T. gondii Infection in Urban and Rural Areas in the Amazon: Where Is the Risk for Toxoplasmosis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Area
2.3. Study Design and Samples Collection
2.4. Serological Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Lyu, C.; Zhao, J.; Shen, B. Sixty years (1957–2017) of research on 255 toxoplasmosis in China-an overview. Front. Microbiol. 2017, 8, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.L.; Kruszon-Moran, D.; Elder, S.; Rivera, H.N.; Press, C.; Montoya, J.G.; McQuillan, G.M. Toxoplasma gondii Infection in the United States, 2011–2014. Am. J. Trop. Med. Hyg. 2018, 98, 551–557. [Google Scholar] [CrossRef]
- Tuon, F.F.; Wollmann, L.C.; Pegoraro, D.; Gouveia, A.M.; Andrejow, A.P.; Schultz, A.T.; Gomes, C.J.; Novaes, O.C.; Salmória, P.A. Seroprevalence of Toxoplasma gondii, cytomegalovirus and Epstein Barr virus in 578 tissue donors in Brazil. J. Infect. Public Health 2019, 12, 289–291. [Google Scholar] [CrossRef]
- Santos, A.L.C.; Trettel, A.C.P.T.; Ribeiro, L.J.B.B.; Vasconcellos, M.L.; Zenazokenae, L.E.; Santos, M.A.; Lemos, E.R.S.; Amendoeira, M.R.R. Serological study on toxoplasmosis in the Haliti-Paresí community of the Utiariti indigenous territory, Campo Novo do Parecis, Mato Grosso, Brazil. Parasite Epidemiol. Control. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Carmo, E.L. Aspectos Epidemiológicos da Toxoplasmose Região Metropolitana de Belém, Pará, Brasil. Ph.D. Thesis, Universidade Federal do Pará, Belém, Brazil, 2011. [Google Scholar]
- Demar, M.; Hommel, D.; Djossou, F.; Peneau, C.; Boukhari, R.; Louvel, D.; Bourbigot, A.M.; Nasser, V.; Ajzenberg, D.; Dardé, M.L.; et al. Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin. Microbiol. Infect. 2012, 18, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of Transmission and Sources of Infection in Outbreaks of Human Toxoplasmosis. Emerg Infect Dis. 2019, 25, 2177–2182. [Google Scholar] [CrossRef] [Green Version]
- Brasil. Ministério da Saúde. Relatório Final Sobre a Investigação do Surto de Toxoplasmose em Rondônia, Brasil; Ministério da Saúde: Brasília, Brazil, 2011; p. 2011. [Google Scholar]
- Morais, R.A.P.B.; Freire, A.B.C.; Barbosa, D.R.B.; Silva, L.C.T.; Pinheiro, A.F.; Costa, S.S.; Ramos, F.L.P.; Bichara, C.N.C.; Lima, L.J.B.; Silva, A.V.; et al. Surto de toxoplasmose aguda no Município de Ponta de Pedras, Arquipélago do Marajó, Estado do Pará, Brasil: Características clínicas, laboratoriais e epidemiológicas. Rev. Pan-Amaz. Saude 2016, 7, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Instituto Brasileiro de Geografia e Estatística. Available online: https://cidades.ibge.gov.br/brasil/pa/ponta-de-pedras/panorama (accessed on 13 March 2021).
