Factors Affecting Rapid Cognitive Decline in Patients with Alzheimer’s Disease: A Longitudinal Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
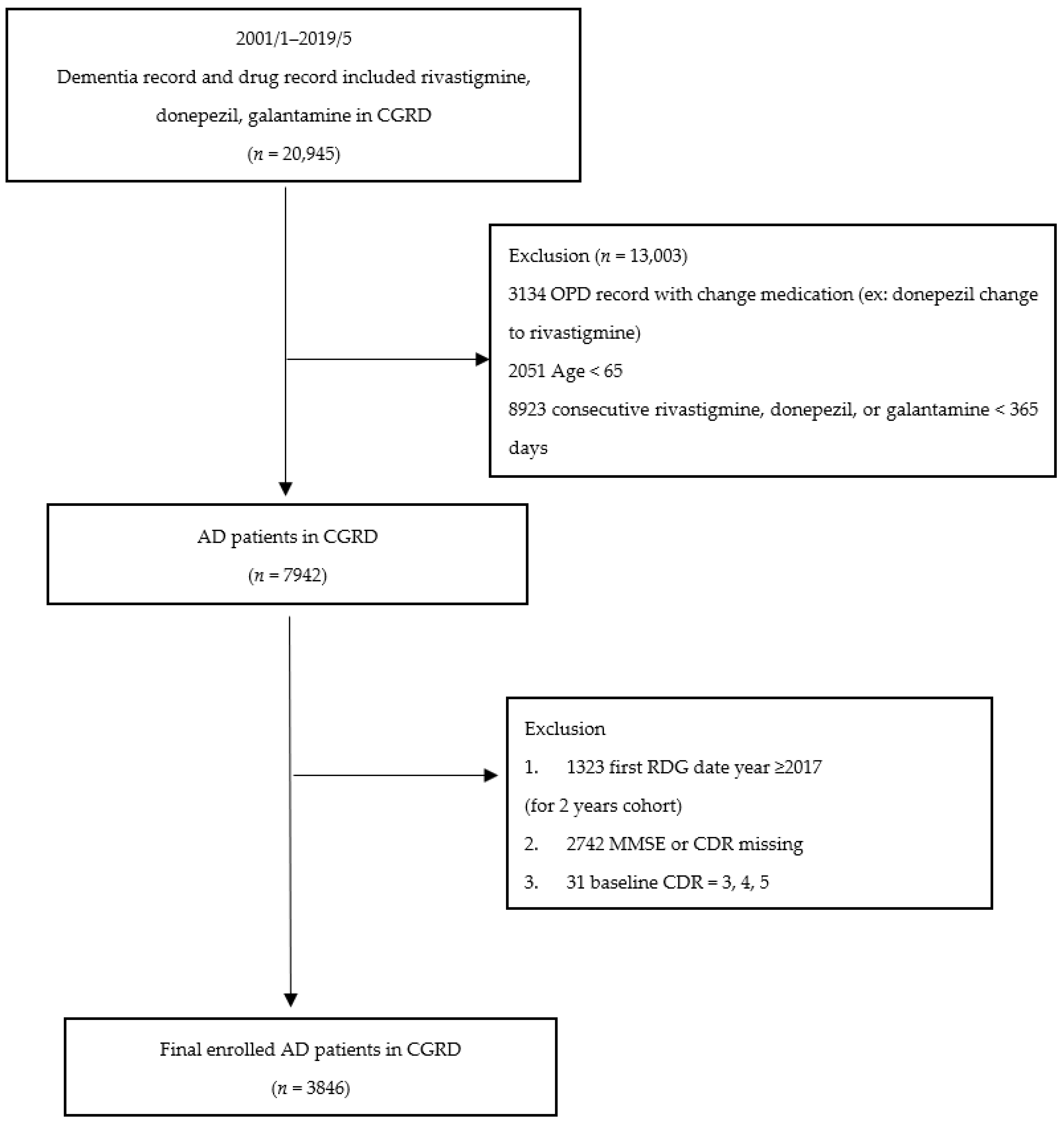
2.2. Selection of Patients with AD and Comorbidities
2.3. Patient Data and Their Anonymity
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
3.2. Risk of Rapid Cognitive Decline among Patients with AD
3.3. Sensitivity Analysis: Risk of Rapid Cognitive Decline among Patients with AD Stratified by Age
3.4. Sensitivity Analysis: Risk of Rapid Cognitive Decline among Patients with AD Stratified by HTN and T2DM
4. Discussion
Strengthens and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Small, G.; Bullock, R. Defining optimal treatment with cholinesterase inhibitors in Alzheimer’s disease. Alzheimers Dement. 2011, 7, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Alexander, G.C.; Knopman, D.S.; Emerson, S.S.; Ovbiagele, B.; Kryscio, R.J.; Perlmutter, J.S.; Kesselheim, A.S. Revisiting FDA Approval of Aducanumab. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Fink, H.A.; Linskens, E.J.; MacDonald, R.; Silverman, P.C.; McCarten, J.R.; Talley, K.M.; Forte, M.L.; Desai, P.J.; Nelson, V.A.; Miller, M.A.; et al. Benefits and harms of prescription drugs and supplements for treatment of clinical Alzheimer-type dementia: A systematic review and meta-analysis. Ann. Intern. Med. 2020, 172, 656–668. [Google Scholar] [CrossRef]
- Likitjaroen, Y.; Meindl, T.; Friese, U.; Wagner, M.; Buerger, K.; Hampel, H.; Teipel, S.J. Longitudinal changes of fractional anisotropy in Alzheimer’s disease patients treated with galantamine: A 12-month randomized, placebo-controlled, double-blinded study. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 341–350. [Google Scholar] [CrossRef]
- Cummings, J.L.; Farlow, M.R.; Meng, X.; Tekin, S.; Olin, J.T. Rivastigmine transdermal patch skin tolerability. Clin. Drug Investig. 2010, 30, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Engedal, K.; Soininen, H.; Verhey, F.; Waldemar, G.; Wimo, A.; Wetterholm, A.L.; Zhang, R.; Haglund, A.; Subbiah, P.; et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001, 57, 489–495. [Google Scholar] [CrossRef]
- Schmidt, C.; Wolff, M.; Weitz, M.; Bartlau, T.; Korth, C.; Zerr, I. Rapidly progressive Alzheimer disease. Arch. Neurol. 2011, 68, 1124–1130. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.C.; Edland, S.; Clark, C.; Galasko, D.; Koss, E.; Mohs, R.; Van Belle, G.; Fillenbaum, G.; Heyman, A. The consortium to establish a registry for Alzheimer’s disease (CERAD): Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology 1993, 43, 2457–2465. [Google Scholar] [CrossRef]
