Rehabilitation after a Complete Avulsion of the Proximal Rectus Femoris Muscle: Considerations from a Case Report
Abstract
1. Background
2. Case Presentation
2.1. Anthropometrics and Injury Mechanism
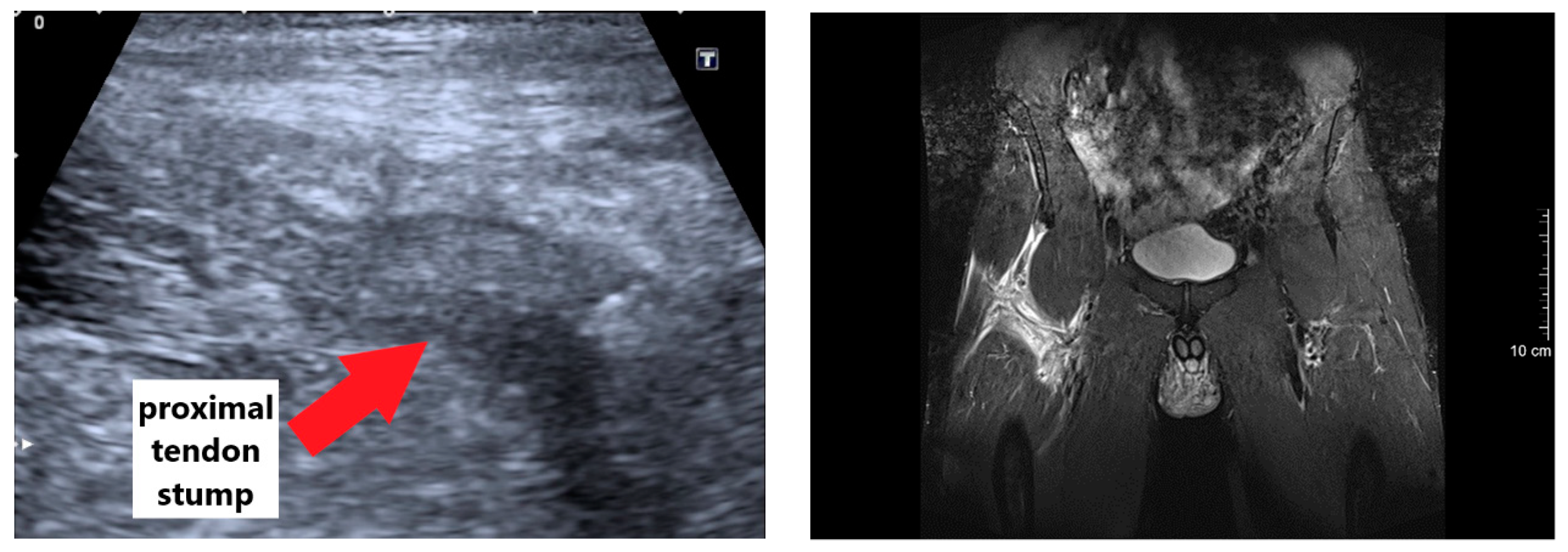
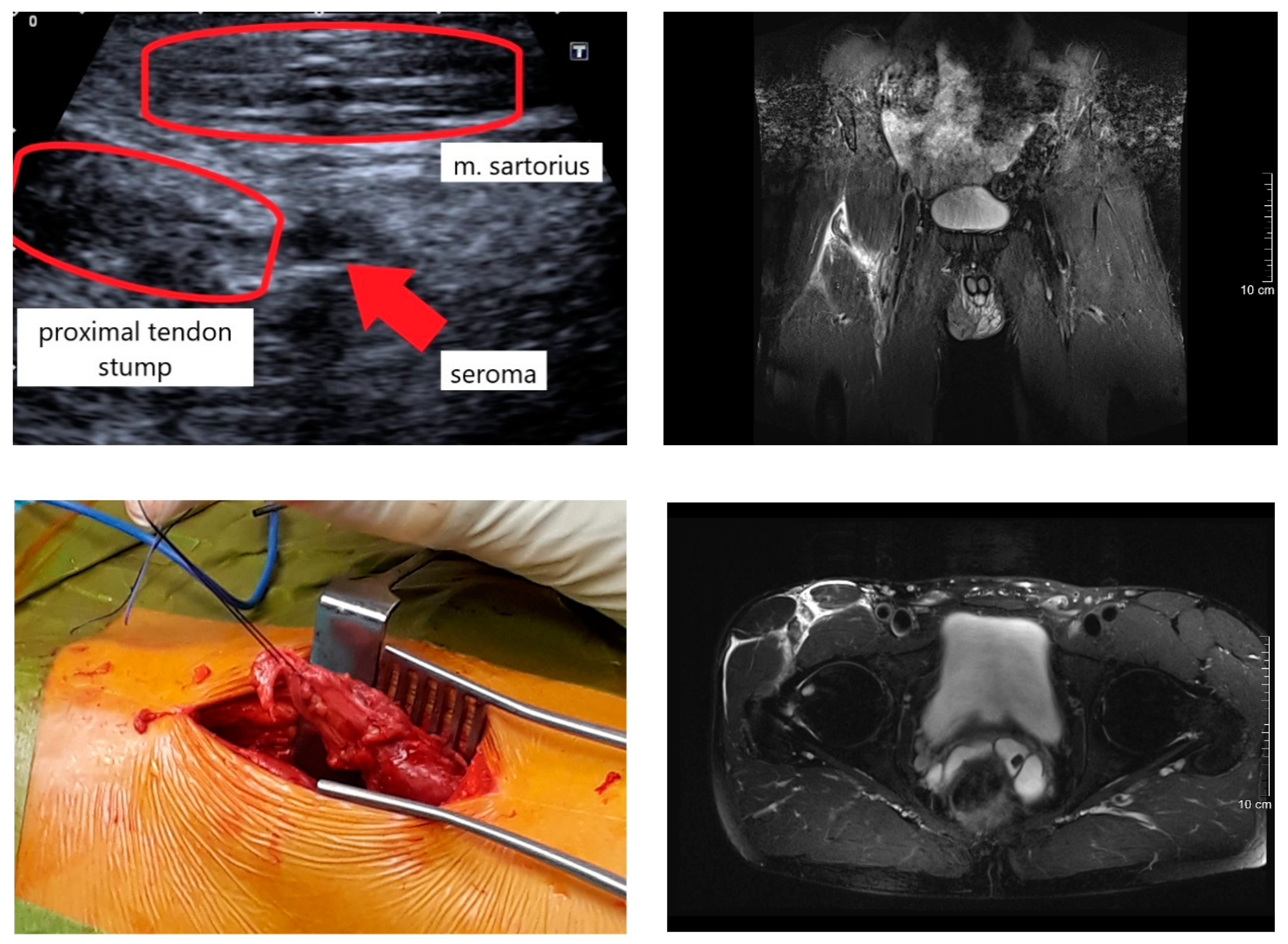
2.2. Diagnosis and Surgery Procedure
2.3. Postoperative and Rehabilitation Procedures
2.4. Testing Procedures
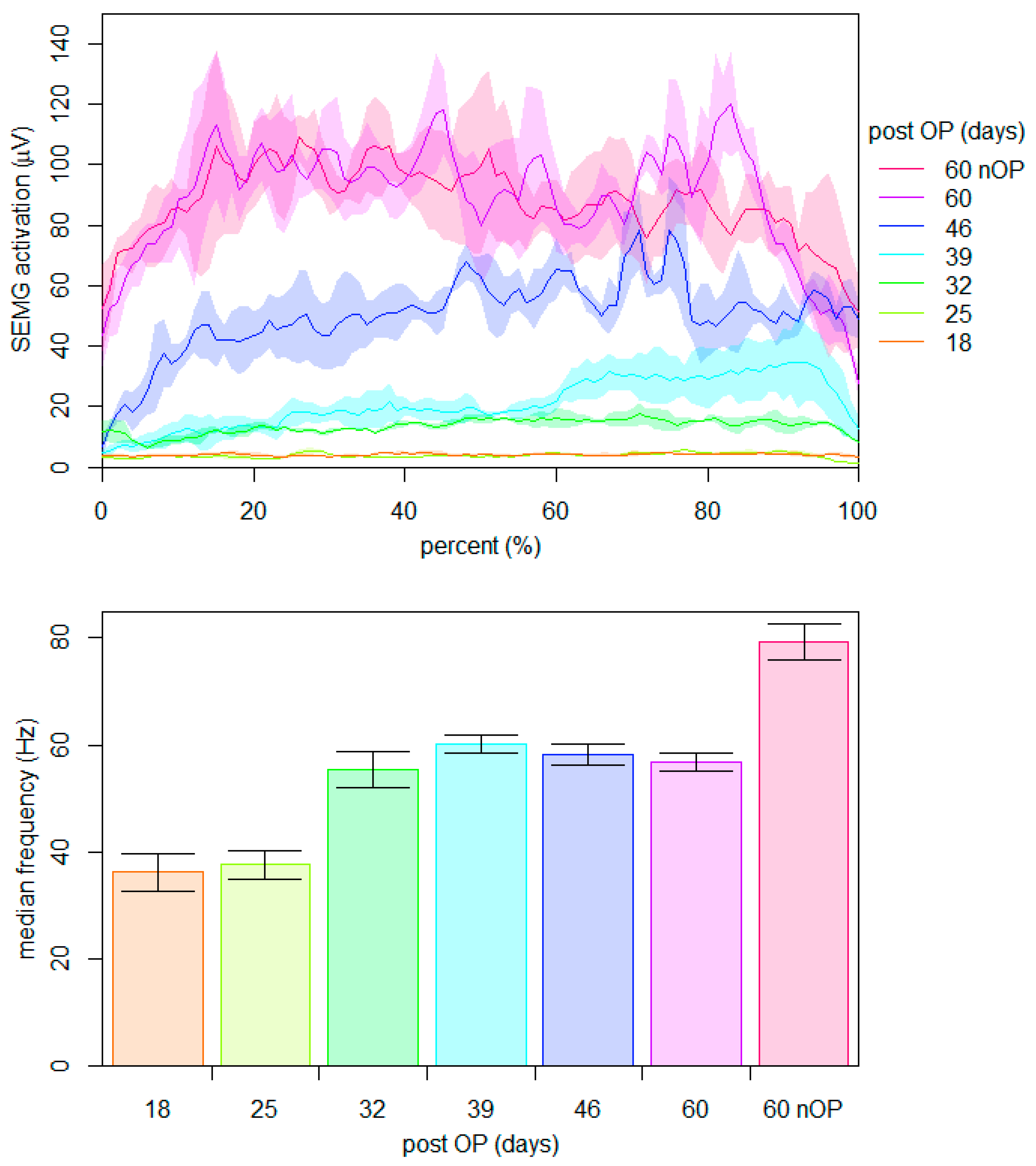
2.4.1. Maximum Voluntary Neuromuscular Activation within the First Nine Weeks Post-Surgery
2.4.2. Crank Torque during Ergometer Cycling within the First 9 Weeks Post-Surgery
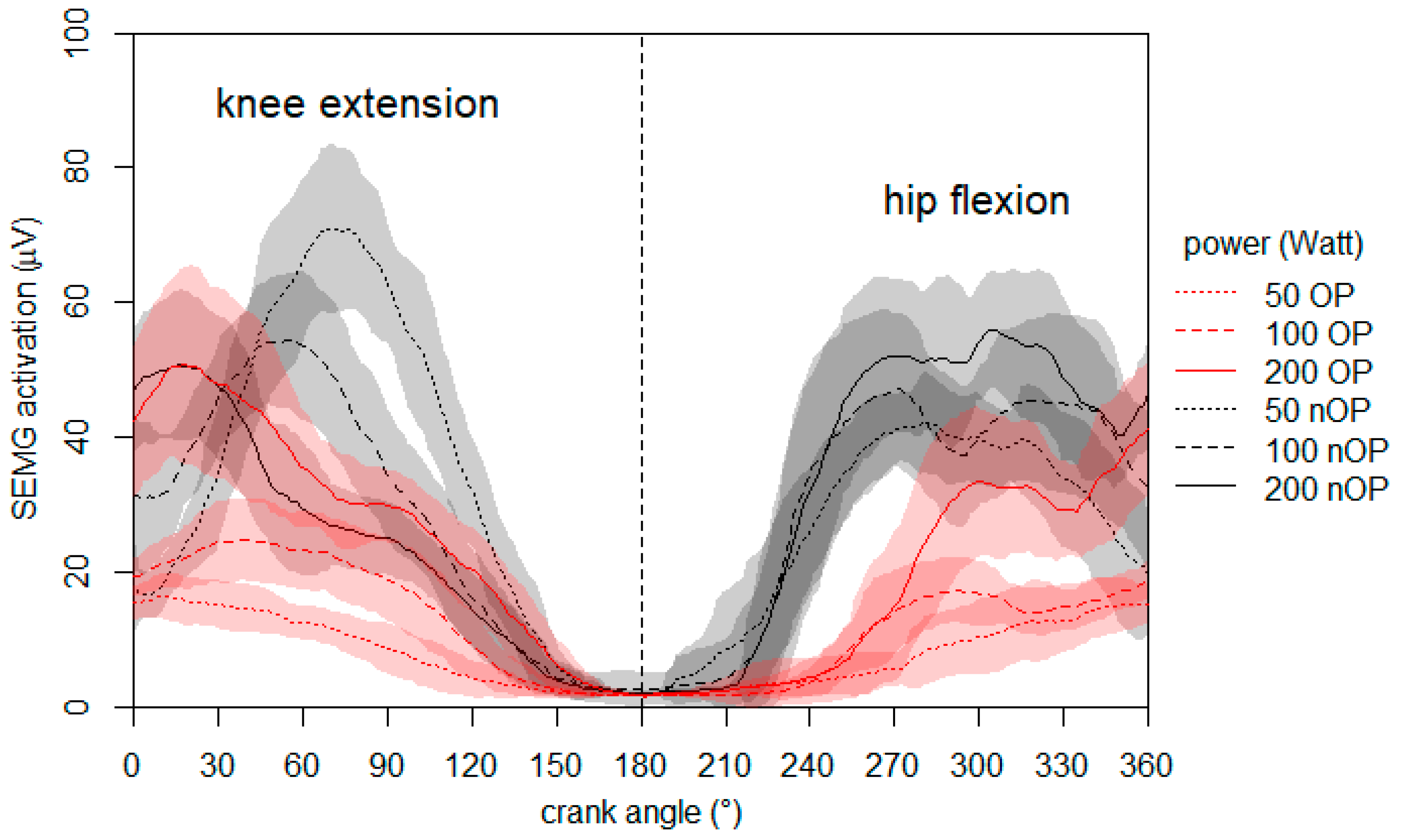
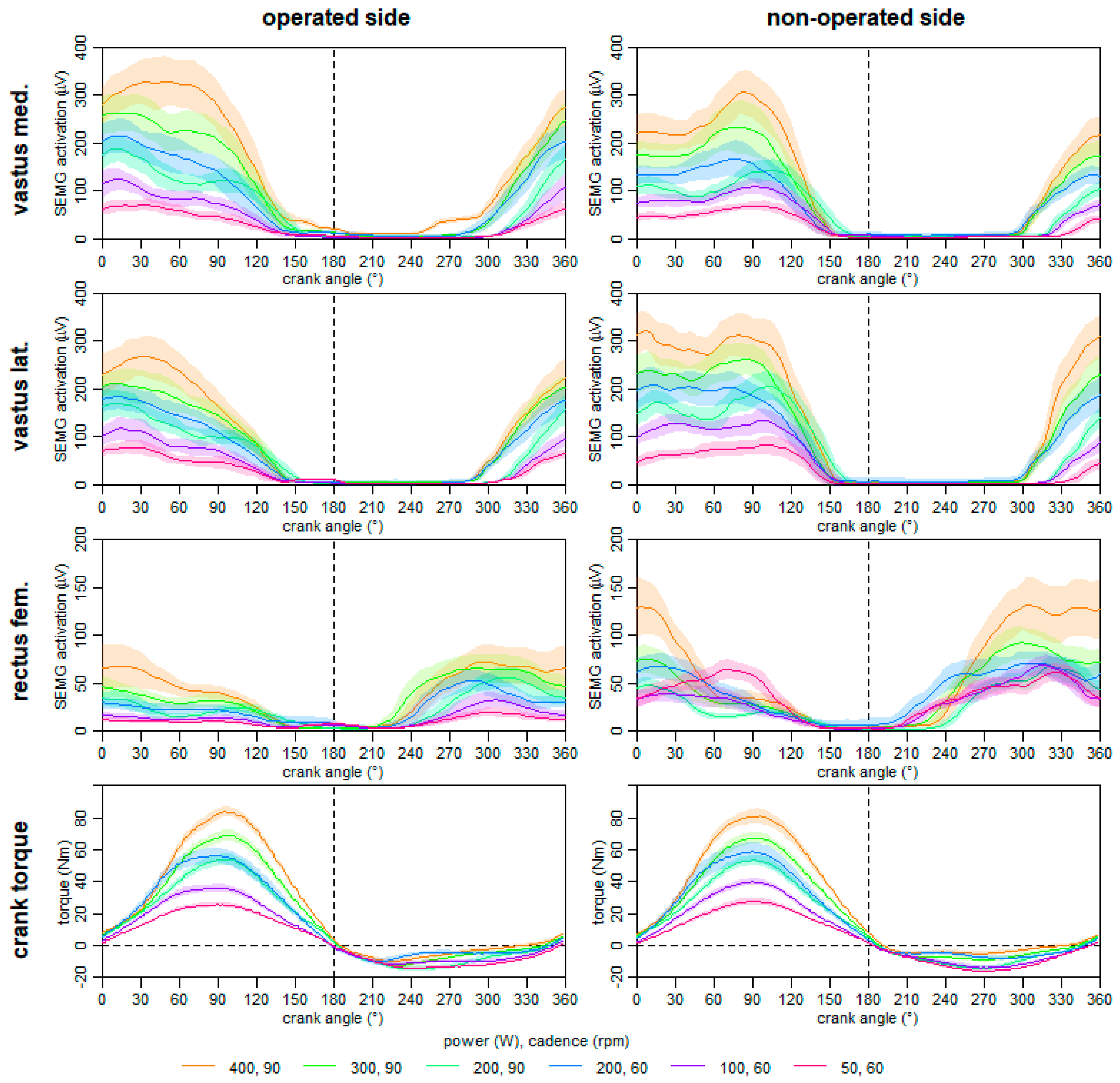
2.4.3. Neuromuscular Activation during Ergometer Cycling on the 60th Day Post-Surgery
2.4.4. Leg Volume during the One-Year Follow-Up Period
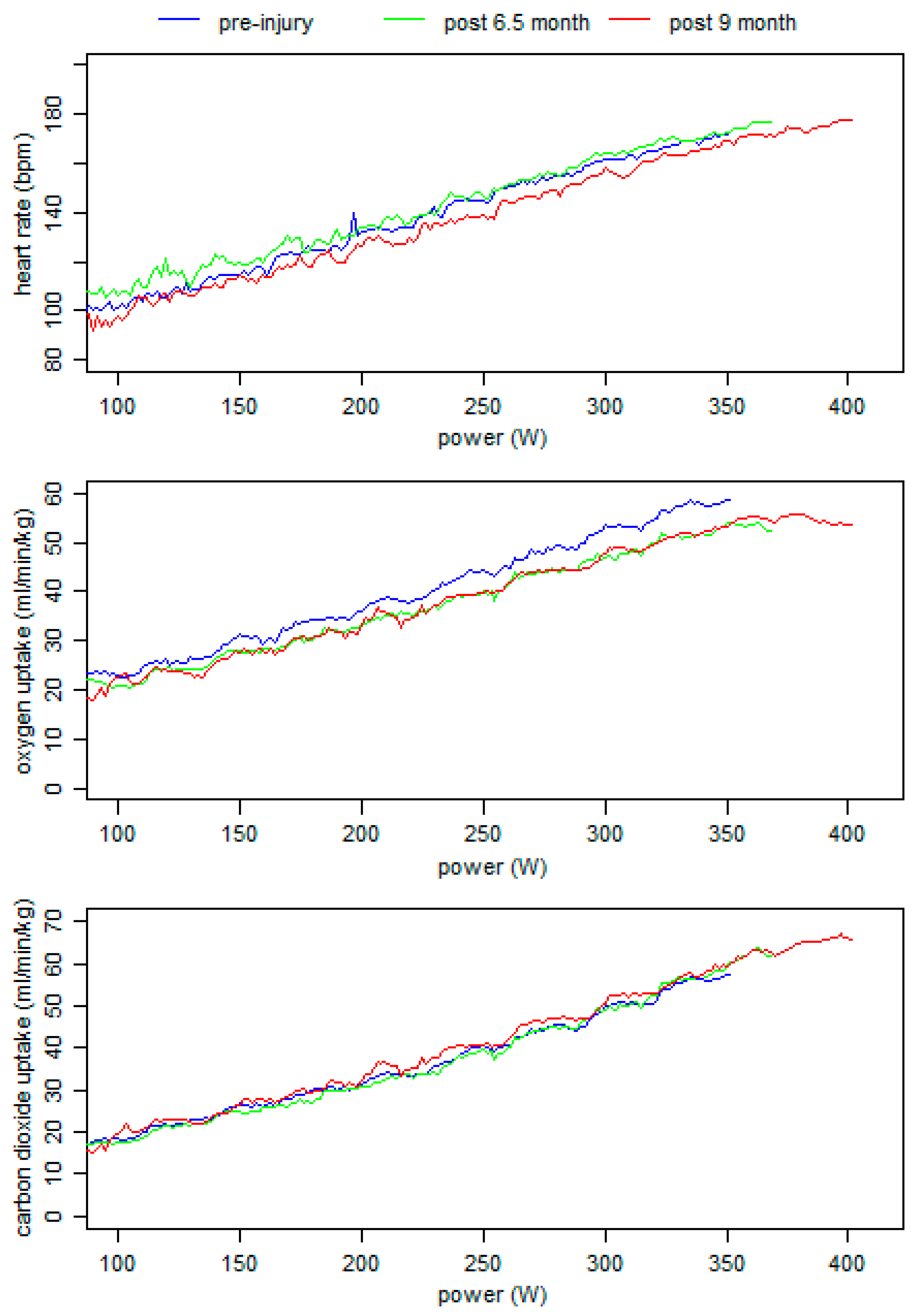
2.4.5. Endurance Performance during the One-Year Follow-Up
2.4.6. Anatomical Quadriceps Characteristics at One Year Post-Surgery
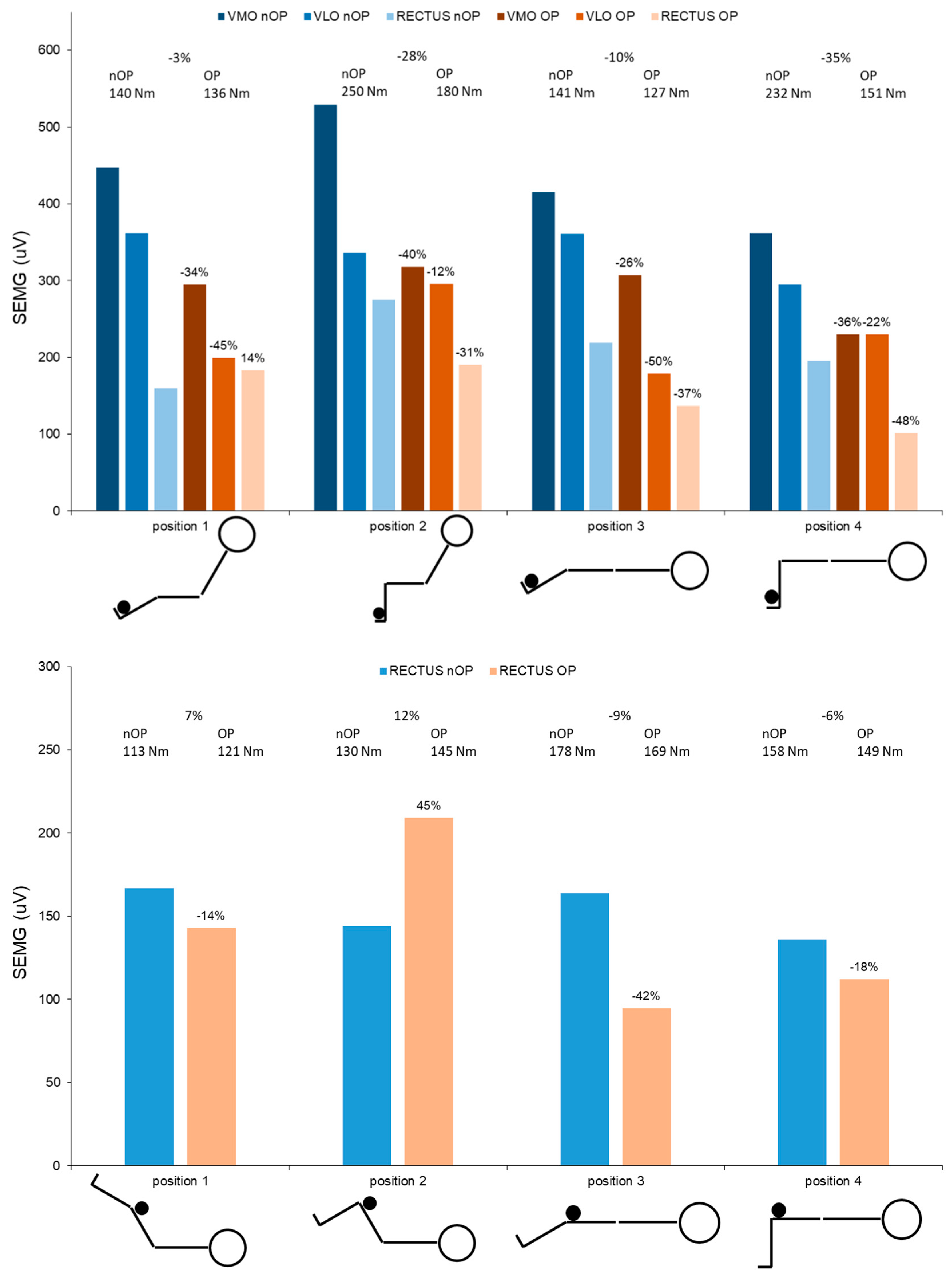
2.4.7. Neuromuscular Activation and Isometric Strength at One Year Post-Surgery
2.4.8. Crank Torque and Neuromuscular Activation during Ergometer Cycling at One Year Post-Surgery
3. Discussion
3.1. Injury Mechanism, Diagnosis, and Surgery Procedure
3.2. Rehabilitation and Testing Procedures until Six Month after Surgery
3.3. One-Year Follow up Testing Procedures
4. Conclusions
- Within the first month post-surgery, an almost total neuromuscular inhibition of the rectus femoris muscle was present.
- A stepwise reduction in inter-limb compensation was observable (e.g., in crank torque) during rehabilitation.