- Lima, V.M.; Costa, S.M.F.; Ribeiro, H. Uma contribuição da metodologia peir para o estudo de uma pequena cidade na Amazônia: Ponta de Pedras, Pará. Saude Soc. 2017, 26, 1071–1086. [Google Scholar] [CrossRef]
- Ferreira, M.U.; Hiramoto, R.M.; Aureliano, D.P.; Silva-Nunes, M.; Silva, N.S.; Malafronte, R.S.; Muniz, P.T. A community-based survey of human toxoplasmosis in rural Amazonia: Seroprevalence, seroconversion rate, and associated risk factors. Am. J. Trop. Med. Hyg. 2009, 81, 171–176. [Google Scholar] [CrossRef]
- Bóia, M.N.; Carvalho-Costa, F.A.; Sodré, F.C.; Pinto, G.M.T.; Amendoeira, M.R.R. Seroprevalence of Toxoplasma gondii infection among indian people living in Iauareté, São Gabriel da Cachoeira, Amazonas, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2008, 50, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitaliano, S.N.; Mendonça, G.M.; Sandres, F.A.M.; Camargo, J.S.A.A.; Tarso, P.; Basano, S.A.; Silva, J.C.D.; Souza, V.K.G.; Cartonilho, G.; Almeida, A.T.S.; et al. Epidemiological aspects of Toxoplasma gondii infection in riverside communities in the Southern Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2015, 48, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrells, A.; Opsteegh, M.; Pollock, K.G.; Alexander, C.L.; Chatterton, J.; Evans, R.; Walker, R.; McKenzie, C.A.; Hill, D.; Innes, E.A.; et al. The prevalence and genotypic analysis of Toxoplasma gondii from individuals in Scotland, 2006–2012. Parasit. Vectors 2016, 9, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, F.L.; Amendoeira, M.R.; Bastos, O.M.; Mattos, D.P.; Fonseca, A.B.; Nicolau, J.L.; Neves, L.B.; Millar, P.R. Prevalence and risk factors for Toxoplasma gondii infection among pregnant and postpartum women attended at public healthcare facilities in the City of Niterói, State of Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.C.; Vilela, M.M.; Sena-Lopes, A.; Farias, N.A.R.; Faria, L.M.J.; Avila, L.F.C.; Berne, M.E.A.; Borsuk, S. Seroprevalence of Toxoplasma gondii and Toxocara canis in a human rural population of Southern Rio Grande do Sul. Rev. Inst. Med. Trop. S. Paulo. 2018, 60, e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmo, E.L.; Morais, R.A.P.B.; Oliveira, A.S.; Figueredo, J.E.; Figueredo, M.C.; Silva, A.V.; Bichara, C.N.C.; Póvoa, M.M. Soroepidemiologia da infecção pelo Toxoplasma gondii no Município de Novo Repartimento, Estado do Pará, Brasil. Rev. Pan-Amaz. Saude 2016, 7, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Dias, R.C.F.; Lopes-Mori, F.M.R.; Mitsuka-Breganó, R.; Dias, R.A.F.; Tokano, D.V.; Reiche, E.M.V.; Freire, R.L.; Navarro, I.T. Factors associated to infection by Toxoplasma gondii in pregnant women attended in Basic Health Units in the city of Rolândia, Paraná, Brazil. Rev. Soc. Bras. Med. Trop. 2011, 53, 185–191. [Google Scholar] [CrossRef]
