Toxicological Comparison of Mancozeb and Zoxamide Fungicides at Environmentally Relevant Concentrations by an In Vitro Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Chemicals
2.2. Cytotoxicity Assays
2.3. Apoptosis–Necrosis Assay
2.4. Reactive Oxygen Species (ROS) Assay
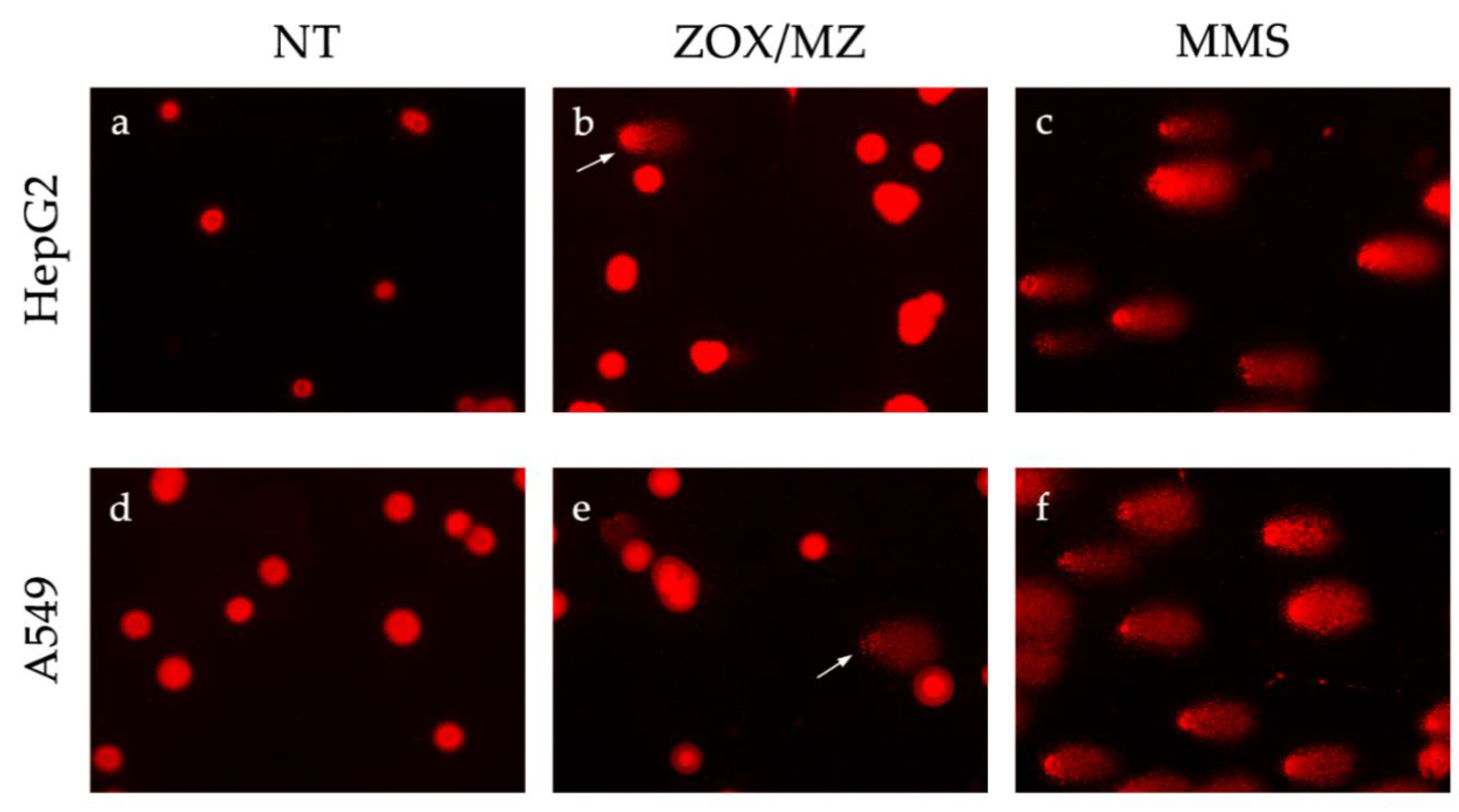
2.5. Alkaline Comet Assay
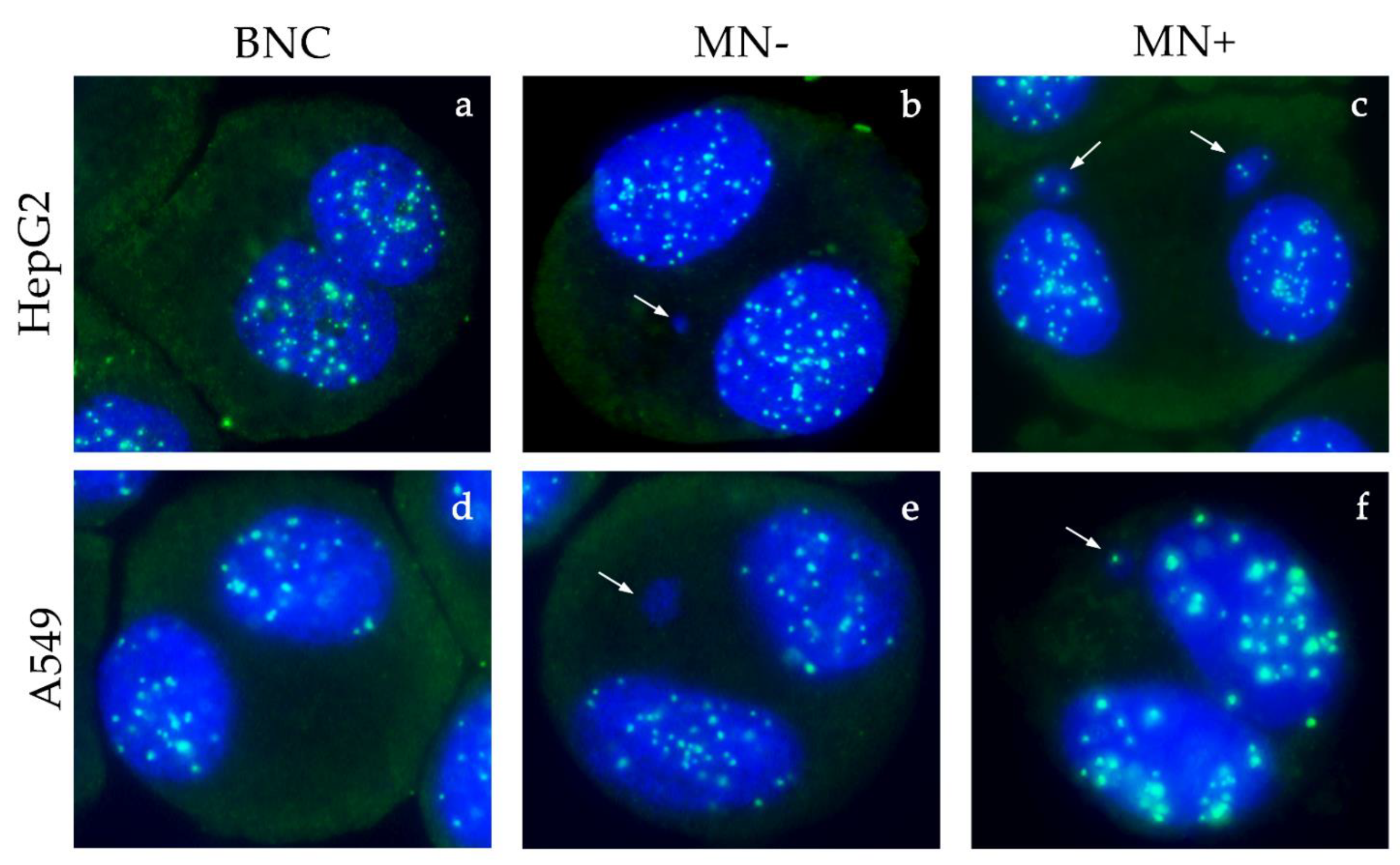
2.6. Cytokinesis-Block Micronucleus Assay (CBMN) and CREST Immunofluorescence
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results
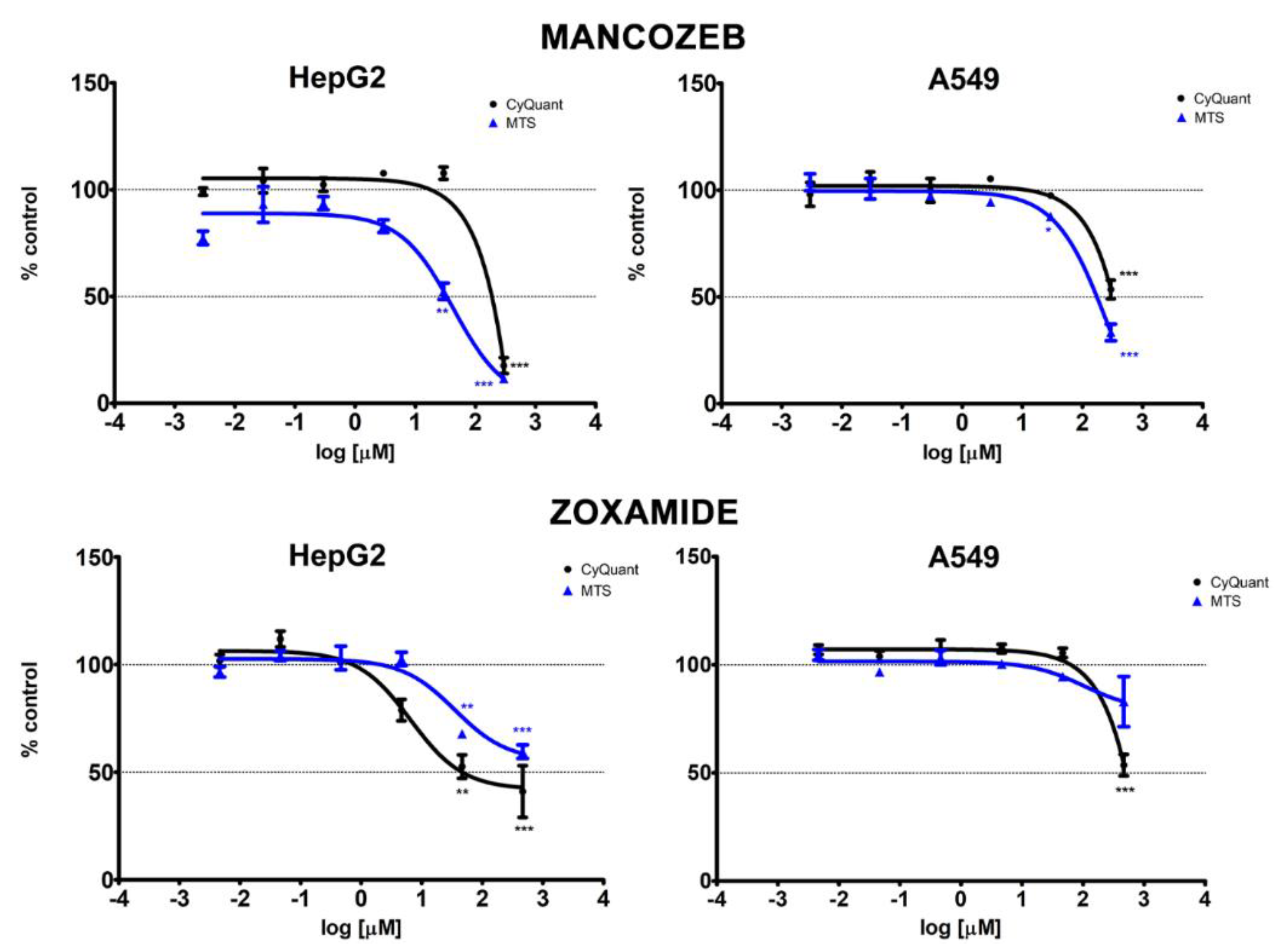
3.1. Cytotoxicity
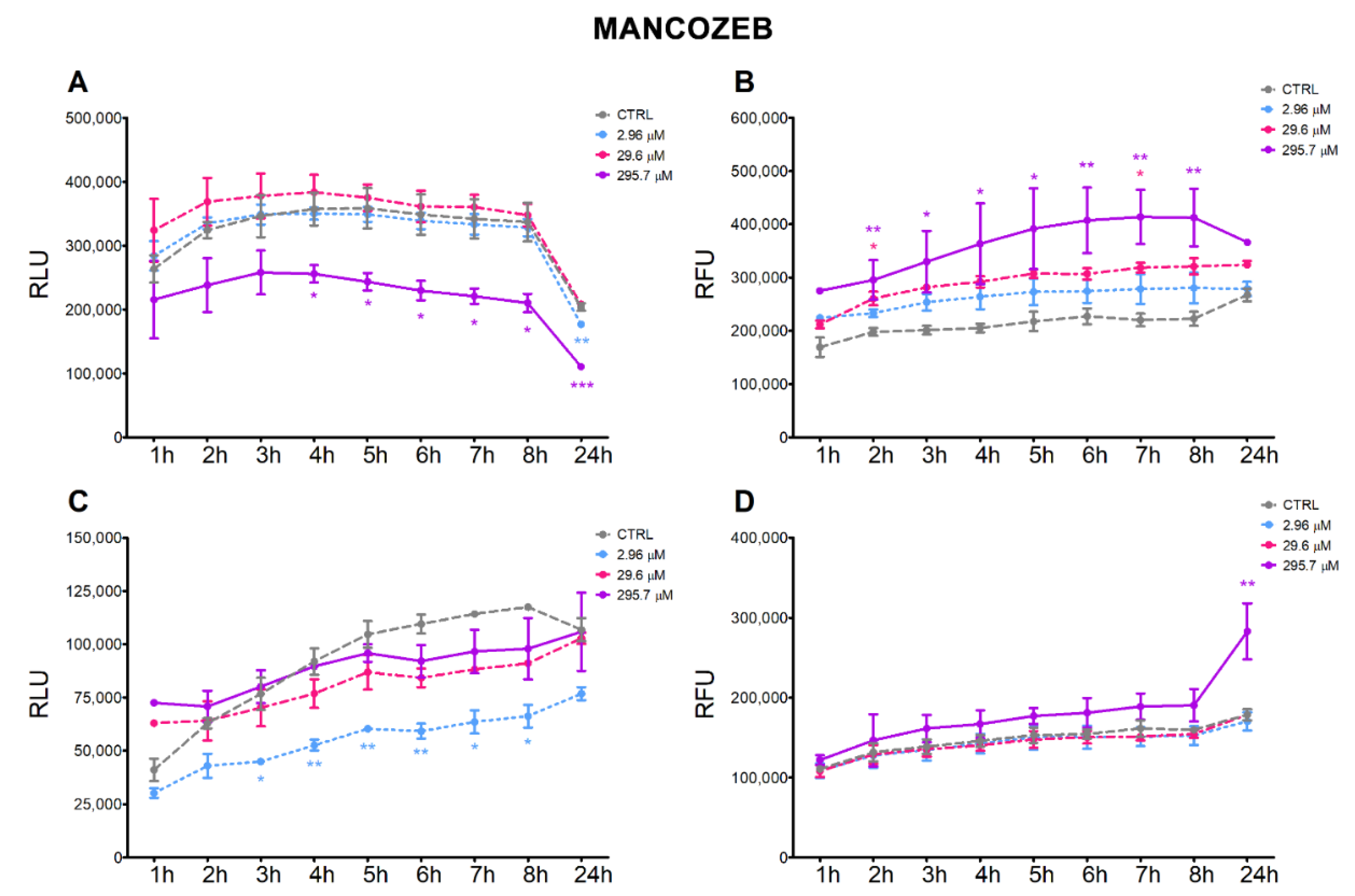
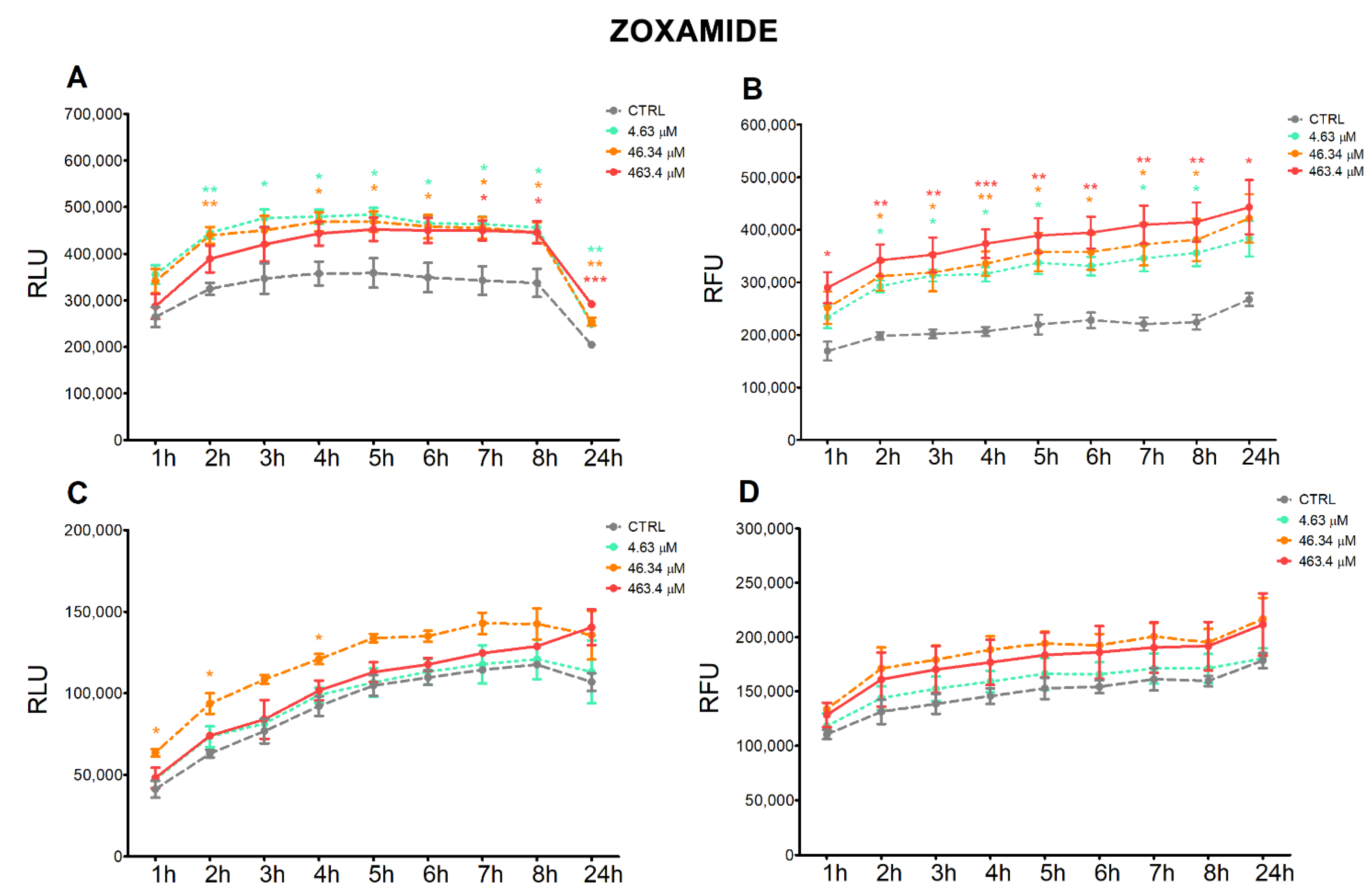
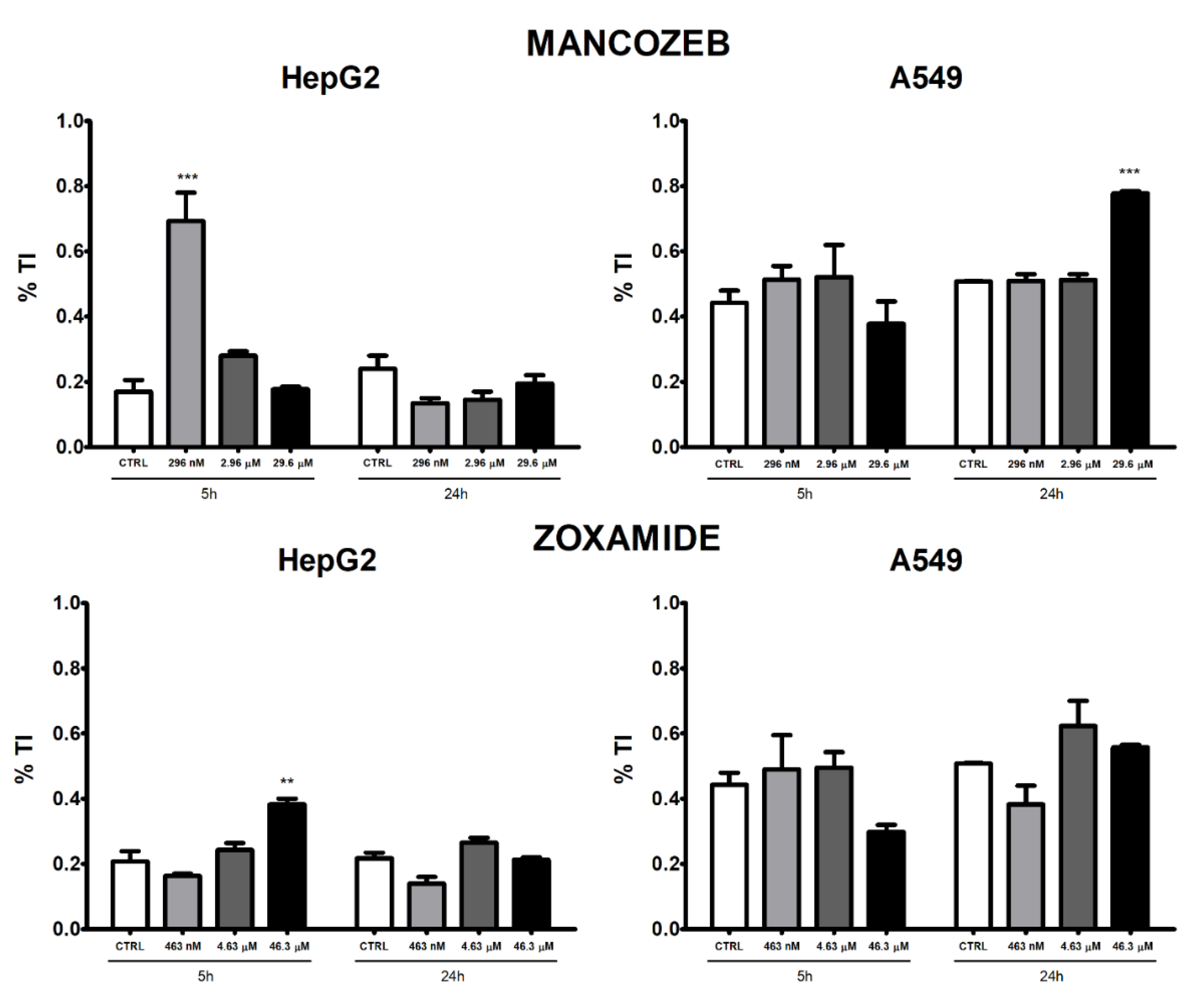
3.2. Apoptosis and Necrosis Time-Course
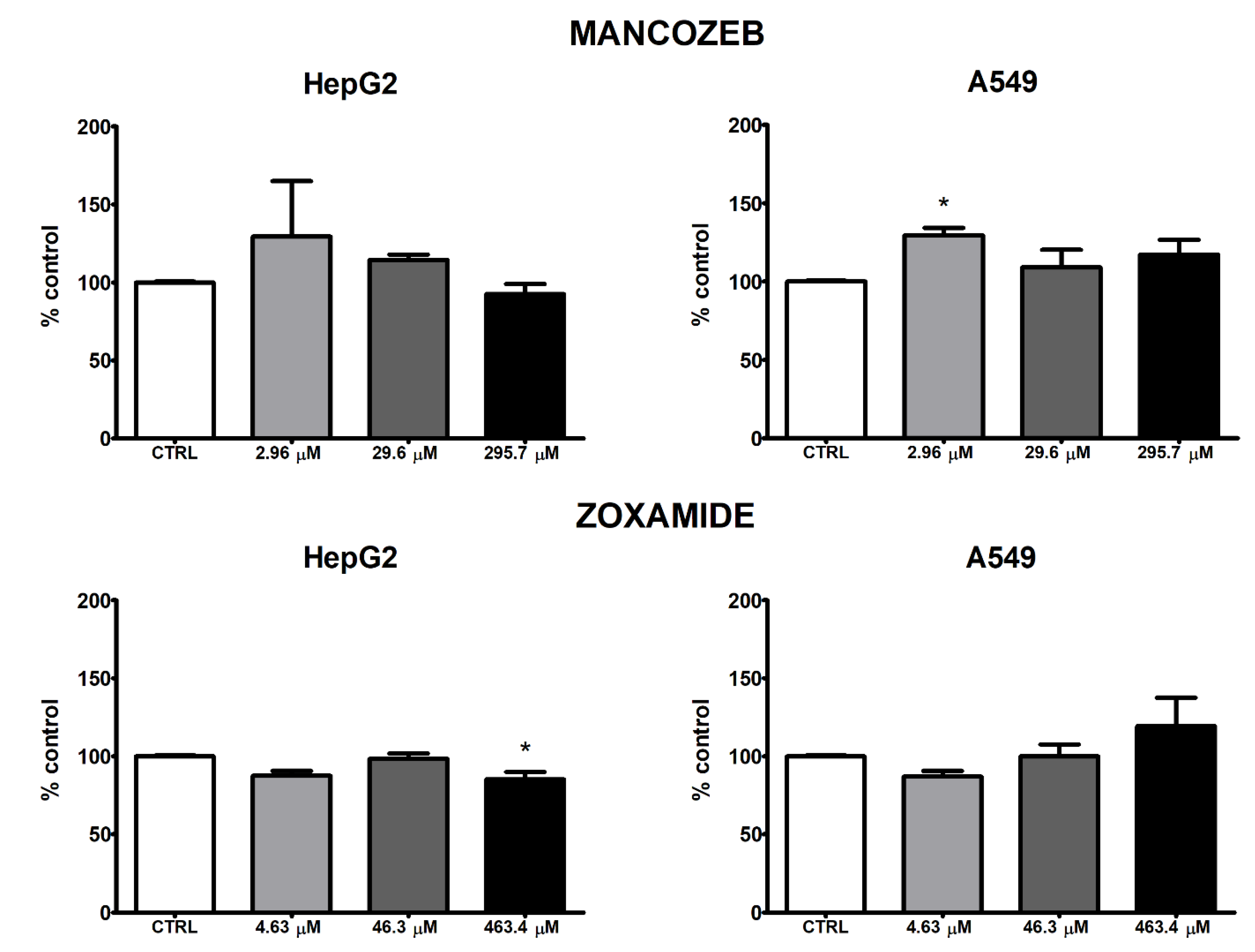
3.3. ROS Intracellular Levels
3.4. Comet Assay
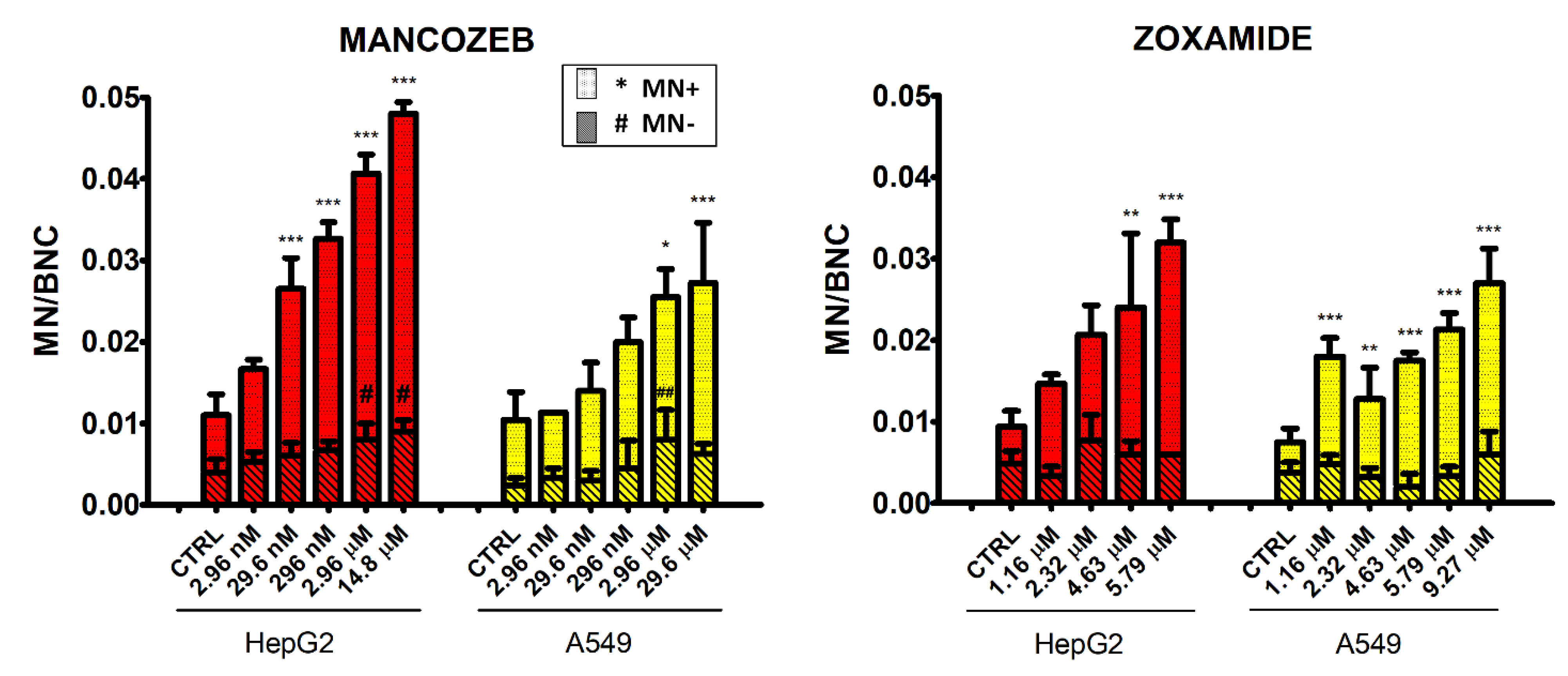
3.5. Micronucleus Test
3.6. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.S.S.L.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent Advances and Remaining Barriers to Producing Novel Formulations of Fungicides for Safe and Sustainable Agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef]
- Casida, J.E. Pest Toxicology: The Primary Mechanisms of Pesticide Action. Chem. Res. Toxicol. 2009, 22, 609–619. [Google Scholar] [CrossRef]
- International Programme on Chemical Safety; UNEP; Internationale Arbeitsorganisation (Eds.) Dithiocarbamate Pesticides, Ethylenethiourea, and Propylenethiourea: A General Introduction; Environmental Health Criteria; World Health Organization: Geneva, Switzerland, 1988; ISBN 978-92-4-154278-4. [Google Scholar]