- Muscular intra-limb compensations were shown at six months post-surgery and even one year after surgery, which were also represented in the long-term adaption of the muscle characteristics and leg volumes.
- A changed motor control strategy can result in asymmetric muscle patterns during ergometer cycling, and can even result in symmetric power output.
- During rehabilitation, there might be a benefit to normalizing neuromuscular muscle activation in ergometer cycling using higher loads.
5. Additional Comments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Consent for Publication
Availability of Data and Materials
Conflicts of Interest
Abbreviations
| MRI | magnetic resonance imaging |
| SEMG | surface electromyography |
References
- Bordalo-Rodrigues, M.; Rosenberg, Z.S. MR imaging of the proximal rectus femoris musculotendinous unit. Magn. Reson. Imaging Clin. N. Am. 2005, 13, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hasselman, C.T.; Best, T.M.; Hughes, C.T.; Martinez, S.; Garrett, W.E., Jr. An explanation for various rectus femoris strain injuries using previously undescribed muscle architecture. Am. J. Sports Med. 1995, 23, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Barbosa, N.C.; Tuteja, S.; Gardon, R.; Daggett, M.; Monnot, D.; Kajetanek, C.; Thaunat, M. Surgical Management of Rectus Femoris Avulsion Among Professional Soccer Players. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef]
- Lempainen, L.; Kosola, J.; Pruna, R.; Puigdellivol, J.; Ranne, J.; Orava, S. Operative Treatment of Proximal Rectus Femoris Injuries in Professional Soccer Players: A Series of 19 Cases. Orthop. J. Sports Med. 2018, 6. [Google Scholar] [CrossRef]