- Antinarelli, L.; Silva, M.R.; Guimarães, R.; Terror, M.S.; Lima, P.E.; Ishii, J.; Muniz, P.F.; Coimbra, E.S. Rural residence remains a risk factor for Toxoplasma infection among pregnant women in a highly urbanized Brazilian area: A robust cross-sectional study. Trans. R. Soc. Trop. Med. Hyg. 2020, traa153. [Google Scholar] [CrossRef]
- Taylor, M.R.; Lennon, B.; Holland, C.V.; Cafferkey, M. Community study of toxoplasma antibodies in urban and rural schoolchildren aged 4 to 18 years. Arch. Dis. Child. 1997, 77, 406–409. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Zanzi, C.; Williams-Nguyen, J.; Belongia, E.A. A sero-survey of toxoplasmosis in farm and non-farm children from Wisconsin, United States, 1997–1999. BMC Public Health 2013, 13, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamba, S.; Cissé, M.; Sangaré, I.; Zida, A.; Ouattara, S.; Guiguemdé, R.T. Seroprevalence and risk factors of Toxoplasma gondii infection in pregnant women from Bobo Dioulasso, Burkina Faso. BMC Infect. Dis. 2017, 17, 482–487. [Google Scholar] [CrossRef]
- Iddawela, D.; Vithana, S.; Ratnayake, C. Seroprevalence of toxoplasmosis and risk factors of Toxoplasma gondii infection among pregnant women in Sri Lanka: A cross sectional study. BMC Public Health 2017, 17, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Huffner, J.G.P.; Oliveira, A.R.F. Crescimento urbano desordenado no município de Ponta de Pedras na Ilha do Marajó: Um estudo de caso do bairro do Carnapijó. InterEspaço: Revista de Geografia e interdisciplinaridade 2018, 3, 159–181. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.T.; Aguilar, D.M.; Camargo, L.M.; Labruna, M.B.; de Andrade, H.F.; Meireles, L.R.; Dubey, J.P.; Thulliez, P.; Dias, R.A.; Gennari, S.M. Seroprevalence of Toxoplasma gondii antibodies in humans from rural Western Amazon, Brazil. J. Parasitol. 2006, 92, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Zanzi, C.; Campbell, C. Seroepidemiology of toxoplasmosis in rural and urban communities from Los Rios Region, Chile. Infect. Ecol. Epidemiol. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Avelar, M.V.; Martinez, V.O.; Moura, D.L.; Barros, I.A.; Primo, A.A.S.; Duarte, A.O.; Soares, N.M.; Lima, F.W.M. Association between seroprevalence of IgG anti-Toxoplasma gondii and risk factors for infection among pregnant women in Climério de Oliveira Maternity, Salvador, Bahia, Brazil. Rev. Inst. Med. Trop. S. Paulo 2017, 59, e90. [Google Scholar] [CrossRef] [Green Version]
