Water Contaminated by Acid Mine Drainage on the Populations of Diatoms in Dams (Iberian Pyrite Belt, SW Spain). Int. J. Environ. Res. Public Health 2019, 16, 4516. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Acid Mine Drainage Prediction. U.S. Environmental Protection Agency; Office of Solid Waste: Washington, DC, USA, 1994.
- Grande, J.A.; Borrego, J.; Morales, J.A. A study of heavy metal pollution in the Tinto-Odiel estuary in southwestern Spain using factor analysis. Environ. Geol. 2000, 39, 1095–1101. [Google Scholar] [CrossRef]
- Dogan, P.A. Characterization of mine waste for prediction of acid mine drainage. In Environmental Impacts of Mining Activities; Azcue, J.M., Ed.; Springer: Berlin, Germany, 1999; Volume 1, pp. 19–38. [Google Scholar]
- Nicholson, R.V. Iron-sulfide oxidation mechanism. In Chemical Weathering Rates of Silicate Minerals; White, A.F., Brantley, R.J., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1994; Volume 31, pp. 173–225. [Google Scholar]
- Förstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment, 2nd ed.; Springer: Berlin, Germany, 1983; p. 488. [Google Scholar]
- EMCBC. The Perpetual Pollution Machine Acid Mine Drainage; BC Mining Control: Canada, 1996; pp. 1–6. [Google Scholar]
- Álvaro, A. Mineralogía y Geoquímica de Sulfatos Secundarios en Ambientes de Drenaje Ácido de Mina. Área Minera del Yacimiento de San Miguel (Faja Pirítica Ibércia). Ph.D. Thesis, Universidad del País Vasco, Lejona, Spain, 2010. [Google Scholar]
- Luís, A.T.; Grande, J.A.; Durães, N.; Dávila, J.M.; Santisteban, M.; Almeida, S.F.P.; de la Torre, M.L.; Sarmiento, A.M.; Fortes, J.C.; Ferreira da Silva, E.F. Biogeochemical characterization of surface waters in the Aljustrel mining area (South Portugal). Environ. Geochem. Health 2019, 41, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- European Community (EC). Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Commun. 2000, 327, 1–72. [Google Scholar]
- Prygiel, J.; Coste, M. Guide Méthodologique Pour la Mise en Oeuvre de L’indice Biologique Diatomées NF T 90–354; Agence de l’eau Artois Picardie: Douai, France, 2000; p. 340. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bacillariophyceae. Bacillariophyceae: (vol.I) Naviculaceae. pp 876; (vol.2) Bacillariaceae, Epithemiaceae, Surirellaceae. pp 596; (vol.3) Centrales, Fragilariaceae, Eunotiaceae. pp 577; vol (4) Achnanthaceae. pp 437. 2; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1991. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology & Morphology of the Genera; University Press: Cambridge, UK, 1990; p. 749. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Plumlee, G.S.; Smith, K.S.; Montour, M.R.; Ficklin, W.H.; Mosier, E.L. Geologic controls on the composition of natural waters and mine waters draining diverse mineral-deposit types. In Reviews in Economic Geology. The Environmental Geochemistry of Mineral Deposits; Filipek, L.H., Plumlee, G.S., Eds.; Society of Economic Geologists, INC. Ed.: Chelsea, MI, USA, 1999; Volume 6B, pp. 373–432. [Google Scholar]
- Siqueiros-Beltrones, D.A.; Argumedo-Hernández, U.; Murillo-Jiménez, J.M.; Marmolejo-Rodríguez, A.J. Diversidad de diatomeas bentónicas marinas en un ambiente ligeramente enriquecido con elementos potencialmente tóxicos. Rev. Mex. Biodiversi. 2014, 85, 1065–1085. [Google Scholar] [CrossRef] [Green Version]
- Neff, J.M. Effect of Contaminants from Oil Well Produced Water, 1st ed.; Elsevier: London, UK, 2002; p. 452. [Google Scholar]
- Bonny, S.M.; Jones, B. Diatom-mediated barite precipitation in microbial mats calcifying at Stinking Springs, a warm sulphur spring system in Northwestern Utah, USA. Sediment. Geol. 2007, 94, 223–244. [Google Scholar] [CrossRef]
- Elias, C.L.; Calapez, A.R.; Almeida, S.F.P.; Feio, M.J. From perennial to temporary streams: An extreme drought as a driving force of freshwater communities’ traits. Mar. Freshwater Res. 2015, 66, 469–480. [Google Scholar] [CrossRef]
