Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2
Abstract
:1. Introduction
2. Materials and Methods
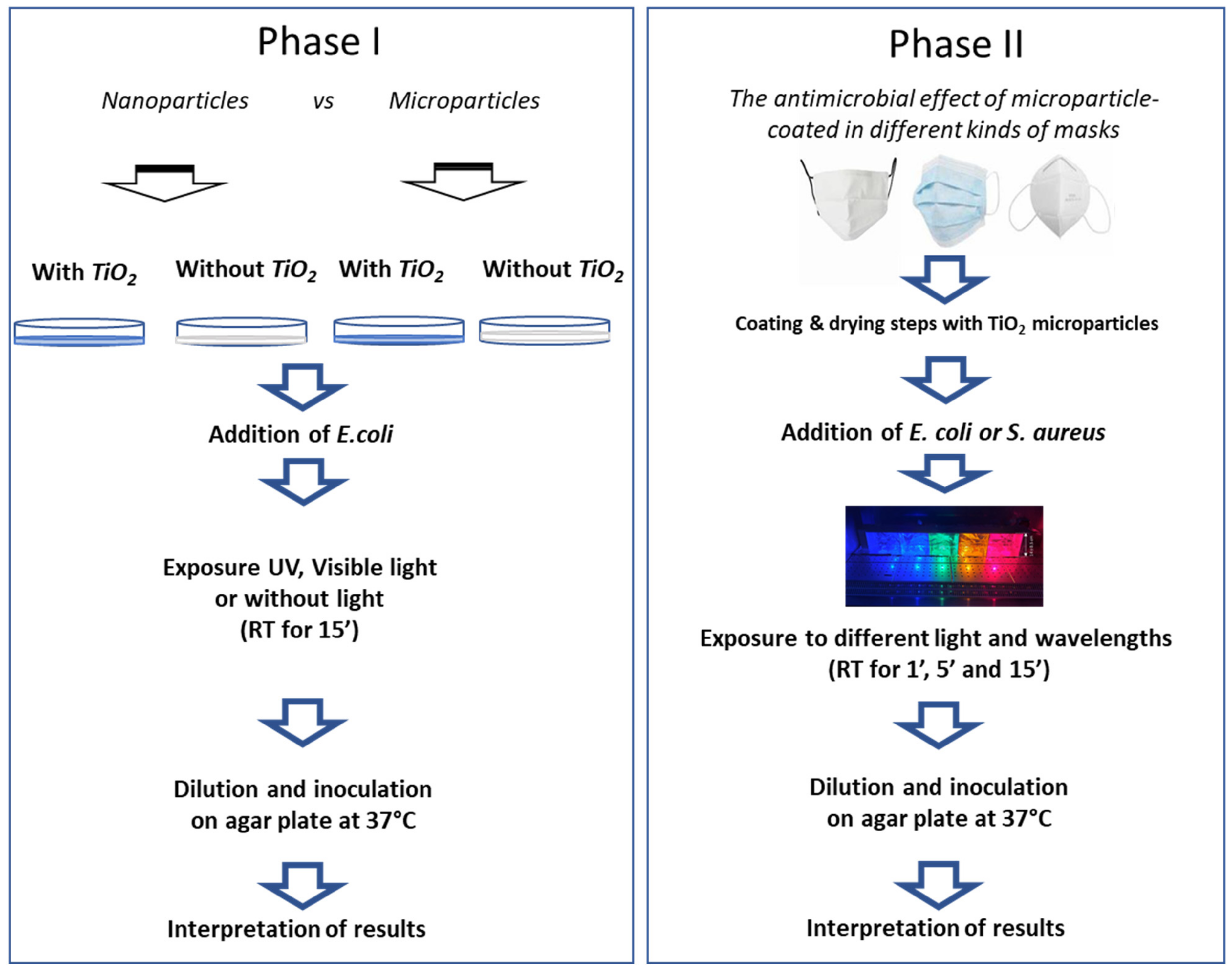
2.1. Design of Study
2.2. Bacterial Strains and Culture Conditions
2.3. Materials
2.4. Exposure
2.5. Phase I: Comparison between Nano (NP)- and Micro-Particles (MP) of TiO2 in Sterile Water Medium
2.6. Phase II: Antimicrobial Effect of Microparticle-Coated Masks
2.6.1. Coating of Mask
2.6.2. Preparation of Test
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 December 2020).