- Langer, P.R.; Selesnick, H. Proximal rectus femoris avulsion in an elite, olympic-level sprinter. Am. J. Orthop. 2010, 39, 543–547. [Google Scholar]
- Garcia, V.V.; Duhrkop, D.C.; Seijas, R.; Ares, O.; Cugat, R. Surgical treatment of proximal ruptures of the rectus femoris in professional soccer players. Arch. Orthop. Trauma Surg. 2012, 132, 329–333. [Google Scholar] [CrossRef]
- Irmola, T.; Heikkila, J.T.; Orava, S.; Sarimo, J. Total proximal tendon avulsion of the rectus femoris muscle. Scand. J. Med. Sci. Sports 2007, 17, 378–382. [Google Scholar] [CrossRef]
- Kannus, P.; Natri, A. Etiology and pathophysiology of tendon ruptures in sports. Scand. J. Med. Sci. Sports 1997, 7, 107–112. [Google Scholar] [CrossRef]
- Keel, M.; Bastian, J. Beckennahe Sehnenverletzungen. OP-Journal 2016, 31, 168–172. [Google Scholar] [CrossRef][Green Version]
- Dean, C.S.; Arbeloa-Gutierrez, L.; Chahla, J.; Pascual-Garrido, C. Proximal Rectus Femoris Avulsion Repair. Arthrosc. Tech. 2016, 5, e545–e549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gamradt, S.C.; Brophy, R.H.; Barnes, R.; Warren, R.F.; Thomas Byrd, J.W.; Kelly, B.T. Nonoperative treatment for proximal avulsion of the rectus femoris in professional American football. Am. J. Sports Med. 2009, 37, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Zlomislic, V.; Du, J.; Huang, B.K.; Chang, E.Y.; Chang, D.G. Nonoperative Management of a Severe Proximal Rectus Femoris Musculotendinous Injury in a Recreational Athlete: A Case Report. PM&R 2018, 10, 1417–1421. [Google Scholar]