- Grande, J.A.; Jiménez, A.; Borrego, J.; María, L.; Gómez, T. Relationships between conductivity and pH in channels exposed to acid mine drainage processes: Study of a large mass of data using classical statistics. Water Resour. Manag. 2010, 24, 4579–4587. [Google Scholar] [CrossRef]
- Valente, T.; Ferreira, M.J.; Grande, J.A.; de la Torre, M.L.; Borrego, J. pH, electric conductivity and sulfate as base parameters to estimate the concentration of metals in AMD using a fuzzy inference system. J. Geochem. Explor. 2013, 124, 22–28. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Lu, T.; Zhang, M.; Ke, M.; Fu, Z.; Pan, X.; Qian, H. A comparison of the effects of copper nanoparticles and copper sulfate on Phaeodactylum tricornutum physiology and transcription. Environ. Toxicol. Pharm. 2017, 56, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lottermoser, B. Mine Wastes; Springer: Berlin, Germany, 2003. [Google Scholar]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.C. Statistics and Data Analysis in Geology, 2nd ed.; J. Wiley: New York, NY, USA, 1986; p. 656. [Google Scholar]
- Grande, J.A.; Gonzalez, A.; Beltran, R.; Sánchez-Rodas, D. Application of Factor Analysis to the Study of Contamination in the Aquifer System of Ayamonte-Huelva (Spain). Groundwater 1996, 34, 155–161. [Google Scholar] [CrossRef]
- de la Torre, M.L.; Grande, J.A.; Valente, T.; Perez-Ostalé, E.; Santisteban, M.; Aroba, J.; Ramos, I. Definition of redox and pH influence in the AMD mine system using a fuzzy qualitative tool (Iberian Pyrite Belt, SW Spain). Environ. Sci. Pollut. Res. 2016, 23, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Brake, S.S.; Hasiotis, S.T. Eukaryote-dominated biofilms and their significance in acidic environments. Geomicrobiol. J. 2010, 27, 534–558. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A. Eukaryotic diversity at pH extremes. Front. Microbiol. 2013, 3, 441. [Google Scholar] [CrossRef] [Green Version]



| pH | Eh (mV) | TDS (ppm) | Sulphate (ppm) | Barium (ppb) | ∑Ficklin (ppb) | ∑REE (ppb) | Shannon Index | Richness | |
|---|---|---|---|---|---|---|---|---|---|
| Number points | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Average | 4.99 | 235.53 | 1787.07 | 3358.95 | 24.23 | 71.7 | 8768.29 | 1.88 | 21.8 |
| Variation coefficient | 36.22% | 70.85% | 146.89% | 235.71% | 78.09% | 266.73% | 94.94% | 45.58% | 64.95% |
| Minimum | 2.64 | 16.0 | 155.0 | 18.0 | 3.58 | 0 | 113.39 | 0.44 | 5.0 |
| Maxima | 7.7 | 477.0 | 9950.0 | 30,707.0 | 78.2 | 741.6 | 2761.,1 | 3.11 | 43.0 |
| Factor Number | Eingenvalue | % of Variance | % of Cumulative Variance |
|---|---|---|---|
| 1 | 4.88 | 67.85 | 67.85 |
| 2 | 1.43 | 19.87 | 87.72 |
| 3 | 0.79 | 1.93 | 98.65 |
| 4 | 0.08 | 1.10 | 99.76 |
| 5 | 0.02 | 0.24 | 100 |
| 6 | 0 | 0 | 100 |
| 7 | 0 | 0 | 100 |
| 8 | 0 | 0 | 100 |
| 9 | 0 | 0 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, J.A.; Luís, A.T.; Córdoba, F.; Leiva, M.; Dávila, J.M.; Fortes, J.C.; Santisteban, M.; Ferreira da Silva, E.; Sarmiento, A.M. Odiel River (SW Spain), a Singular Scenario Affected by Acid Mine Drainage (AMD): Graphical and Statistical Models to Assess Diatoms and Water Hydrogeochemistry Interactions. Int. J. Environ. Res. Public Health 2021, 18, 8454. https://doi.org/10.3390/ijerph18168454
Grande JA, Luís AT, Córdoba F, Leiva M, Dávila JM, Fortes JC, Santisteban M, Ferreira da Silva E, Sarmiento AM. Odiel River (SW Spain), a Singular Scenario Affected by Acid Mine Drainage (AMD): Graphical and Statistical Models to Assess Diatoms and Water Hydrogeochemistry Interactions. International Journal of Environmental Research and Public Health. 2021; 18(16):8454. https://doi.org/10.3390/ijerph18168454
Chicago/Turabian StyleGrande, José A., Ana Teresa Luís, Francisco Córdoba, Mercedes Leiva, José Miguel Dávila, Juan Carlos Fortes, María Santisteban, Eduardo Ferreira da Silva, and Aguasanta Miguel Sarmiento. 2021. "Odiel River (SW Spain), a Singular Scenario Affected by Acid Mine Drainage (AMD): Graphical and Statistical Models to Assess Diatoms and Water Hydrogeochemistry Interactions" International Journal of Environmental Research and Public Health 18, no. 16: 8454. https://doi.org/10.3390/ijerph18168454