- Lobo, M.L.; Patrocinio, G.; Sevivas, T.; Sousa, B.; Matos, O. Portugal and Angola: Similarities and differences in Toxoplasma gondii seroprevalence and risk factors in pregnant women. Epidemiol. Infect. 2017, 145, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Elsheikha, H.M.; Zhou, N.; Peng, P.; Qin, S.Y.; Meng, Q.F.; Qian, A.D. Prevalence of antibodies against Toxoplasma gondii in pets and their owners in Shandong province, Eastern China. BMC Infect. Dis. 2018, 18, 430. [Google Scholar] [CrossRef] [PubMed]
- Carme 2001. Exposition a Toxoplasma gondii et risque de foetopathie toxoplasmique. Med. Trop. 2001, 61, 6. [Google Scholar]
- Guigue, N.; Léon, L.; Hamane, S.; Gits-Muselli, M.; Le Strat, Y.; Alanio, A.; Bretagne, S. Continuous Decline of Toxoplasma gondii Seroprevalence in Hospital: A 1997-2014 Longitudinal Study in Paris, France. Front. Microbiol. 2018, 9, 2369. [Google Scholar] [CrossRef] [PubMed]

| Rural Area | Urban Area | Total | ||||
|---|---|---|---|---|---|---|
| Variables | n | IgG Positive (%) | n | IgG Positive (%) | n | IgG Positive (%) |
| Sex | ||||||
| Male | 283 | 185 (65.4) | 163 | 133 (81.6) | 446 | 318 (71.3) |
| Female | 388 | 235 (60.6) | 306 | 251 (82.0) | 694 | 486 (70.0) |
| Age group | ||||||
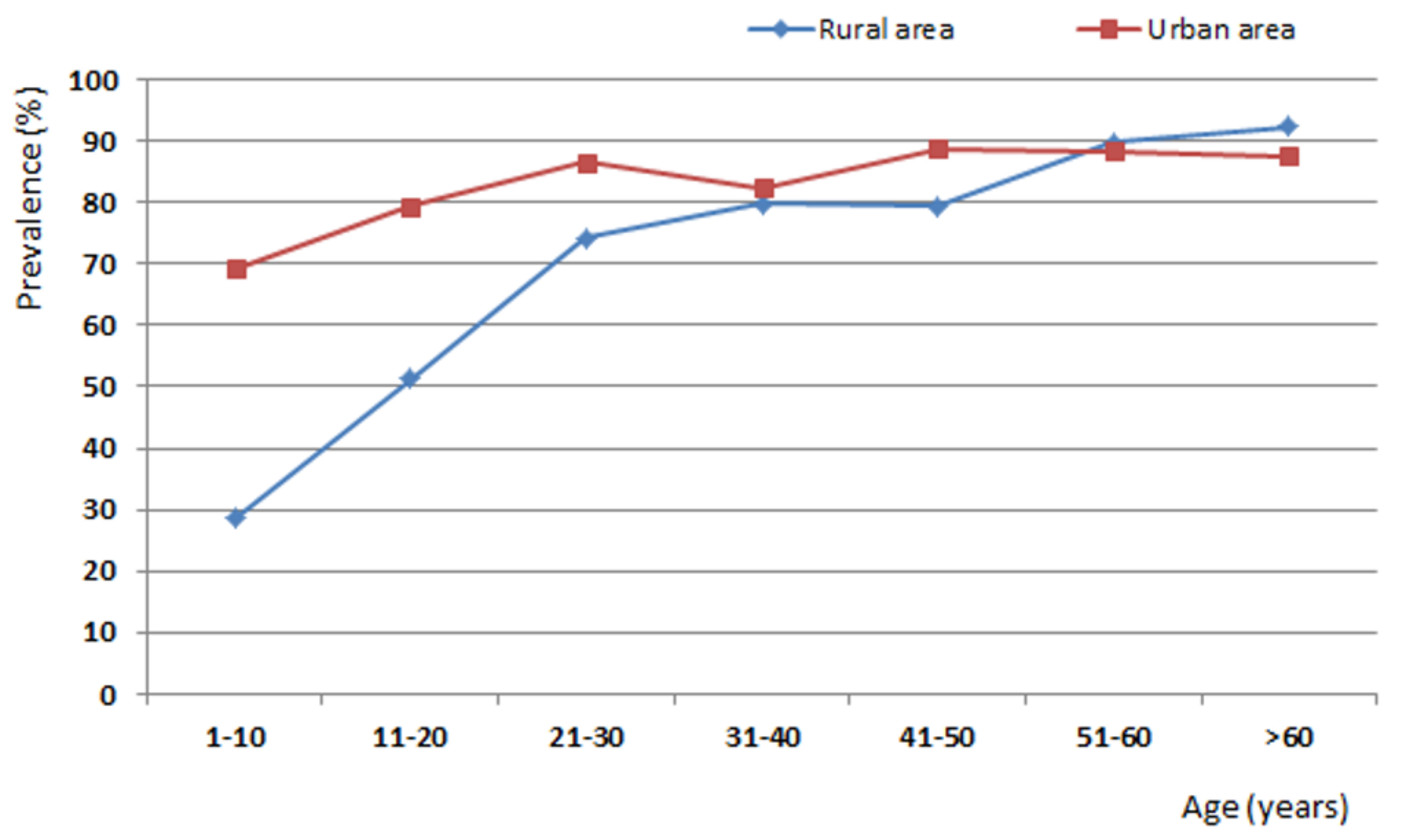
| 1–10 | 147 | 42 (28.6) | 88 | 61 (69.3) | 235 | 103 (43.8) |
| 11–20 | 158 | 81 (51.3) | 87 | 69 (79.3) | 245 | 150 (61.2) |
| 21–30 | 113 | 84 (74.3) | 74 | 64 (86.5) | 187 | 148 (79.1) |
| 31–40 | 84 | 67 (79.8) | 74 | 61 (82.4) | 158 | 128 (81.0) |
| 41–50 | 68 | 54 (79.4) | 63 | 56 (88.8) | 131 | 110 (84.0) |
| 51–60 | 49 | 44 (89.8) | 43 | 38 (88.4) | 92 | 82 (89.1) |
| >60 | 52 | 48 (92.3) | 40 | 35 (87.5) | 92 | 83 (90.2) |
| Soil contact | ||||||
| Yes | 583 | 218 (37.4) | 284 | 234 (82.4) | 867 | 452 (52.1) |
| No | 88 | 55 (62.5) | 185 | 150 (81.1) | 273 | 205 (75.1) |
| Having a cat at home | ||||||
| Yes | 203 | 126 (62.1) | 128 | 103 (80.5) | 331 | 229 (69.2) |
| No | 468 | 294 (62.8) | 341 | 281 (82.4) | 809 | 575 (71.1) |
| Contact with cats | ||||||
| Yes | 486 | 318 (65.4) | 393 | 326 (82.9) | 879 | 644 (73.3) |
| No | 185 | 102 (55.1) | 76 | 58 (76.3) | 261 | 160 (61.3) |
| River water intake | ||||||
| Yes | 216 | 132 (61.1) | 45 | 38 (84.4) | 261 | 170 (65.1) |
| No | 455 | 288 (63.3) | 424 | 346 (81.6) | 879 | 634 (72.1) |
| Water treatment by boiling or filtering | ||||||
| Yes | 192 | 118 (61.5) | 216 | 185 (85.6) | 408 | 303 (74.3) |
| No | 479 | 302 (63.0) | 253 | 199 (78.6) | 732 | 501 (68.4) |
| Having yard with land or sand | ||||||
| Yes | 635 | 400 (63.0) | 389 | 323 (83.0) | 1024 | 723 (70.6) |