- Esser, S.; Jantz, D.; Hurdle, M.F.; Taylor, W. Proximal Rectus Femoris Avulsion: Ultrasonic Diagnosis and Nonoperative Management. J. Athl. Train. 2015, 50, 778–780. [Google Scholar] [CrossRef]
- Kim, J.W.; Hwang, Y.S.; Moon, K.P.; Kim, K.T.; Song, J.Y.; Kang, M.S. Treatment of Avulsion Fracture of Proximal Rectus Femoris with Suture Anchor. Korean J. Sports Med. 2016, 34, 83. [Google Scholar] [CrossRef]
- Straw, R.; Colclough, K.; Geutjens, G. Surgical repair of a chronic rupture of the rectus femoris muscle at the proximal musculotendinous junction in a soccer player. Br. J. Sports Med. 2003, 37, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Wohlfahrt, H.W.; Haensel, L.; Mithoefer, K.; Ekstrand, J.; English, B.; McNally, S.; Orchard, J.; van Dijk, C.N.; Kerkhoffs, G.M.; Schamasch, P.; et al. Terminology and classification of muscle injuries in sport: The Munich consensus statement. Br. J. Sports Med. 2013, 47, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Cassidy, F.H.; Yokoo, T.; Aganovic, L.; Hanna, R.F.; Bydder, M.; Middleton, M.S.; Hamilton, G.; Chavez, A.D.; Schwimmer, J.B.; Sirlin, C.B. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics 2009, 29, 231–260. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Ueblacker, P.; Muller-Wohlfahrt, H.W.; Hinterwimmer, S.; Imhoff, A.B.; Feucht, M.J. Suture anchor repair of proximal rectus femoris avulsions in elite football players. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2590–2594. [Google Scholar] [CrossRef]
