The Relationship of Tobacco, Alcohol, and Betel Quid with the Formation of Oral Potentially Malignant Disorders: A Community-Based Study from Northeastern Thailand
Abstract
:1. Introduction
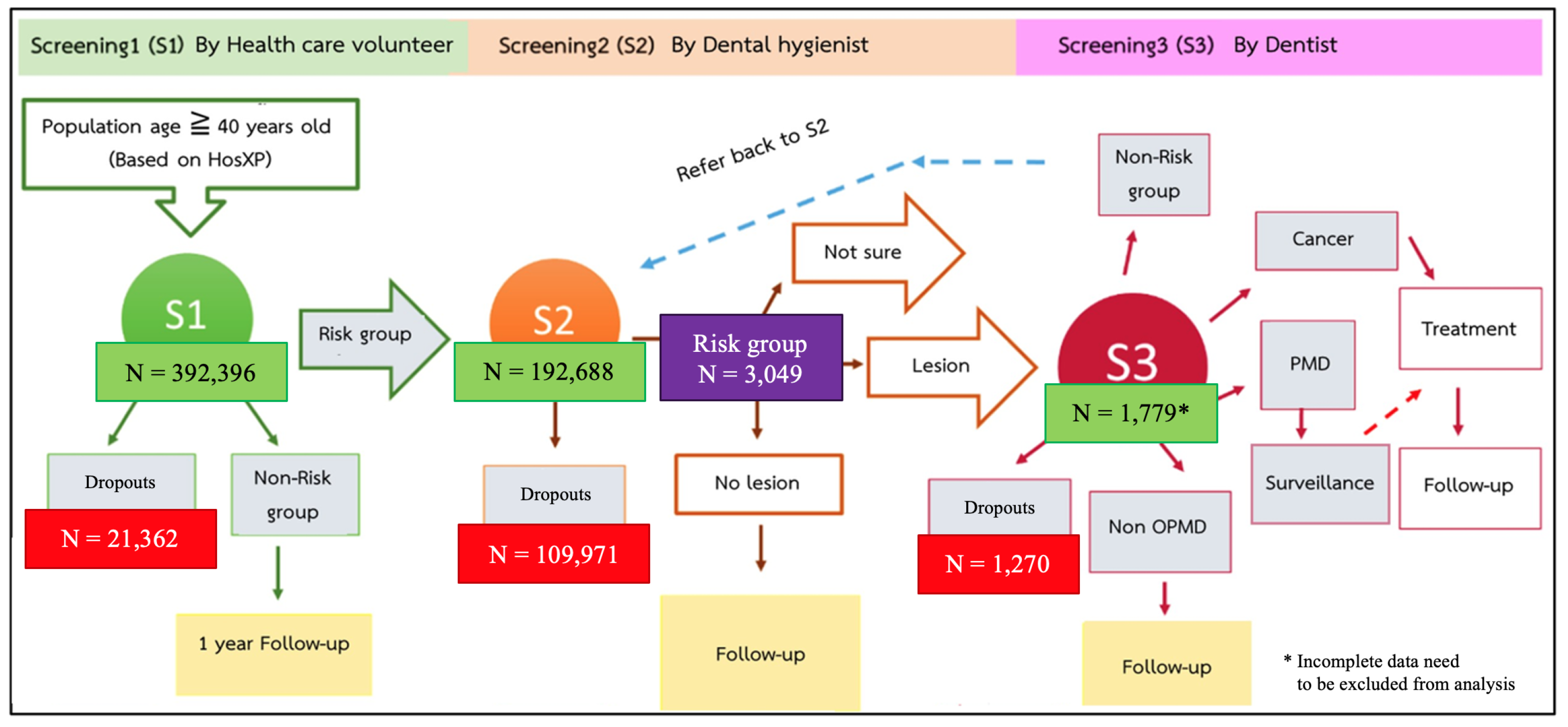
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OPMDs | oral potentially malignant disorders |
| SLT | smokeless tobacco |
| BQ | betel quid |
| SS | secondhand smoking |
| OR | odds ratio |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampangsri, W.; Vatanasapt, P.; Kamsa-Ard, S.; Suwanrungruang, K.; Promthet, S. Betel quid chewing and upper aerodigestive tract cancers: A prospective cohort study in khon kaen, Thailand. Asian Pac. J. Cancer Prev. 2013, 14, 4335–4338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Maxillofac. Pathol. 2016, 45, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.; Gueiros, L.A.; Leao, J.C.; Fedele, S. Risk factors and etiopathogenesis of potentially premalignant oral epithelial lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 603–611. [Google Scholar] [CrossRef] [Green Version]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.C.; Mehta, F.S.; Daftary, D.K.; Pindborg, J.J.; Bhonsle, R.B.; Jalnawalla, P.N.; Sinor, P.N.; Pitkar, V.K.; Murti, P.R.; Irani, R.R.; et al. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow-up study of Indian villagers. Community Dent. Oral Epidemiol. 1980, 8, 283–333. [Google Scholar] [CrossRef]
- Mehta, F.S.; Pindborg, J.J.; Gupta, P.C.; Daftary, D.K. Epidemiologic and histologic study of oral cancer and leukoplakia among 50,915 villagers in India. Cancer 1969, 24, 832–849. [Google Scholar] [CrossRef]
- Smith, L.W.; Bhargava, K.; Mani, N.J.; Silverman, S.; Malaowalla, A.M.; Billimoria, K.F. Oral cancer and precancerous lesions in 57,518 industrial workers in Gujarat, India. Indian J. Cancer. 1975, 12, 118–123. [Google Scholar]
- Silverman, S.J.; Bhargava, K.; Mani, N.J.; Smith, L.W.; Malaowalla, A.M. Malignant transformation and natural history of oral leukoplakia in 57,518 industrial workers of Gujarat, India. Cancer 1976, 38, 1790–1795. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Tail, Y.H.; Wang, W.C.; Chen, C.Y.; Kao, Y.H.; Chen, Y.K.; Chen, C.H. Malignant transformation in 5071 southern Taiwanese patients with potentially malignant oral mucosal disorders. BMC Oral Health 2014, 14, 99. [Google Scholar] [CrossRef]