- Blom, K.; Vaartjes, I.; Peters, S.A.; Koek, H.L. The influence of vascular risk factors on cognitive decline in patients with Alzheimer’s disease. Maturitas 2014, 79, 96–99. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wu, M.N.; Chou, P.S.; Su, H.C.; Lin, S.H.; Sung, P.S. Longitudinal neuropsychological outcome in Taiwanese Alzheimer’s disease patients treated with medication. Curr. Alzheimer Res. 2018, 15, 474–481. [Google Scholar] [CrossRef]
- Domínguez, R.O.; Marschoff, E.R.; González, S.E.; Repetto, M.G.; Serra, J.A. Type 2 diabetes and/or its treatment leads to less cognitive impairment in Alzheimer’s disease patients. Diabetes Res. Clin. Pract. 2012, 98, 68–74. [Google Scholar] [CrossRef]
- Brainard, J.; Wilsher, S.H.; Salter, C.; Loke, Y.K. Methodological review: Quality of randomized controlled trials in health literacy. BMC Health Serv. Res. 2016, 16, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sona, A.; Zhang, P.; Ames, D.; Bush, A.I.; Lautenschlager, N.T.; Martins, R.; Masters, C.L.; Rowe, C.C.; Szoeke, C.; Taddei, K.; et al. Predictors of rapid cognitive decline in Alzheimer’s disease: Results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of ageing. Int. Psychogeriatr. 2012, 24, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.M.; Storandt, M.; Roe, C.M.; Morris, J.C. Progression of Alzheimer’s disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement. 2013, 9, S39–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, H.; Hanyu, H.; Sato, T.; Kanetaka, H.; Shimizu, S.; Hirao, K.; Kikukawa, M.; Iwamoto, T. Vascular risk factors and progression in Alzheimer’s disease. Geriatr. Gerontol. Int. 2011, 11, 211–214. [Google Scholar] [CrossRef]
- Musicco, M.; Palmer, K.; Salamone, G.; Lupo, F.; Perri, R.; Mosti, S.; Spalletta, G.; Di Iulio, F.; Pettenati, C.; Cravello, L.; et al. Predictors of progression of cognitive decline in Alzheimer’s disease: The role of vascular and sociodemographic factors. J. Neurol. 2009, 256, 1288–1295. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C.C. The Chang Gung Research Database—A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Andrieu, S.; Cantet, C.; Reynish, E.; Ousset, P.-J.; Arbus, C.; Gillette-Guyonnet, S.; Nourhashémi, F.; Vellas, B.; REAL.FR Group. Predictive value of rapid decline in Mini Mental State Examination in clinical practice for prognosis in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 26, 109–116. [Google Scholar] [CrossRef]
- Wilkinson, D.; Passmore, A.; Bullock, R.; Hopker, S.; Smith, R.; Potocnik, F.; Maud, C.; Engelbrecht, I.; Hock, C.; Ieni, J.; et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int. J. Clin. Pract. 2002, 56, 441–446. [Google Scholar]
- Hansen, R.A.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, C.G.; Jonas, D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging. 2008, 3, 211–215. [Google Scholar]