- Hotfiel, T.; Seil, R.; Bily, W.; Bloch, W.; Gokeler, A.; Krifter, R.M.; Mayer, F.; Ueblacker, P.; Weisskopf, L.; Engelhardt, M. Nonoperative treatment of muscle injuries-recommendations from the GOTS expert meeting. J. Exp. Orthop. 2018, 5, 24. [Google Scholar] [CrossRef]
- Cross, T.M.; Gibbs, N.; Houang, M.T.; Cameron, M. Acute quadriceps muscle strains: Magnetic resonance imaging features and prognosis. Am. J. Sports Med. 2004, 32, 710–719. [Google Scholar] [CrossRef]
- Kassarjian, A.; Rodrigo, R.M.; Santisteban, J.M. Current concepts in MRI of rectus femoris musculotendinous (myotendinous) and myofascial injuries in elite athletes. Eur. J. Radiol. 2012, 81, 3763–3771. [Google Scholar] [CrossRef]
- Pogliacomi, F.; Visigalli, A.; Valenti, P.G.; Pedrazzini, A.; Bernuzzi, G.; Concari, G.; Vaienti, E.; Ceccarelli, F. Rectus femoris myotendinous lesion treated with PRP: A case report. Acta Biomed. 2019, 90, 178–183. [Google Scholar]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef]
- Davi, S.M.; Lepley, A.S.; Denegar, C.R.; DiStefano, L.J.; Edgar, C.M.; Lepley, L.K. Quadriceps Inhibition After Naturally Occurring Patellar Tendon Damage and Pain. J. Athl. Train. 2020, 55, 608–614. [Google Scholar] [CrossRef]
- Boyer, M.I.; Goldfarb, C.A.; Gelberman, R.H. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J. Hand Ther. 2005, 18, 80–85. [Google Scholar] [CrossRef]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon healing: Repair and regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Celik, D.; Argut, S.K.; Turker, N.; Kilicoglu, O.I. The effectiveness of superimposed neuromuscular electrical stimulation combined with strengthening exercises on patellofemoral pain: A randomized controlled pilot trial. J. Back Musculoskelet. Rehabil. 