- Dost, F.; Lê Cao, K.A.; Ford, P.J.; Farah, C.S. A retrospective analysis of clinical features of oral malignant and potentially malignant disorders with and without oral epithelial dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 725–733. [Google Scholar] [CrossRef]
- Bánóczy, J. Follow-up studies in oral leukoplakia. J. Maxillofac. Surg. 1977, 5, 69–75. [Google Scholar] [CrossRef]
- Schepman, K.P.; van der Meij, E.H.; Smeele, L.E.; van der Waal, I. Malignant transformation of oral leukoplakia: A follow-up study of a hospital-based population of 166 patients with oral leukoplakia in The Netherlands. Oral Oncol. 1998, 34, 270–275. [Google Scholar] [CrossRef]
- Dost, F.; Lê Cao, K.; Ford, P.J.; Ades, C.; Farah, C.S. Malignant transformation of oral epithelial dysplasia: A real-world evaluation of histopathologic grading. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reibel, J. Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Crit. Rev. Oral Biol. Med. 2003, 14, 47–62. [Google Scholar] [CrossRef]
- Chuang, S.L.; Su, W.W.; Chen, S.L.; Yen, A.M.; Wang, C.P.; Fann, J.C. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer 2017, 123, 1597–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzmann, E.J.; Donovan, M.J. Effective early detection of oral cancer using a simple and inexpensive point of care device in oral rinses. Expert Rev. Mol. Diagn. 2018, 18, 837–844. [Google Scholar] [CrossRef]
- Kao, S.Y.; Lim, E. An overview of detection and screening of oral cancer in Taiwan. Chin J. Dent. Res. 2015, 18, 7–12. [Google Scholar] [PubMed]
- Shiu, M.N.; Chen, T.H. Impact of betel quid, tobacco and alcohol on 3-stage disease natural history of oral leukoplakia and cancer: Implication for prevention of oral cancer. Eur. J. Cancer Prev. 2004, 13, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Ishii, T.; Iida, S.; Kawai, T. Epidemiological study of oral leukoplakia based on mass screening for oral mucosal diseases in a selected Japanese population. Community Dent. Oral Epidemiol. 1991, 19, 160–163. [Google Scholar] [CrossRef]
- Dietrich, T.; Reichart, P.A.; Scheifele, C. Clinical risk factors of oral leukoplakia in a representative sample of the US population. Oral Oncol. 2004, 40, 158–163. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Su, C.C.; Lin, Y.Y.; Chung, J.A.; Lian, B. Quantification of betel quid chewing and cigarette smoking in oral cancer patients. Community Dent. Oral Epidemiol. 2009, 37, 555–561. [Google Scholar] [PubMed]