- Houeto, P.; Bindoula, G.; Hoffman, J.R. Ethylenebisdithiocarbamates and Ethylenethiourea: Possible Human Health Hazards. Environ. Health Perspect. 1995, 103, 568–573. [Google Scholar] [CrossRef] [PubMed]
- European Commission. COMMISSION WORKING DOCUMENT-Review Report for the Active Substance Mancozeb 2009; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- Maranghi, F.; De Angelis, S.; Tassinari, R.; Chiarotti, F.; Lorenzetti, S.; Moracci, G.; Marcoccia, D.; Gilardi, E.; Di Virgilio, A.; Eusepi, A.; et al. Reproductive Toxicity and Thyroid Effects in Sprague Dawley Rats Exposed to Low Doses of Ethylenethiourea. Food Chem. Toxicol. 2013, 59, 261–271. [Google Scholar] [CrossRef]
- Runkle, J.; Flocks, J.; Economos, J.; Dunlop, A.L. A Systematic Review of Mancozeb as a Reproductive and Developmental Hazard. Environ. Int. 2017, 99, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, A.V.A.; Stellavato, A.; La Gatta, A.; Lamberti, M.; Schiraldi, C. Mancozeb, a Fungicide Routinely Used in Agriculture, Worsens Nonalcoholic Fatty Liver Disease in the Human HepG2 Cell Model. Toxicol. Lett. 2016, 249, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.; Hardej, D. Ethylene Bisdithiocarbamate Pesticides Cause Cytotoxicity in Transformed and Normal Human Colon Cells. Environ. Toxicol. Pharmacol. 2012, 34, 556–573. [Google Scholar] [CrossRef]
- Kumar, K.; Sabarwal, A.; Singh, R.P. Mancozeb Selectively Induces Mitochondrial-Mediated Apoptosis in Human Gastric Carcinoma Cells through ROS Generation. Mitochondrion 2019, 48, 1–10. [Google Scholar] [CrossRef]
- Calviello, G.; Piccioni, E.; Boninsegna, A.; Tedesco, B.; Maggiano, N.; Serini, S.; Wolf, F.; Palozza, P. DNA Damage and Apoptosis Induction by the Pesticide Mancozeb in Rat Cells: Involvement of the Oxidative Mechanism. Toxicol. Appl. Pharmacol. 