2019, 33, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.L.; Kelly, J.; Hohmann, E. Neuromuscular adaptations and correlates of knee functionality following ACL reconstruction. J. Orthop. Res. 2008, 26, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, W.I.; Cramp, M.C.; Scott, O.M. Changes in muscle strength and EMG median frequency after anterior cruciate ligament reconstruction. Eur. J. Appl. Physiol. 2006, 98, 613–623. [Google Scholar] [CrossRef]
- Lepley, L.K.; Davi, S.M.; Burland, J.P.; Lepley, A.S. Muscle Atrophy After ACL Injury: Implications for Clinical Practice. Sports Health 2020, 12, 579–586. [Google Scholar] [CrossRef]
- Buddhadev, H.H.; Crisafulli, D.L.; Suprak, D.N.; San Juan, J.G. Individuals With Knee Osteoarthritis Demonstrate Interlimb Asymmetry in Pedaling Power During Stationary Cycling. J. Appl. Biomech. 2018, 34, 306–311. [Google Scholar] [CrossRef]
- Hunt, M.A.; Sanderson, D.J.; Moffet, H.; Inglis, J.T. Interlimb asymmetry in persons with and without an anterior cruciate ligament deficiency during stationary cycling. Arch. Phys. Med. Rehabil. 2004, 85, 1475–1478. [Google Scholar] [CrossRef]
- Roos, P.E.; Button, K.; van Deursen, R.W. Motor control strategies during double leg squat following anterior cruciate ligament rupture and reconstruction: An observational study. J. Neuroeng. Rehabil. 2014, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Messer, D.J.; Shield, A.J.; Williams, M.D.; Timmins, R.G.; Bourne, M.N. Hamstring muscle activation and morphology are significantly altered 1-6 years after anterior cruciate ligament reconstruction with semitendinosus graft. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Gokeler, A.; Bisschop, M.; Benjaminse, A.; Myer, G.D.; Eppinga, P.; Otten, E. Quadriceps function following ACL reconstruction and rehabilitation: Implications for optimisation of current practices. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1163–1174. [Google Scholar] [CrossRef]
- Baumgart, C.; Schubert, M.; Hoppe, M.W.; Gokeler, A.; Freiwald, J. Do ground reaction forces during unilateral and bilateral movements exhibit compensation strategies following ACL reconstruction? Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1385–1394. [Google Scholar] [CrossRef]
- Grooms, D.; Appelbaum, G.; Onate, J. Neuroplasticity following anterior cruciate ligament injury: A framework for visual-motor training approaches in rehabilitation. J. Orthop. Sports Phys. Ther. 2015, 45, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Gokeler, A.; Verhagen, E.; Hirschmann, M.T. Let us rethink research for ACL injuries: A call for a more complex scientific approach. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1303–1304. [Google Scholar] [CrossRef]
- Loureiro, A.; Constantinou, M.; Diamond, L.E.; Beck, B.; Barrett, R. Individuals with mild-to-moderate hip osteoarthritis have lower limb muscle strength and volume deficits. BMC Musculoskelet. Disord. 2018, 19, 303. [Google Scholar] [CrossRef] [PubMed]


| Side | M. Rectus Femoris | M. Vastus Intermedius | M. Vastus Lateralis | M. Vastus Medialis | Femur | Pelvis | |
|---|---|---|---|---|---|---|---|
| Fat fraction | nOP | 0.105 | 0.112 | 0.109 | 0.112 | - | - |