- Mungas, D.; Reed, B.R.; Ellis, W.G.; Jagust, W.J. The effects of age on rate of progression of Alzheimer disease and dementia with associated cerebrovascular disease. Arch. Neurol. 2001, 58, 1243–1247. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.S.; Beckett, L.A.; Barnes, L.L.; Schneider, J.A.; Bach, J.; Evans, D.A.; Bennett, D.A. Individual differences in rates of change in cognitive abilities of older persons. Psychol. Aging. 2002, 17, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.J.; Hansen, L.A.; Alford, M.F.; Foster, K.; Salmon, D.P.; Galasko, D.; Thal, L.J.; Masliah, E. Age at onset is associated with disease severity in Lewy body variant and Alzheimer’s disease. Neuroreport 2002, 13, 1825–1828. [Google Scholar] [CrossRef]
- Bernick, C.; Cummings, J.; Raman, R.; Sun, X.; Aisen, P. Age and rate of cognitive decline in Alzheimer disease: Implications for clinical trials. Arch. Neurol. 2012, 69, 901–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.N.; Mormino, E.C.; Rogalski, E.J.; Kawas, C.H.; Willis, R.J.; Park, D.C. What are the later life contributions to reserve, resilience, and compensation? Neurobiol. Aging. 2019, 83, 140–144. [Google Scholar] [CrossRef]
- Golchert, J.; Roehr, S.; Luck, T.; Wagner, M.; Fuchs, A.; Wiese, B.; van den Bussche, H.; Brettschneider, C.; Werle, J.; Bickel, H.; et al. Women Outperform Men in Verbal Episodic Memory Even in Oldest-Old Age: 13-Year Longitudinal Results of the AgeCoDe/AgeQualiDe Study. J. Alzheimers Dis. 2019, 69, 857–869. [Google Scholar] [CrossRef]
- Van Exel, E.; Gussekloo, J.; De Craen, A.J.; Bootsma-Van Der Wiel, A.; Houx, P.; Knook, D.L.; Westendorp, R.G. Cognitive function in the oldest old: Women perform better than men. J. Neurol. Neurosurg. Psychiatry 2001, 71, 29–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, C.; Andrieu, S.; Sinclair, A.; Hanaire, H.; Vellas, B. Diabetes is associated with a slower rate of cognitive decline in Alzheimer disease. Neurology 2009, 73, 1359–1366. [Google Scholar] [CrossRef]
- Secnik, J.; Cermakova, P.; Fereshtehnejad, S.-M.; Dannberg, P.; Johnell, K.; Fastbom, J.; Winblad, B.; Eriksdotter, M.; Religa, D. Diabetes in a large dementia cohort: Clinical characteristics and treatment from the Swedish dementia registry. Diabetes Care 2017, 40, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, M.; Xu, Z.-Q.; Gao, C.-Y.; Fang, C.-Q.; Deng, J.; Yan, J.-C.; Wang, Y.-J.; Zhou, H.-D. Vascular risk aggravates the progression of Alzheimer’s disease in a Chinese cohort. J. Alzheimers Dis. 2010, 20, 491–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielke, M.; Rosenberg, P.; Tschanz, J.; Cook, L.; Corcoran, C.; Hayden, K.; Norton, M.; Rabins, P.; Green, R.; Welsh-Bohmer, K.; et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology 2007, 69, 1850–1858. [Google Scholar] [CrossRef]