2006, 211, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Ali, W.; Singh, R.; Bhui, K.; Tyagi, S.; Al-Khedhairy, A.A.; Srivastava, P.K.; Musarrat, J.; Shukla, Y. Mancozeb-Induced Genotoxicity and Apoptosis in Cultured Human Lymphocytes. Life Sci. 2012, 90, 815–824. [Google Scholar] [CrossRef]
- Paz-Trejo, C.; Gómez-Arroyo, S. Genotoxic Evaluation of Common Commercial Pesticides in Human Peripheral Blood Lymphocytes. Toxicol. Ind. Health 2017, 33, 938–945. [Google Scholar] [CrossRef]
- Goldoni, A.; Klauck, C.R.; Da Silva, S.T.; Da Silva, M.D.; Ardenghi, P.G.; Da Silva, L.B. DNA Damage in Wistar Rats Exposed to Dithiocarbamate Pesticide Mancozeb. Folia Biol. 2014, 60, 202–204. [Google Scholar]
- Belpoggi, F.; Soffritti, M.; Guarino, M.; Lambertini, L.; Cevolani, D.; Maltoni, C. Results of Long-Term Experimental Studies on the Carcinogenicity of Ethylene-Bis-Dithiocarbamate (Mancozeb) in Rats. Ann. N. Y. Acad. Sci. 2002, 982, 123–136. [Google Scholar] [CrossRef]
- Young, D.H.; Slawecki, R.A. Mode of Action of Zoxamide (RH-7281), a New Oomycete Fungicide. Pestic. Biochem. Physiol. 2001, 69, 100–111. [Google Scholar] [CrossRef]
- Young, D.H.; Rubio, F.M.; Danis, P.O. A Radioligand Binding Assay for Antitubulin Activity in Tumor Cells. J. Biomol. Screen. 2006, 11, 82–89. [Google Scholar] [CrossRef][Green Version]
- European Commission. COMMISSION WORKING DOCUMENT-Review Report for the Active Substance Zoxamide 2004; European Commission: Brussels, Belgium, 2004. [Google Scholar]
- European Food Safety Authority (EFSA); Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Zoxamide. EFS2 2017, 15, 4980. [Google Scholar] [CrossRef][Green Version]