| OP | 0.106 | 0.109 | 0.105 | 0.114 | - | - | |
| OP/nOP | −1% | 3% | 4% | −2% | |||
| Muscle volume (cm³) | nOP | 355.2 | 897.9 | 1033.0 | 730.8 | 590.6 | 303.5 |
| OP | 287.3 | 654.0 | 931.5 | 617.4 | 586.0 | 295.4 | |
| OP/nOP | 19% | 27% | 10% | 16% | 1% | 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgart, C.; Grim, C.; Heiss, R.; Ehrenstein, P.; Freiwald, J.; Hoppe, M.W. Rehabilitation after a Complete Avulsion of the Proximal Rectus Femoris Muscle: Considerations from a Case Report. Int. J. Environ. Res. Public Health 2021, 18, 8727. https://doi.org/10.3390/ijerph18168727
Baumgart C, Grim C, Heiss R, Ehrenstein P, Freiwald J, Hoppe MW. Rehabilitation after a Complete Avulsion of the Proximal Rectus Femoris Muscle: Considerations from a Case Report. International Journal of Environmental Research and Public Health. 2021; 18(16):8727. https://doi.org/10.3390/ijerph18168727
Chicago/Turabian StyleBaumgart, Christian, Casper Grim, Rafael Heiss, Philipp Ehrenstein, Jürgen Freiwald, and Matthias Wilhelm Hoppe. 2021. "Rehabilitation after a Complete Avulsion of the Proximal Rectus Femoris Muscle: Considerations from a Case Report" International Journal of Environmental Research and Public Health 18, no. 16: 8727. https://doi.org/10.3390/ijerph18168727
APA StyleBaumgart, C., Grim, C., Heiss, R., Ehrenstein, P., Freiwald, J., & Hoppe, M. W. (2021). Rehabilitation after a Complete Avulsion of the Proximal Rectus Femoris Muscle: Considerations from a Case Report. International Journal of Environmental Research and Public Health, 18(16), 8727. https://doi.org/10.3390/ijerph18168727