- Feng, S.; Shen, C.; Xia, N.; Song, W.; Fan, M.; Cowling, B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, 434–436. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory Data Repository. Available online: https://apps.who.int/gho/data/view.main.BMIPLUS2REGv (accessed on 8 March 2021).
- Food and Drug Administration (FDA). Face Masks, Including Surgical Masks, and Respirators for COVID. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/face-masks-including-surgical-masks-and-respirators-covid-19#using (accessed on 1 June 2021).
- National Institute for Occupational Safety and Health (NIOSH). Use of Respirators and Surgical Masks for Protection against Healthcare Hazards 2020 (updated 19 November 201823 April 2020). Available online: https://www.cdc.gov/niosh/topics/healthcarehsps/respiratory.html (accessed on 1 June 2021).
- European Centre for Disease Prevention and Control (ECDC). Safe Use of Personal Protective Equipment in the Treatment of Infectious Diseases of High Consequence 2014 (cited 23 April 2020). Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/safe-use-of-ppe.pdf (accessed on 1 June 2021).
- World Health Organization (WHO). Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19) and Considerations during Severe Shortages: Interim Guidance, 6 April 2020. (14 April 2020). Available online: https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf (accessed on 1 June 2021).
- Mahdavi, A. Efficiency Measurement of N95 Filtering Facepiece Respirators against Ultrafine Particles under Cyclic and Constant Flows. Doctoral Dissertation, Concordia University, Montreal, QC, Canada, 2013. [Google Scholar]
- Richardson, A.W.; Eshbaugh, J.P.; Hofacre, K.C.; Gardner, P.D. Respirator Filter Efficiency Testing against Particulate and Biological Aerosols under Moderate to High Flow Rates; Battelle Memorial Inst: Columbus, OH, USA, 2006. [Google Scholar]
- Rengasamy, S.; Eimer, B.; Shaffer, R. Simple respiratory protection—Evaluation of the filtration performance of cloth masks and common fabric materials against 20–1000 nm size particles. Ann. Occup. Hyg. 2010, 54, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Willeke, K.; Grinshpun, S.A.; Donnelly, J.; Coffey, C.C. Performance of N95 respirators: Filtration efficiency for air-borne microbial and inert particles. Am. Ind. Hyg. Assoc. J. 1998, 59, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; McCaffrey, B.; Hariharan, P.; Myers, M.R. Quantification of leakage of sub-micron aerosols through surgical masks and facemasks for pediatric use. J. Occup. Environ. Hyg. 2017, 14, 214–223. [Google Scholar] [CrossRef]
- Moradmand, P.A.; Khaloozadeh, H. An experimental study of modeling and self-tuning regulator design for an electro-hydro servo-system. In Proceedings of the 2017 5th International Conference on Control, Instrumentation, and Automation (ICCIA), Shiraz, Iran, 21–23 November 2017; Institute of Electrical and Electronics Engineers (IEEE): Washington, DC, USA, 2017; pp. 126–131. [Google Scholar]
- Eshbaugh, J.P.; Gardner, P.D.; Richardson, A.W.; Hofacre, K.C. N95 and P100 respirator filter efficiency under high constant and cyclic flow. J. Occup. Environ. Hyg. 2008, 6, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Kasper, G. Filtration efficiency of nanometer-size aerosol particles. J. Aerosol Sci. 1991, 22, 31–41. [Google Scholar] [CrossRef]
- Kim, C.S.; Bao, L.; Okuyama, K.; Shimada, M.; Niinuma, H. Filtration efficiency of a fibrous filter for nanoparticles. J. Nanoparticle Res. 2006, 8, 215–221. [Google Scholar] [CrossRef]
- Xiao, X.; Qian, L. Investigation of humidity-dependent capillary force. Langmuir 2000, 16, 8153–8158. [Google Scholar] [CrossRef]
- Givehchi, R.; Tan, Z. The effect of capillary force on airborne nanoparticle filtration. J. Aerosol Sci. 2015, 83, 12–24. [Google Scholar] [CrossRef]