- Helzner, E.P.; Luchsinger, J.A.; Scarmeas, N.; Cosentino, S.; Brickman, A.M.; Glymour, M.M.; Stern, Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch. Neurol. 2009, 66, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Bellew, K.M.; Pigeon, J.G.; Stang, P.E.; Fleischman, W.; Gardner, R.M.; Baker, W.W. Hypertension and the rate of cognitive decline in patients with dementia of the Alzheimer type. Assoc. Disord. 2004, 18, 208–213. [Google Scholar]
- Safouris, A.; Hambye, A.S.; Sculier, C.; Papageorgiou, S.G.; Vasdekis, S.N.; Gazagnes, M.D.; Tsivgoulis, G. Chronic brain hypoperfusion due to multi-vessel extracranial atherosclerotic disease: A potentially reversible cause of cognitive impairment. J. Alzheimers Dis. 2015, 43, 23–27. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5); American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Zhao, Q.F.; Li, J.Q.; Wang, J.; Yu, J.T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
| Variables | Mean ± SD or Percentage (%) |
|---|---|
| Patients, number | 3846 |
| Age | 77.8 ± 6.2 |
| Gender | |
| Male | 1503 (39.1) |
| Female | 2343 (60.9) |
| Physical comorbidities | |
| Pulmonary disease | 386 (10.0) |
| Renal disease | 366 (9.5) |
| Liver disease | 317 (8.2) |
| T2DM | 931 (24.2) |
| HTN | 2035 (52.9) |
| Hyperlipidemia | 1142 (29.7) |
| Medication | |
| Rivastigmine | 1833 (47.7) |
| Donepezil | 1736 (45.1) |
| Galantamine | 277 (7.2) |
| Duration of AChEI use days | 658.7 ± 21.9 |
| Consecutive AChEI Prescription | |
| ≤22 months | 1949 (50.7) |
| >22 months | 1897 (49.3) |
| Baseline cognitive test before AChEIs | |
| MMSE | 17.2 ± 5.3 |
| CDR | |
| 0.5 | 2281 (59.3) |
| 1 | 1196 (31.1) |
| 2 | 369 (9.6) |
| Cognitive test following 2 years follow-up | |
| MMSE | 15.8 ± 6.3 |
| CDR | |
| 0.5 | 1561 (40.6) |
| 1 | 1558 (40.5) |
| 2 | 639 (16.6) |
| ≥3 | 88 (2.3) |
| Cognitive decline (defined by MMSE) | |
| Slow (decline ≤3 in 2 years), n (%) | 3536 (91.9) |
| Rapid (decline >3 in 2 years), n (%) | 310 (8.1) |
| Adjusted HRs * | 95% CI | p Value | |
|---|---|---|---|
| Consecutive Drug Prescription | |||
| ≤22 months | Ref | ||
| >22 months | 0.41 | 0.33–0.52 | <0.001 |
| Age group | |||
| 65–75 | Ref | ||
| 76–85 | 0.73 | 0.57–0.93 | <0.05 |
| >85M | 0.53 | 0.36–0.79 | <0.01 |
| Gender | |||
| Male | Ref | ||
| Female | 0.83 | 0.66–1.05 | 0.122 |
| Medication | |||
| Galantamine | - | ||
| Rivastigmine | 1.57 | 0.92–2.67 | 0.098 |
| Donepezil | 1.45 | 0.85–2.48 | 0.176 |
| Baseline CDR | |||
| 0.5 | Ref | ||
| 1 | 1.61 | 1.26–2.07 | <0.001 |
| 2 | 2.64 | 1.90–3.65 | <0.001 |