| No | 36 | 20 (55.5) | 80 | 61 (76.2) | 116 | 81 (69.8) |
| Açaí juice intake | ||||||
| Yes | 662 | 417 (63.0) | 454 | 372 (81.9) | 1116 | 789 (70.7) |
| No | 9 | 3 (33.3) | 15 | 12 (80.0) | 24 | 15 (62.5) |
| Raw/undercooked meat intake | ||||||
| Yes | 177 | 115 (64.9) | 130 | 103 (79.2) | 307 | 218 (71.0) |
| No | 494 | 305 (61.7) | 339 | 281 (82.9) | 833 | 586 (70.3) |
| Hunted meat intake | ||||||
| Yes | 448 | 290 (64.7) | 189 | 152 (80.4) | 637 | 442 (69.4) |
| No | 202 | 117 (57.9) | 280 | 232 (82.8) | 482 | 349 (72.4) |
| Habit of entering the forest | ||||||
| Yes | 403 | 262 (65.0) | 176 | 147 (83.5) | 579 | 409 (70.6) |
| No | 250 | 149 (59.6) | 291 | 235 (80.7) | 541 | 384 (71.0) |
| Variables | n | IgG Positive (%) | OR (CI 95%) | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 283 | 185 (65.4) | 1.2 (0.9–1.7) | 0.234 |
| Female | 388 | 235 (60.6) | ||
| Age group | ||||
| 1–10 | 147 | 42 (28.6) | Refer. | - |
| 11–20 | 158 | 81 (51.3) | 2.6 (1.6–4.2) | <0.0001 |
| 21–30 | 113 | 84 (74.3) | 7.2 (4.1–12.6) | <0.0001 |
| 31–40 | 84 | 67 (79.8) | 9.8 (5.2–18.7) | <0.0001 |
| 41–50 | 68 | 54 (79.4) | 9.6 (5.8–19.2) | <0.0001 |
| 51–60 | 49 | 44 (89.8) | 22.0 (8.2–59.3) | <0.0001 |
| >60 | 52 | 48 (92.3) | 30.0 (10.2–88.4) | <0.0001 |
| Soil contact | ||||
| Yes | 583 | 218 (37.4) | 1.0 (0.6–1.6) | 0.921 |
| No | 88 | 55 (62.5) | ||
| Having a cat at home | ||||
| Yes | 203 | 126 (62.1) | 0.9 (0.7–1.4) | 0.922 |
| No | 468 | 294 (62.8) | ||
| Contact with cats | ||||
| Yes | 486 | 318 (65.4) | 1.5 (1.1–2.2) | 0.017 |
| No | 185 | 102 (55.1) | ||
| River water intake | ||||
| Yes | 216 | 132 (61.1) | 0.9 (0.6–1.3) | 0.645 |
| No | 455 | 288 (63.3) | ||
| Water treatment by boiling or filtering | ||||
| Yes | 192 | 118 (61.5) | 0.9 (0.7–1.3) | 0.767 |
| No | 479 | 302 (63.0) | ||
| Having yard with land or sand | ||||
| Yes | 635 | 400 (63.0) | 1.4 (0.7–2.7) | 0.471 |
| No | 36 | 20 (55.5) | ||
| Açaí juice intake | ||||
| Yes | 662 | 417 (63.0) | 3.4 (0.8–13.7) | 0.139 |
| No | 9 | 3 (33.3) | ||
| Raw/undercooked meat intake | ||||
| Yes | 177 | 115 (64.9) | 1.1 (0.8–1.6) | 0.502 |
| No | 494 | 305 (61.7) | ||
| Hunted meat intake | ||||
| Yes | 448 | 290 (64.7) | 1.3 (0.9–1.9) | 0.116 |
| No | 202 | 117 (57.9) | ||
| Habit of entering the forest | ||||
| Yes | 403 | 262 (65.0) | 1.3 (0.9–1.7) | 0.191 |
| No | 250 | 149 (59.6) |
| Variable | OR | CI 95% | p-Value |
|---|---|---|---|
| Rural area | |||
| Age (≥21years ) | 6.217 | 4.33–8.92 | <0.001 |
| Contact with cat | 1.799 | 1.22–2.65 | 0.003 |
| Urban area | |||
| Age (≥21 years ) | 2.118 | 1.31–3.41 | 0.002 |