- Jones, R.M.; Rempel, D. Standards for surgical respirators and masks: Relevance for Protecting healthcare workers and the public during pandemics. Ann. Work. Expo. Health 2021, 65, 495–504. [Google Scholar] [CrossRef]
- Bailar, J.C.; Burke, D.S.; Brosseau, L.M.; Cohen, H.J.; Gallagher, E.J.; Gensheimber, K.F. Reusability of Facemasks during an Influenza Pandemic; The National Academies Press: Washington, DC, USA, 2006; pp. 1–106. [Google Scholar]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Cheng, J.; Venditti, R.A. Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 2019, 142, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.C. Gender, face mask perceptions, and face mask wearing: Are men being dangerous during the COVID-19 pan-demic? Personal. Individ. Differ. 2021, 170, 110417. [Google Scholar] [CrossRef] [PubMed]
- Celis, J.E.; Espejo, W.; Paredes-Osses, E.; Contreras, S.A.; Chiang, G.; Bahamonde, P. Plastic residues produced with confirm-atory testing for COVID-19: Classification, quantification, fate, and impacts on human health. Sci. Total Environ. 2020, 760, 144167. [Google Scholar] [CrossRef]
- Bergman, M.S.; Viscusi, D.J.; Palmiero, A.J.; Powell, J.B.; Shaffer, R.E. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J. Int. Soc. Respir. Prot. 2011, 28, 48. [Google Scholar]
- Lore, M.B.; Heimbuch, B.K.; Brown, T.L.; Wander, J.D.; Hinrichs, S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann. Occup. Hyg. 2011, 56, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.D.; Sambol, A.R.; Shaffer, R.E. Evaluation of multiple (3-cycle) de-contamination processing for filtering facepiece respirators. J. Eng. Fibers Fabr. 2010, 5, 33–41. [Google Scholar]
- Fisher, E.M.; Williams, J.L.; Shaffer, R.E. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS ONE 2011, 6, e18585. [Google Scholar] [CrossRef] [Green Version]
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 respirators be reused after disinfection? How many times? ACS Nano 2020, 14, 6348–6356. [Google Scholar] [CrossRef]
- Kenney, P.A.; Chan, B.K.; Kortright, K.E.; Cintron, M.; Russi, M.; Epright, J.; Lee, L.; Balcezak, T.J.; Havill, N.L.; Martinello, R.A. Hydrogen peroxide vapor decontamination of N95 respirators for reuse. Infect. Control. Hosp. Epidemiol. 2021, 1–3. [Google Scholar] [CrossRef]
- Fischer, R.J.; Morris, D.H.; van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Effectiveness of N95 respirator decontamina-tion and reuse against SARS-CoV-2 virus. Emerg. Infect. Dis. 2020, 26, 2253–2255. [Google Scholar] [CrossRef]
- Feldmann, F.; Shupert, W.L.; Haddock, E.; Twardoski, B.; Feldmann, H. Gamma irradiation as an effective method for inac-tivation of emerging viral pathogens. Am. J. Trop. Med. Hyg. 2019, 100, 1275. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Shaffer, R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece res-pirators. J. Appl. Microbiol. 2011, 110, 287–295. [Google Scholar] [CrossRef]
- Mills, D.; Harnish, D.A.; Lawrence, C.; Sandoval-Powers, M.; Heimbuch, B.K. Ultraviolet germicidal irradiation of influen-za-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control 2018, 46, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margarucci, L.M.; Spica, V.R.; Protano, C.; Gianfranceschi, G.; Giuliano, M.; Di Onofrio, V.; Mucci, N.; Valeriani, F.; Vitali, M.; Romano, F. Potential antimicrobial effects of photocatalytic nanothecnologies in hospital settings. Ann Ig 2019, 31, 461–473. [Google Scholar] [PubMed]
- European Union. Biocidal Products Regulation (EU) 528/regulation (eu) no 528/2012 of the european parliament and of the council of 22 May 2012 concerning the making available on the market and use of biocidal products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02012R0528-20140425&from=EN (accessed on 7 June 2021).