- Natarajan, A.T.; Darroudi, F. Use of Human Hepatoma Cells for in Vitro Metabolic Activation of Chemical Mutagens/Carcinogens. Mutagenesis 1991, 6, 399–403. [Google Scholar] [CrossRef]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 Cell Line as a Type II Pulmonary Epithelial Cell Model for Drug Metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, J.; Lassila, A.; Päivärinta, K.; Valanne, S.; Sarpo, S.; Hakkola, J.; Pelkonen, O.; Raunio, H. Induction and Regulation of Xenobiotic-Metabolizing Cytochrome P450s in the Human A549 Lung Adenocarcinoma Cell Line. Am. J. Respir. Cell Mol. Biol. 2000, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Knasmüller, S.; Mersch-Sundermann, V.; Kevekordes, S.; Darroudi, F.; Huber, W.W.; Hoelzl, C.; Bichler, J.; Majer, B.J. Use of Human-Derived Liver Cell Lines for the Detection of Environmental and Dietary Genotoxicants; Current State of Knowledge. Toxicology 2004, 198, 315–328. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2016; ISBN 978-92-64-26486-1. [Google Scholar]
- Weis, G.C.C.; Assmann, C.E.; Cadoná, F.C.; Bonadiman, B.d.S.R.; Alves, A.d.O.; Machado, A.K.; Duarte, M.M.M.F.; da Cruz, I.B.M.; Costabeber, I.H. Immunomodulatory Effect of Mancozeb, Chlorothalonil, and Thiophanate Methyl Pesticides on Macrophage Cells. Ecotoxicol. Environ. Saf. 2019, 182, 109420. [Google Scholar] [CrossRef] [PubMed]
- Oesch, F.; Metzler, M.; Fabian, E.; Kamp, H.; Bernshausen, T.; Damm, G.; Triebel, S.; Döhmer, J.; Landsiedel, R.; Van Ravenzwaay, B. In Vitro Mammalian Metabolism of the Mitosis Inhibitor Zoxamide and the Relationship to Its In Vitro Toxicity. Xenobiotica 2010, 40, 72–82. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Marchi, S.; Petroni, G.; Kroemer, G.; Galluzzi, L.; Pervaiz, S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020, 30, 537–555. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Oh, S.; Velasco, M.; Ta, C.; Montalvo, J.; Calderone, A. RTP801 Regulates Maneb- and Mancozeb-Induced Cytotoxicity via NF-ΚB. J. Biochem. Mol. Toxicol. 2014, 28, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Domico, L.M.; Cooper, K.R.; Bernard, L.P.; Zeevalk, G.D. Reactive Oxygen Species Generation by the Ethylene-Bis-Dithiocarbamate (EBDC) Fungicide Mancozeb and Its Contribution to Neuronal Toxicity in Mesencephalic Cells. Neurotoxicology 2007, 28, 1079–1091. [Google Scholar] [CrossRef]
- Domico, L.M.; Zeevalk, G.D.; Bernard, L.P.; Cooper, K.R. Acute Neurotoxic Effects of Mancozeb and Maneb in Mesencephalic Neuronal Cultures Are Associated with Mitochondrial Dysfunction. NeuroToxicology 2006, 27, 816–825. [Google Scholar] [CrossRef]
- Iorio, R.; Castellucci, A.; Rossi, G.; Cinque, B.; Cifone, M.G.; Macchiarelli, G.; Cecconi, S. Mancozeb Affects Mitochondrial Activity, Redox Status and ATP Production in Mouse Granulosa Cells. Toxicol. Vitr. 2015, 30, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider Roles in Cancer Progression and Prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef]
- Dietrich, C. Antioxidant Functions of the Aryl Hydrocarbon Receptor. Stem Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Zangar, R. Mechanisms That Regulate Production of Reactive Oxygen Species by Cytochrome P450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Vlach, M.; Quesnot, N.; Dubois-Pot-Schneider, H.; Ribault, C.; Verres, Y.; Petitjean, K.; Rauch, C.; Morel, F.; Robin, M.