- Kumar, S.; Narayanan, V.S.; Ananda, S.R.; Kavitha, A.P.; Krupashankar, R. Prevalence and Risk Factors for Oral Potentially Malignant Disorders in Indian Population. Adv. Prev. Med. 2015, 7, e208519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaw, K.-K.; Ohnmar, M.; Hlaing, M.-M.; Oo, Y.-T.-N.; Win, S.-S.; Htike, M.-M.-T.; Aye, P.-P.; Shwe, S.; Htwe, M.-T.; Thein, Z.-M. Betel Quid and Oral Potentially Malignant Disorders in a Periurban Township in Myanmar. PLoS ONE 2016, 11, e0162081. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ko, Y.C.; Huang, H.L.; Chao, Y.Y.; Tsai, C.C.; Shieh, T.Y.; Lin, L.M. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br. J. Cancer. 2003, 88, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Mehta, F.S.; Shroff, B.C.; Gupta, P.C.; Daftary, D.K. Oral leukoplakia in relation to tobacco habits. A ten-year follow-up study of Bombay policemen. Oral Surg. Oral Med. Oral Pathol. 1972, 34, 426–433. [Google Scholar]
- Pindborg, J.J.; Mehta, F.S.; Daftary, D.K. Incidence of oral cancer among 30,000 villagers in India in a 7-year follow-up study of oral precancerous lesions. Community Dent. Oral Epidemiol. 1975, 3, 86–88. [Google Scholar] [CrossRef]
- Aittiwarapoj, A.; Juengsomjit, R.; Kitkumthorn, N.; Lapthanasupkul, P. Oral Potentially Malignant Disorders and Squamous Cell Carcinoma at the Tongue: Clinicopathological Analysis in a Thai Population. Eur. J. Dent. 2019, 13, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Amarasinghe, H.K.; Usgodaarachchi, U.S.; Johnson, N.W.; Lalloo, R.; Warnakulasuriya, S. Betel-quid chewing with or without tobacco is a major risk factor for oral potentially malignant disorders in Sri Lanka: A case-control study. Oral Oncol. 2010, 46, 297–301. [Google Scholar] [CrossRef]
- Gupta, P.C. A study of dose-response relationship between tobacco habits and oral leukoplakia. Br. J. Cancer. 1984, 50, 527–531. [Google Scholar] [CrossRef]
- Baric, J.M.; Alman, J.E.; Feldman, R.S.; Chauncey, H.H. Influence of cigarette, pipe, and cigar smoking, removable partial dentures, and age on oral leukoplakia. Oral Surg. Oral Med. Oral Pathol. Oral Radio 1982, 54, 424–429. [Google Scholar] [CrossRef]
- Macigo, F.G.M.D.; Guthua, S.W. The association between oral leukoplakia and use of tobacco, alcohol and khat based on relative risks assessment in Kenya. Eur. J. Oral Sci. 1995, 103, 268–273. [Google Scholar] [CrossRef]