| Comorbidity | |||
| Pulmonary disease | 1.22 | 0.86–1.73 | 0.267 |
| Renal disease | 1.11 | 0.74–1.65 | 0.616 |
| Liver disease | 0.85 | 0.55–1.30 | 0.446 |
| T2DM | 0.83 | 0.62–1.10 | 0.202 |
| HTN | 1.19 | 0.93–1.51 | 0.159 |
| Hyperlipidemia | 0.96 | 0.73–1.24 | 0.731 |
| Aged 65–75 | Aged 76–85 | Aged > 85 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted HRs * | 95% CI | p Value | Adjusted HRs * | 95% CI | p Value | Adjusted HRs * | 95% CI | p Value | |
| Consecutive Drug Prescription | |||||||||
| ≤22 month | Ref | Ref | Ref | ||||||
| >22 months | 0.37 | 0.25–0.54 | <0.001 | 0.47 | 0.34–0.66 | <0.001 | 0.25 | 0.12–0.53 | <0.001 |
| Gender | |||||||||
| Male | Ref | Ref | Ref | ||||||
| Female | 0.76 | 0.52–1.09 | 0.138 | 1.06 | 0.76–1.48 | 0.747 | 0.35 | 0.16–0.75 | <0.001 |
| Medication | |||||||||
| Galantamine | - | - | - | ||||||
| Rivastigmine | 1.50 | 0.64–3.48 | 0.350 | 1.40 | 0.67–2.91 | 0.369 | 2.49 | 0.32–19.59 | 0.387 |
| Donepezil | 1.06 | 0.45–2.50 | 0.898 | 1.44 | 0.69–3.00 | 0.333 | 4.64 | 0.60–36.06 | 0.143 |
| Baseline CDR | |||||||||
| 0.5 | Ref | Ref | Ref | ||||||
| 1 | 2.06 | 1.40–3.03 | <0.001 | 1.37 | 0.96–1.95 | 0.082 | 1.82 | 0.77–4.30 | 0.172 |
| 2 | 2.85 | 1.57–5.17 | <0.001 | 2.57 | 1.66–3.97 | <0.001 | 3.71 | 1.41–9.79 | <0.001 |
| Comorbidity | |||||||||
| Pulmonary diseas | 1.45 | 0.84–2.51 | 0.183 | 1.33 | 0.79–2.21 | 0.281 | 0.55 | 0.19–1.63 | 0.284 |
| Renal disease | 1.09 | 0.57–2.05 | 0.800 | 1.17 | 0.65–2.10 | 0.607 | 1.17 | 0.37–3.75 | 0.790 |
| Liver disease | 1.36 | 0.74–2.50 | 0.326 | 0.54 | 0.28–1.08 | 0.081 | 0.59 | 0.12–2.81 | 0.509 |
| T2DM | 0.68 | 0.42–1.09 | 0.111 | 0.88 | 0.59–1.30 | 0.525 | 1.50 | 0.61–3.71 | 0.380 |
| HTN | 1.14 | 0.77–1.68 | 0.523 | 1.27 | 0.91–1.78 | 0.164 | 1.14 | 0.53–2.44 | 0.736 |
| Hyperlipidemia | 0.90 | 0.59–1.37 | 0.623 | 0.91 | 0.63–1.32 | 0.633 | 1.56 | 0.64–3.79 | 0.324 |
| Patients with T2DM | Patients without T2DM | |||||
|---|---|---|---|---|---|---|
| Adjusted HRs * | 95% CI | p Value | Adjusted HRs * | 95% CI | p Value | |
| Consecutive Drug Prescription | ||||||
| ≤22 months | Ref | Ref | ||||
| >22 months | 0.54 | 0.33–0.90 | <0.05 | 0.37 | 0.29–0.49 | <0.001 |
| Age group | ||||||
| 65–75 | Ref | Ref | ||||
| 76–85 | 0.94 | 0.56–1.59 | 0.829 | 0.69 | 0.52–0.90 | <0.01 |
| >85 | 1.13 | 0.49–2.60 | 0.766 | 0.44 | 0.28–0.69 | <0.001 |
| Gender | ||||||
| Male | Ref | Ref | ||||
| Female | 0.74 | 0.45–1.20 | 0.220 | 0.87 | 0.67–1.13 | 0.297 |
| Medication | ||||||
| Galantamine | - | - | ||||
| Rivastigmine | 1.78 | 0.55–5.80 | 0.336 | 1.50 | 0.83–2.73 | 0.181 |
| Donepezil | 1.27 | 0.38–4.25 | 0.698 | 1.47 | 0.81–2.68 | 0.206 |
| Baseline CDR | ||||||
| 0.5 | Ref | Ref | ||||