-A.; Corlu, A.; et al. Cytochrome P450 1A1/2, 2B6 and 3A4 HepaRG Cell-Based Biosensors to Monitor Hepatocyte Differentiation, Drug Metabolism and Toxicity. Sensors 2019, 19, 2245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, D.; Zhu, X.; Yu, L.; Mao, M.; Liu, Y. Anticancer Evaluation of a Novel Dithiocarbamate Hybrid as the Tubulin Polymerization Inhibitor. Investog. New Drugs 2020, 38, 525–532. [Google Scholar] [CrossRef]
- Soloneski, S.; Reigosa, M.A.; Larramendy, M.L. Effect of the Dithiocarbamate Pesticide Zineb and Its Commercial Formulation, the Azzurro. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2003, 536, 121–129. [Google Scholar] [CrossRef]
- Manandhar, M.; Boulware, K.S.; Wood, R.D. The ERCC1 and ERCC4 (XPF) Genes and Gene Products. Gene 2015, 569, 153–161. [Google Scholar] [CrossRef]
- Allgayer, J.; Kitsera, N.; von der Lippen, C.; Epe, B.; Khobta, A. Modulation of Base Excision Repair of 8-Oxoguanine by the Nucleotide Sequence. Nucleic Acids Res. 2013, 41, 8559–8571. [Google Scholar] [CrossRef]
- Collins, A. Investigating Oxidative DNA Damage and Its Repair Using the Comet Assay. Mutat. Res./Rev. Mutat. Res. 2009, 681, 24–32. [Google Scholar] [CrossRef]
- Rundell, M.S.; Wagner, E.D.; Plewa, M.J. The Comet Assay: Genotoxic Damage or Nuclear Fragmentation? Environ. Mol. Mutagen. 2003, 42, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Young, D.H. Fungicides Acting on Mitosis and Cell Division: Zoxamide, an Antitubulin Fungicide for Control of Oomycete Pathogens. In Modern Crop Protection Compounds; Jeschke, P., Witschel, M., Krämer, W., Schirmer, U., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 785–795. ISBN 978-3-527-69926-1. [Google Scholar]