- Petersen, B.R. Effect on oral leukoplakia of reducing or ceasing tobacco smoking. Acta Derm.-Venereol. 1982, 62, 164–167. [Google Scholar]
- Gupta, P.; Pindborg, J.; Bhonsle, R.B.; Murti, P.R.; Mehta, F.; Aghi, M.B.; Daftary, D.K.; Shah, H.T.; Sinor, P.N. Intervention study for primary prevention of oral cancer among 36000 Indian tobacco users. Lancet 1986, 327, 1235–1239. [Google Scholar] [CrossRef]
- Morse, D.E.; Katz, R.V.; Pendrys, D.G.; Holford, T.R.; Krutchkoff, D.J.; Eisenberg, E.; Kosis, D.; Mayne, S.T. Smoking and drinking in relation to oral epithelial dysplasia. Cancer Epidemiol. Biomark. Prev. 1996, 5, 769–777. [Google Scholar]
- Kulasegaram, R.; Downer, M.C.; Jullien, J.A.; Zakrzewska, J.M.; Speight, P.M. Case-control study of oral dysplasia and risk habits among patients of a dental hospital. Eur. J. Cancer B Oral Oncol. 1995, 31, 227–231. [Google Scholar] [CrossRef]
- Hashibe, M.; Mathew, B.; Kuruvilla, B.; Thomas, G.; Sankaranarayanan, R.; Parkin, D.M.; Zhang, Z.F. Chewing Tobacco, Alcohol, and the Risk of Erythroplakia. Cancer Epidemiol. Biomark. Prev. 2000, 9, 639–645. [Google Scholar]
- Bile, K.M.; Shaikh, J.A.; Afridi, H.U.R.; Khan, Y. Smokeless tobacco use in Pakistan and its association with oropharyngeal cancer. East Mediterr. Health J. 2010, 16, 24–30. [Google Scholar] [CrossRef]
- Winn, D. Snuff dipping and oral cancer among women in the southern United States. N. Engl. J. Med. 1981, 304, 745–749. [Google Scholar] [CrossRef]
- Grady, D.; Greene, J.; Daniels, T.E.; Ernster, V.L.; Robertson, P.B.; Hauck, W.; Greenspan, D.; Greenspan, J.; Silverman, S. Oral mucosal lesions found in SLT users. J. Am. Dent. Assoc. 1990, 121, 117–123. [Google Scholar] [CrossRef]
- Martin, G.C.; Brown, J.P.; Eifler, C.W.; Houston, G.D. Oral Leukoplakia Status Six Weeks After Cessation of SLT Use. J. Am. Dent. Assoc. 1999, 130, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Johnson, N.W. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS ONE 2014, 9, e113385. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Psoter, W.J.; Buxó, C.J.; Elias, A.; Cuadrado, L.; Morse, D.E. Smoking and drinking in relation to oral potentially malignant disorders in Puerto Rico: A case-control study. BMC Cancer 2011, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Martin, S.P.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F. Smoking and Drinking in Relation to Oral and Pharyngeal Cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar]