- Park, G.W.; Cho, M.; Cates, E.L.; Lee, D.; Oh, B.-T.; Vinje, J.; Kim, J. Fluorinated TiO2 as an ambient light-activated virucidal surface coating material for the control of human norovirus. J. Photochem. Photobiol. B Biol. 2014, 140, 315–320. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Arribas, B.; Coaut, C.; Blanco, R.; Coaut, D. Understandng the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar]
- Wiener, J.; Quinn, J.P.; Bradford, P.; Goering, R.; Nathan, C.; Bush, K.; Weinstein, R.A. Multiple antibiotic–resistant Klebsiella and Escherichia coli in nursing homes. JAMA 1999, 281, 517–523. [Google Scholar] [CrossRef]
- Josset, S.; Keller, N.; Lett, M.-C.; LeDoux, M.J.; Keller, V. Numeration methods for targeting photoactive materials in the UV-A photocatalytic removal of microorganisms. Chem. Soc. Rev. 2008, 37, 744–755. [Google Scholar] [CrossRef]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Giovanni, E.; Radi, R.; Roberts, L.J.; et al. Even free radicals should follow some rules: A guide to free radical re-search terminology and methodology. Free. Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnology 2018, 16, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risks to humans. In Some Antiviral and Antineoplastic Drugs, and Other Pharmaceutical Agents; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). EFSA Statement on the Review of the Risks Related to the Exposure to the Food Additive Titanium Dioxide (E 171) Performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, e05714. [Google Scholar]
- Lin, Y.-C.; Fang, Y.-P.; Hung, C.-F.; Yu, H.-P.; Alalaiwe, A.; Wu, Z.-Y.; Fang, J.-Y. Multifunctional TiO2/SBA-15 mesoporous silica hybrids loaded with organic sunscreens for skin application: The role in photoprotection and pollutant adsorption with reduced sunscreen permeation. Colloids Surf. B: Biointerfaces 2021, 202, 111658. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, N.; Qiblawi, S.; Norton, S.A.; Fivenson, D. Sunscreens: UV filters to protect us: Part 1: Changing regulations and choices for optimal sun protection. Int. J. Womens Dermatol. 2021, 7, 28–44. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of titanium dioxide nanoparticles ex-posure on human health—A review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canu, I.G.; Fraize-Frontier, S.; Michel, C.; Charles, S. Weight of epidemiological evidence for titanium dioxide risk as-sessment: Current state and further needs. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 430–435. [Google Scholar] [CrossRef]
- Huang, H.; Fan, C.; Li, M.; Nie, H.-L.; Wang, F.-B.; Wang, H.; Wang, R.; Xia, J.; Zheng, X.; Zuo, X.; et al. COVID-19: A Call for Physical Scientists and Engineers. ACS Nano 2020, 14, 3747–3754. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Li, Z.; Xie, Z.; Sokolova, I.M.; Song, L.; Peijnenburg, W.J.G.M.; Hu, M.; Wang, Y. Rethinking nano-TiO2 Safety: Overview of toxic effects in humans and aquatic animals. Small 2020, 16, e2002019. [Google Scholar] [CrossRef]
- Palmieri, V.; De Maio, F.; De Spirito, M.; Papi, M. Face masks and nanotechnology: Keep the blue side up. Nano Today 2021, 37, 101077. [Google Scholar] [CrossRef]
- Margarucci, L.M.; Spica, V.R.; Gianfranceschi, G.; Valeriani, F. Untouchability of natural spa waters: Perspectives for treatments within a personalized water safety plan. Environ. Int. 2019, 133, 105095. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free. Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.-C.; Horvatovich, P.; Gies, J.-P.; Keller, V.; Keller, N.; Andre, P. TiO 2 photocatalysis damages lipids and proteins in escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 nanoparticles to escherichia coli: Effects of particle size, crystal phase and water chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 20743:2013 Textiles—Determination of Antibacterial Activity of Textile Products. Available online: https://www.iso.org/standard/59586.html (accessed on 7 June 2021).
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updates 2017, 33, 1–22. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Using Face Masks in the Community: First Update. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-face-masks-community-first-update.pdf (accessed on 3 March 2021).