- Cai, M.; Miao, J.; Song, X.; Lin, D.; Bi, Y.; Chen, L.; Liu, X.; Tyler, B.M. C239S Mutation in the β-Tubulin of Phytophthora Sojae Confers Resistance to Zoxamide. Front. Microbiol. 2016, 7, 762. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Y.; Ni, H.; Gao, J.; Yang, Y.; Xu, W.; Tao, L. Evaluation of the Cytotoxic Effects of Glyphosate Herbicides in Human Liver, Lung, and Nerve. J. Environ. Sci. Health Part B 2019, 54, 737–744. [Google Scholar] [CrossRef]
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C.; et al. A Lung/Liver-on-a-Chip Platform for Acute and Chronic Toxicity Studies. Lab Chip 2018, 18, 3814–3829. [Google Scholar] [CrossRef] [PubMed]
- Oshimura, M.; Barrett, J.C. Chemically Induced Aneuploidy in Mammalian Cells: Mechanisms and Biological Significance in Cancer. Environ. Mutagen. 1986, 8, 129–159. [Google Scholar] [CrossRef]


| Gene | qPCR Primers (5′-3′) | |
|---|---|---|
| GAPDH | fw: | ACTCCTCCACCTTTGACGCT |
| rev: | CTTCAAGGGGTCTACATGGC | |
| BAX | fw: | GTCTTTTTCCGAGTGGCAGC |
| rev: | GACAGGGACATCAGTCGCTT | |
| BCL2 | fw: | CTTTGAGTTCGGTGGGGTCA |
| rev: | GGGCCGTACAGTTCCACAAA | |
| NRF2 | fw: | ACAAGATGGGCTGCTGCACTGG |
| rev: | TCCCCGAGGAACCCGCTGAAAA | |
| CYP1A1 | fw: | CCCCCACAGCACAACAAGAG |
| rev: | GGGTGAGAAACCGTTCAGGT | |
| ERCC1 | fw: | GGCTCGAGAAAGACAGGCTCC |
| rev: | CATATTCGGCGTAGGTCTGAGG | |
| OGG1 | fw: | GCCTGATGGCCCTAGACAAG |
| rev: | GCACTGAACAGCACCGCTT | |
| Chemical | Test | HepG2 | A549 | ||||
|---|---|---|---|---|---|---|---|
| EC10 (95% CI) | EC20 (95% CI) | EC50 (95% CI) | EC10 (95% CI) | EC20 (95% CI) | EC50 (95% CI) | ||
| Mancozeb | MTS | 3.61 (<2.96 × 10−3–8.04) | 8.67 (1.01–16.34) | 38.73 (19.11–58.35) | 22.89 (7.82–37.96) | 47.13 (24.66–69.61) | 162.03 (121.68–202.38) |
| CyQUANT | 142.43 (<2.96 × 10−3–677.98) | 166.45 (<2.96 × 10−3–658.65) | 217.96 (<2.96 × 10−3–560.64) | 69.39 (<2.96 × 10−3–181.19) | 121.89 (0.16–243.59) | 319.24 (251.16–387.32) | |