| Variables | n | IgG Positive (%) | OR (CI 95%) | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 163 | 133 (81.6) | 0.9 (0.6–1.6) | 0.991 |
| Female | 306 | 251 (82.0) | ||
| Age group | ||||
| 1–10 | 88 | 61 (69.3) | Refer. | - |
| 11–20 | 87 | 69 (79.3) | 1.7 (0.8–3.7) | 0.180 |
| 21–30 | 74 | 64 (86.5) | 2.8 (1.3–6.3) | 0.016 |
| 31–40 | 74 | 61 (82.4) | 2.1 (0.9–4.5) | 0.073 |
| 41–50 | 63 | 56 (88.8) | 3.5 (1.4–8.7) | 0.0083 |
| 51–60 | 43 | 38 (88.4) | 3.4 (1.2–9.5) | 0.030 |
| >60 | 40 | 35 (87.5) | 3.1 (1.1–8.8) | 0.047 |
| Soil contact | ||||
| Yes | 284 | 234 (82.4) | 1.09 (0.7–1.8) | 0.811 |
| No | 185 | 150 (81.1) | ||
| Having a cat at home | ||||
| Yes | 128 | 103 (80.5) | 0.8 (0.5–1.5) | 0.726 |
| No | 341 | 281 (82.4) | ||
| Contact with cats | ||||
| Yes | 393 | 326 (82.9) | 1.51 (0.8–2.7) | 0.225 |
| No | 76 | 58 (76.3) | ||
| River water intake | ||||
| Yes | 45 | 38 (84.4) | 1.22 (0.5–2.8) | 0.789 |
| No | 424 | 346 (81.6) | ||
| Water treatment by boiling or filtering | ||||
| Yes | 216 | 185 (85.6) | 1.61 (0.9–2.6) | 0.066 |
| No | 253 | 199 (78.6) | ||
| Having yard with land or sand | ||||
| Yes | 389 | 323 (83.0) | 1.5 (0.8–2.7) | 0.202 |
| No | 80 | 61 (76.2) | ||
| Açaí juice intake | ||||
| Yes | 454 | 372 (81.9) | 1.1 (0.3–4.1) | 0.801 |
| No | 15 | 12 (80.0) | ||
| Raw/undercooked meat intake | ||||
| Yes | 130 | 103 (79.2) | 0.8 (0.5–1.3) | 0.431 |
| No | 339 | 281 (82.9) | ||
| Hunted meat intake | ||||
| Yes | 189 | 152 (80.4) | 0.8 (0.5–1.4) | 0.583 |
| No | 280 | 232 (82.8) | ||
| Habit of entering the forest | ||||
| Yes | 176 | 147 (83.5) | 1.2 (0.7–1.9) | 0.531 |
| No | 291 | 235 (80.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, R.d.A.P.B.; Carmo, E.L.d.; Costa, W.S.; Marinho, R.R.; Póvoa, M.M. T. gondii Infection in Urban and Rural Areas in the Amazon: Where Is the Risk for Toxoplasmosis? Int. J. Environ. Res. Public Health 2021, 18, 8664. https://doi.org/10.3390/ijerph18168664
Morais RdAPB, Carmo ELd, Costa WS, Marinho RR, Póvoa MM. T. gondii Infection in Urban and Rural Areas in the Amazon: Where Is the Risk for Toxoplasmosis? International Journal of Environmental Research and Public Health. 2021; 18(16):8664. https://doi.org/10.3390/ijerph18168664
Chicago/Turabian StyleMorais, Rafaela dos Anjos Pinheiro Bogoevich, Ediclei Lima do Carmo, Wanda Silva Costa, Rodrigo Rodrigues Marinho, and Marinete Marins Póvoa. 2021. "T. gondii Infection in Urban and Rural Areas in the Amazon: Where Is the Risk for Toxoplasmosis?" International Journal of Environmental Research and Public Health 18, no. 16: 8664. https://doi.org/10.3390/ijerph18168664