- Hayes, R.B.; Bravo-Otero, E.; Kleinman, D.V.; Brown, L.M.; Fraumeni, J.F.; Harty, L.C.; Winn, D.M. Tobacco and Alcohol Use and Oral Cancer in Puerto Rico. Cancer Causes Control 1999, 10, 27–33. [Google Scholar] [CrossRef]
- Martinez, I. Factors associated with cancer of the esophagus, mouth, and pharynx in Puerto Rico. J. Natl. Cancer Inst. 1969, 42, 1069–1094. [Google Scholar]
- Choi, S.Y.; Kahyo, H. Effect of cigarette smoking and alcohol consumption in the aetiology of cancer of the oral cavity, pharynx and larynx. Int. J. Epidemiol. 1991, 20, 878–885. [Google Scholar] [CrossRef]
- Lubin, J.H.; Purdue, M.; Kelsey, K.; Zhang, Z.F.; Winn, D.; Wei, Q.; Talamini, R. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: A pooled analysis of case-control studies. Am. J. Epidemiol. 2009, 170, 937–947. [Google Scholar] [CrossRef]
- Wight, A.J.; Ogden, G.R. Possible mechanisms by which alcohol may influence the development of oral cancer—A review. Oral Oncol. 1998, 34, 441–447. [Google Scholar] [CrossRef]
- Mayne, S.T.; Morse, D.E.; Winn, D.M. Cancer of the oral cavity and pharynx. In Cancer Epidemiology & Prevention; Schottenfeld, D., Fraumeni, J.F., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 46–54. [Google Scholar]
- Petti, S.; Scully, C. Association between different alcoholic beverages and leukoplakia among non- to moderate-drinking adults: A matched case-control study. Eur. J. Cancer. 2006, 42, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Evstifeeva, T.V.; Zaridze, D.G. Nass use, cigarette smoking, alcohol consumption and risk of oral and oesophageal precancer. Eur. J. Cancer B Oral Oncol. 1992, 28, 29–35. [Google Scholar] [CrossRef]
- Nagao, T.; Warnakulasuriya, S.; Gelbier, S.; Yuasa, H.; Tsuboi, S.; Nakagaki, H. Oral pre-cancer and the associated risk factors among industrial workers in Japan’s overseas enterprises in the UK. J. Oral Maxillofac. Pathol. 2003, 32, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Sankaranarayanan, R.; Thomas, G.; Kuruvilla, B.; Mathew, B.; Somanathan, T.; Parkin, D.M.; Zhang, Z.F. Alcohol drinking, body mass index and the risk of oral leukoplakia in an Indian population. Int. J. Cancer 2000, 88, 129–134. [Google Scholar] [CrossRef]
- Fisher, M.A.; Bouquot, J.E.; Shelton, B.J. Assessment of risk factors for oral leukoplakia in West Virginia. Community Dent. Oral Epidemiol. 2005, 33, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.C. Epidemiologic study of the association between alcohol habits and oral leukoplakia. Community Dent. Oral Epidemiol. 1984, 12, 47–50. [Google Scholar] [CrossRef]