| Zoxamide | MTS | 14.45 (<4.63 × 10−3–41.14) | 61.87 (<4.63 × 10−3–135.78) | NA | NA | NA | NA |
| CyQUANT | 0.75 (<4.63 × 10−3–2.09) | 1.79 (<4.63 × 10−3–4.09) | 7.90 (<4.63 × 10−3–18.50) | 200.57 (<4.63 × 10−3–589.47) | 274.82 (<4.63 × 10−3–608.30) | 470.84 (420.00–521.68) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lori, G.; Tassinari, R.; Narciso, L.; Udroiu, I.; Sgura, A.; Maranghi, F.; Tait, S. Toxicological Comparison of Mancozeb and Zoxamide Fungicides at Environmentally Relevant Concentrations by an In Vitro Approach. Int. J. Environ. Res. Public Health 2021, 18, 8591. https://doi.org/10.3390/ijerph18168591
Lori G, Tassinari R, Narciso L, Udroiu I, Sgura A, Maranghi F, Tait S. Toxicological Comparison of Mancozeb and Zoxamide Fungicides at Environmentally Relevant Concentrations by an In Vitro Approach. International Journal of Environmental Research and Public Health. 2021; 18(16):8591. https://doi.org/10.3390/ijerph18168591
Chicago/Turabian StyleLori, Gabriele, Roberta Tassinari, Laura Narciso, Ion Udroiu, Antonella Sgura, Francesca Maranghi, and Sabrina Tait. 2021. "Toxicological Comparison of Mancozeb and Zoxamide Fungicides at Environmentally Relevant Concentrations by an In Vitro Approach" International Journal of Environmental Research and Public Health 18, no. 16: 8591. https://doi.org/10.3390/ijerph18168591
APA StyleLori, G., Tassinari, R., Narciso, L., Udroiu, I., Sgura, A., Maranghi, F., & Tait, S. (2021). Toxicological Comparison of Mancozeb and Zoxamide Fungicides at Environmentally Relevant Concentrations by an In Vitro Approach. International Journal of Environmental Research and Public Health, 18(16), 8591. https://doi.org/10.3390/ijerph18168591