- Pullangott, G.; Kannan, U.; Gayathri, S.; Kiran, D.V.; Maliyekkal, S.M. A comprehensive review on antimicrobial face masks: An emerging weapon in fighting pandemics. RSC Adv. 2021, 11, 6544–6576. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Effect of size and crystalline phase of TiO₂ nanoparticles on photocatalytic inactivation of escherichia coli. J. Nanosci. Nanotechnol. 2019, 19, 8172–8179. [Google Scholar] [CrossRef]
- Erdem, A.; Metzler, D.; Cha, D.; Huang, C.P. Inhibition of bacteria by photocatalytic nano-TiO2 particles in the absence of light. Int. J. Env. Sci. Technol. 2015, 12, 2987–2996. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef]
- Hadi, J.; Dunowska, M.; Wu, S.; Brightwell, G. Control measures for SARS-CoV-2: A review on light-based inactivation of single-stranded RNA viruses. Pathogens 2020, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb Pathog. 2018, 823, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Higashimoto, S. Titanium-dioxide-based visible-light-sensitive photocatalysis: Mechanistic insight and applications. Catalysts 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Valdiglesias, V.; Laffon, B. The impact of nanotechnology in the current universal COVID-19 crisis. Let’s not forget nanosafety! Nanotoxicology 2020, 14, 1013–1016. [Google Scholar] [CrossRef]
- Aksit, A.; Camlibel, N.O.; Zeren, E.T.; Kutlu, B. Development of antibacterial fabrics by treatment with Ag-doped TiO2 nanoparticles. J. Text. Inst. 2017, 108, 2046–2056. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, C.-E.; Qi, Z.; Zhou, Q.; Wang, C. Microwave-assisted preparation of pyrite and its sensitisation of titani-um dioxide in self-cleaning aramid fabrics. Coloration Technol. 2018, 134, 284–291. [Google Scholar] [CrossRef]
- Halimi, S.U.; Abu Bakar, N.F.; Ismail, S.N.; Hashib, S.A.; Naim, M.N. Electrospray deposition of titanium dioxide (TiO2) nanoparticles. In AIP Conference Proceedings; American Institute of Physics: Melville, NY, USA, 2014. [Google Scholar]
- Zahid, M.; Papadopoulou, E.L.; Suarato, G.; Binas, V.D.; Kiriakidis, G.; Gounaki, I.; Moira, O.; Venieri, D.; Bayer, I.S.; Athanassiou, A. Fabrication of visible light-induced antibacterial and self-cleaning cotton fabrics using manganese doped TiO2 nanoparticles. ACS Appl. Bio Mater. 2018, 1, 1154–1164. [Google Scholar] [CrossRef]
- Rilda, Y.; Damara, D.; Putri, Y.E.; Refinel, R.; Agustien, A.; Pardi, H. Pseudomonas aeruginosa antibacterial textile cotton fiber construction based on ZnO–TiO2 nanorods template. Heliyon 2020, 6, e03710. [Google Scholar] [CrossRef]
- Mahdieh, Z.M.; Shekarriz, S.; Taromi, F.A.; Montazer, M. A new method for in situ synthesis of Ag–TiO2 nanocomposite particles on polyester/cellulose fabric by photoreduction and self-cleaning properties. Cellulose 2018, 25, 2355–2366. [Google Scholar] [CrossRef]
- Shahidi, S.; Ahmadi, M.T.; Rashidi, A.; Ghoranneviss, M. Effect of plasma treatment on self-cleaning of textile fabric using titanium dioxide. Micro Nano Lett. 2015, 10, 408–413. [Google Scholar] [CrossRef]
- Bedford, N.M.; Steckl, A.J. Photocatalytic self cleaning textile fibers by coaxial electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 2448–2455. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Shi, H.; Wang, X.; Qi, K.; Zhou, X.; Xin, J.H. Carboxymethyl chitosan coating to block photocatalytic activity of TiO2 nanoparticles. Text. Res. J. 2010, 80, 2214–2220. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Zhang, F.-R.; Li, H.-S. Anti-ultraviolet and physical properties of woolen fabrics cured with citric acid and TiO2/chitosan. J. Appl. Polym. Sci. 2006, 100, 4311–4319. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on Titanium Dioxide (nano form) Coated with Cetyl Phosphate, Manganese Dioxide or Triethoxycaprylylsilane as UV-Filter in Dermally Applied Cosmetic and Corrigendum. The SCCS Adopted This Opinion by Written Procedure on 22 July 2013. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_238.pdf (accessed on 10 July 2021).