| 1 | 2.09 | 1.26–3.49 | <0.01 | 1.51 | 1.13–2.01 | <0.01 |
| 2 | 1.44 | 0.61–3.42 | 0.404 | 2.95 | 2.07–4.20 | <0.001 |
| Comorbidity | ||||||
| Pulmonary disease | 0.70 | 0.29–1.65 | 0.412 | 1.37 | 0.93–2.02 | 0.107 |
| Renal disease | 0.88 | 0.47–1.68 | 0.709 | 1.25 | 0.75–2.06 | 0.392 |
| Liver disease | 0.99 | 0.48–2.01 | 0.967 | 0.78 | 0.45–1.34 | 0.368 |
| HTN | 0.91 | 0.52–1.60 | 0.752 | 1.25 | 0.96–1.63 | 0.093 |
| Hyperlipidemia | 1.06 | 0.65–1.73 | 0.805 | 0.90 | 0.66–1.24 | 0.523 |
| Patients with HTN | Patients without HTN | |||||
|---|---|---|---|---|---|---|
| Adjusted HRs * | 95% CI | p Value | Adjusted HRs * | 95% CI | p Value | |
| Consecutive Drug Prescription | ||||||
| ≤22 months | Ref | Ref | ||||
| >22 months | 0.46 | 0.33–0.63 | <0.001 | 0.35 | 0.25–0.50 | <0.001 |
| Age group | ||||||
| 65–75 | Ref | Ref | ||||
| 76–85 | 0.75 | 0.65–1.05 | 0.092 | 0.70 | 0.49–0.99 | <0.05 |
| >85 | 0.51 | 0.30–0.88 | <0.05 | 0.57 | 0.32–1.03 | 0.064 |
| Gender | ||||||
| Male | Ref | Ref | ||||
| Female | 0.79 | 0.57–1.08 | 0.138 | 0.91 | 0.65–1.28 | 0.607 |
| Medication | ||||||
| Galantamine | - | - | ||||
| Rivastigmine | 0.99 | 0.51–1.93 | 0.987 | 2.48 | 0.98–6.34 | 0.050 |
| Donepezil | 0.92 | 0.47–1.79 | 0.800 | 2.30 | 0.92–5.75 | 0.074 |
| Baseline CDR | ||||||
| 0.5 | Ref | Ref | ||||
| 1 | 1.95 | 1.39–2.75 | <0.001 | 1.25 | 0.86–1.81 | 0.082 |
| 2 | 3.55 | 2.29–5.51 | <0.001 | 1.84 | 1.13–3.01 | <0.05 |
| Comorbidity | ||||||
| Pulmonary disease | 1.30 | 0.82–2.04 | 0.262 | 1.10 | 0.62–1.95 | 0.735 |
| Renal disease | 0.66 | 0.38–1.15 | 0.141 | 2.94 | 1.64–5.24 | <0.001 |
| Liver disease | 0.91 | 0.55–1.52 | 0.720 | 0.63 | 0.27–1.44 | 0.273 |
| T2DM | 0.79 | 0.56–1.11 | 0.179 | 0.98 | 0.59–1.65 | 0.951 |
| Hyperlipidemia | 1.13 | 0.82–1.57 | 0.447 | 0.68 | 0.42–1.10 | 0.114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.-C.; Chu, C.-S.; Chen, C.-L.; Chuang, Y.-C.; Chen, N.-C. Factors Affecting Rapid Cognitive Decline in Patients with Alzheimer’s Disease: A Longitudinal Follow-Up Study. Int. J. Environ. Res. Public Health 2021, 18, 8576. https://doi.org/10.3390/ijerph18168576
Pan C-C, Chu C-S, Chen C-L, Chuang Y-C, Chen N-C. Factors Affecting Rapid Cognitive Decline in Patients with Alzheimer’s Disease: A Longitudinal Follow-Up Study. International Journal of Environmental Research and Public Health. 2021; 18(16):8576. https://doi.org/10.3390/ijerph18168576
Chicago/Turabian StylePan, Chih-Chuan, Che-Sheng Chu, Chien-Liang Chen, Yao-Chung Chuang, and Nai-Ching Chen. 2021. "Factors Affecting Rapid Cognitive Decline in Patients with Alzheimer’s Disease: A Longitudinal Follow-Up Study" International Journal of Environmental Research and Public Health 18, no. 16: 8576. https://doi.org/10.3390/ijerph18168576