- Avino, P.; Protano, C.; Vitali, M.; Manigrasso, M. Benchmark study on fine-mode aerosol in a big urban area and relevant doses deposited in the human respiratory tract. Environ. Pollut. 2016, 216, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Laganà, P.; Caruso, G. The COVID-19 pandemic and its implications on the environment. Environ. Res. 2021, 201, 111648. [Google Scholar] [CrossRef]
- Piana, A.; Colucci, M.E.; Valeriani, F.; Marcolongo, A.; Sotgiu, G.; Pasquarella, C.; Margarucci, L.M.; Petrucca, A.; Gianfranceschi, G.; Babudieri, S.; et al. Monitoring COVID-19 transmission risks by quantitative real-time PCR tracing of droplets in hospital and living environments. mSphere 2021, 6, 01070-20. [Google Scholar] [CrossRef]







| E. coli | Cloth Face Mask | Surgical Face Mask | FFP2 | |||
| Contact time | Coated | Uncoated | Coated | Uncoated | Coated | Uncoated |
| Time 0 | 5.0 ± 1.6 × 103 | 5.0 ± 1 × 103 | 5.0 ± 1.8 × 103 | 5.0 ± 1.8 × 103 | 9 ± 3 × 103 | 9 ± 3 × 103 |
| Time 15’ | 0.0 | 2 ± 3 × 102 | 3 ± 3 × 102 | 1 × 103 | 2 ± 4 × 102 | 9 ± 1 × 102 |
| % of reduction | 100.0 | 96.0 | 97.0 | 80.0 | 98.0 | 90.0 |
| S. aureus | Cloth Face Mask | Surgical Face Mask | FFP2 | |||
| Contact time | Coated | Uncoated | Coated | Uncoated | Coated | Uncoated |
| Time 0 | 5.0 ± 1.8 × 103 | 5.0 ± 1.8 × 103 | 1.3 ± 1.0 × 103 | 8 ± 1 × 102 | 9 ± 3 × 103 | 9 ± 3 × 103 |
| Time 15’ | 0.0 | 5 ± 3 × 102 | 1.6 ± 1.0 × 102 | 5 ± 1 × 102 | 0.0 | 3 ± 1 × 102 |
| % of reduction | 100.0 | 80.0 | 88.0 | 62.5 | 100.0 | 98.0 |
| Application Method | Description | Reference |
|---|---|---|
| Immersion, pad-dry cure | Fabric immersion in a TiO2 suspension, following by a drying step at 80 °C for 5 min, cured at 180 °C for 3 min. The process is then repeated, then washed with deionized water at 60 °C for 5 min and dried at 60 °C again. | [71,72] |
| Electrospray | A suspension of TiO2 is dissolved in a polar solvent, nebulized at atmospheric pressure inside the ionization chamber through a needle held at a high electric potential. | [73] |
| Dip coating, spray coating | Deposition of a wet liquid film by immersion of the fabric into a solution containing hydrolysable metal compounds (or readily formed particles) and its withdrawal at a constant speed into an atmosphere containing water vapor. | [74,75] |
| Irradiation under UV-A | Textile immersion in a suspension of TiO2, then squeezed and immediately irradiated under UVA 100 W lamp for 1 h. Finally, the coated fabric is dried at 130 °C for 5 min and cured at 150 °C for 3 min. | [76] |
| Plasma treatment with direct current magnetron sputtering | Plasma treatment makes fabrics and technical felts hydrophilic and allow wettability with aqueous processing substances in particular stages of the process, such as dyeing, printing, or textile finishing. Then, samples are impregnated with TiO2. | [77] |
| Electrospinning | Electrospinning is a process by which a polymer in solution or spindle can be spun into small diameter fibers, thanks to a high potential electric field. Cellulose acetate is used as the core phase, which, after a deacetylation step, becomes cellulose. A dispersion of TiO2 particles is used as the sheath phase to disperse titania nanoparticles along the fiber outer surfaces. | [78] |
| Some polymers and proteins functionalized with TiO2 particles | By different coating techniques, such as a dip–pad–dry cure process, a chitosan–titanium dioxide (CS–TiO2) composite or CS-TiO2 with citric acid can be applied into a cotton matrix. | [79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margarucci, L.M.; Gianfranceschi, G.; Romano Spica, V.; D’Ermo, G.; Refi, C.; Podico, M.; Vitali, M.; Romano, F.; Valeriani, F. Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2. Int. J. Environ. Res. Public Health 2021, 18, 8662. https://doi.org/10.3390/ijerph18168662
Margarucci LM, Gianfranceschi G, Romano Spica V, D’Ermo G, Refi C, Podico M, Vitali M, Romano F, Valeriani F. Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2. International Journal of Environmental Research and Public Health. 2021; 18(16):8662. https://doi.org/10.3390/ijerph18168662
Chicago/Turabian StyleMargarucci, Lory Marika, Gianluca Gianfranceschi, Vincenzo Romano Spica, Giuseppe D’Ermo, Cristiano Refi, Maurizio Podico, Matteo Vitali, Ferdinando Romano, and Federica Valeriani. 2021. "Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2" International Journal of Environmental Research and Public Health 18, no. 16: 8662. https://doi.org/10.3390/ijerph18168662