- Maserejian, N.N.; Joshipura, K.J.; Rosner, B.A.; Giovannucci, E.; Zavras, A.I. Prospective study of alcohol consumption and risk of oral premalignant lesions in men. Cancer Epidemiol. Biomark. Prev. 2006, 15, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loyha, K.; Vatanasapt, P.; Promthet, S.; Parkin, D.M. Risk factors for oral cancer in northeast Thailand. Asian Pac. J. Cancer Prev. 2012, 13, 5087–5090. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Cheng, A.J.; Lee, L.Y.; Huang, Y.C.; Chang, J.T. Multifaceted Mechanisms of Areca Nuts in Oral Carcinogenesis: The Molecular Pathology from Precancerous Condition to Malignant Transformation. J. Cancer 2019, 10, 4054–4062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Ko, A.M.S.; Yen, C.F.; Chu, K.S.; Gao, Y.J.; Warnakulasuriya, S.; Ibrahim, S.O.; Zain, R.B.; Patrick, W.K.; Ko, Y.C. Betel-quid dependence and oral potentially malignant disorders in six Asian countries. Br. J. Psychiatry 2012, 201, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.H.; Yang, Y.H.; Wang, T.Y.; Shieh, T.Y.; Warnakulasuriya, S. Oral precancerous disorders associated with areca quid chewing, smoking, and alcohol drinking in southern Taiwan. J. Oral Pathol. Med. 2005, 34, 460–466. [Google Scholar] [CrossRef]
- Lee, C.H.; Ko, A.M.S.; Warnakulasuriya, S.; Yin, B.L.; Zain, R.B.; Ibrahim, S.O.; Liu, Z.W.; Li, W.H.; Zhang, S.S.; Utomo, B.; et al. Intercountry prevalences and practices of betel-quid use in south, southeast and eastern Asia regions and associated oral preneoplastic disorders: An international collaborative study by Asian betel-quid consortium of south and east Asia. Int. J. Cancer. 2011, 129, 1741–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papke, R.L.; Bhattacharyya, I.; Hatsukami, D.K.; Moe, I.; Glatman, S. Betel Nut (areca) and Smokeless Tobacco Use in Myanmar. Subst. Use Misuse 2020, 55, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
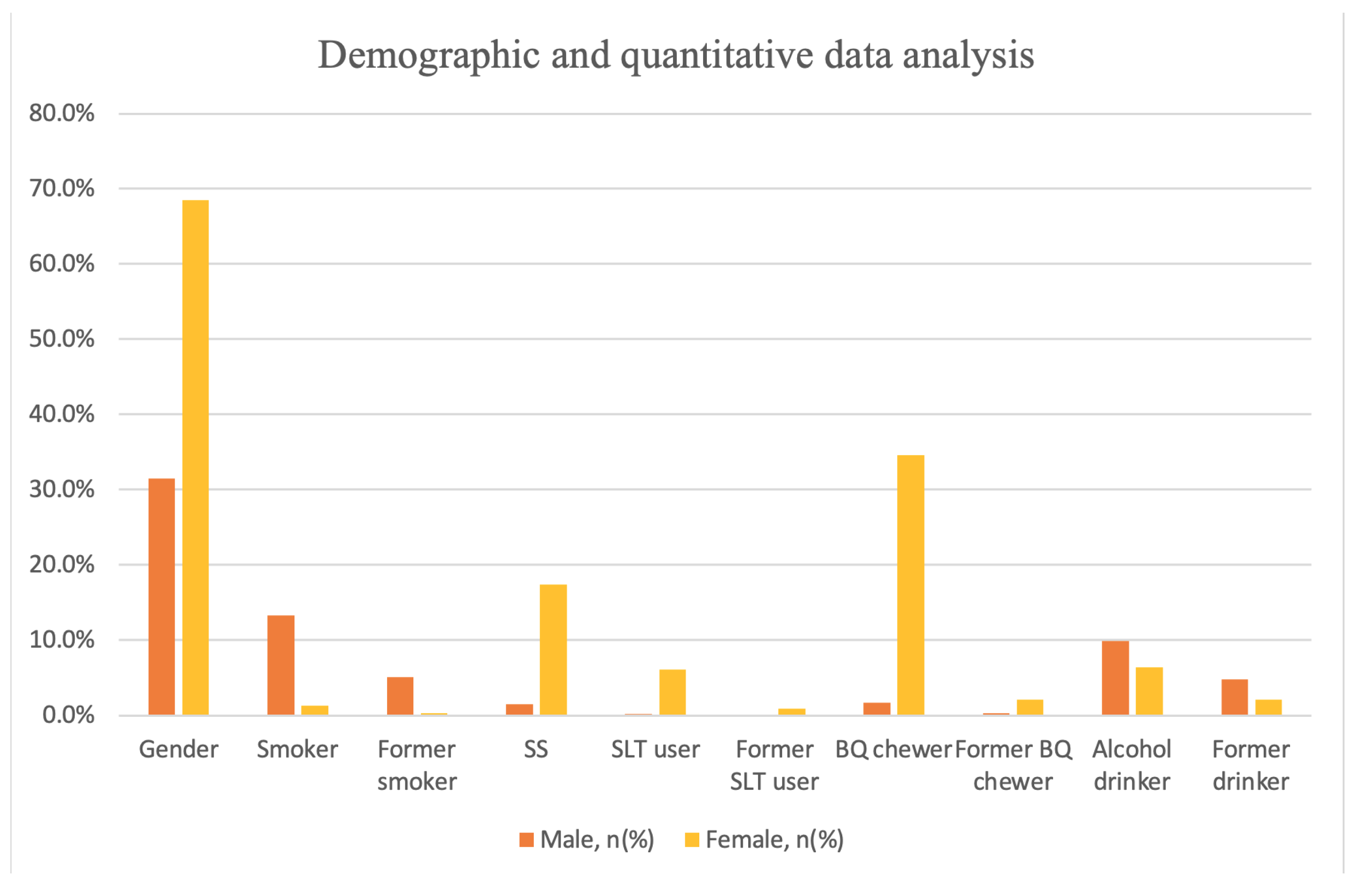

| Male, n% | Female, n% | Total | |
|---|---|---|---|
| Gender, n% | 456 (31.5) | 992 (68.5) | 1448 |
| Age (year), mean ± SD | 65.06 | 66.93 | 66.34 ± 10.15 |
| Tobacco, n% | |||
| Smoker | 192 (13.3) | 19 (1.3) | 211 (14.6) |
| Former smoker | 74 (5.1) | 5 (0.3) | 79 (5.5) |
| SS | 22 (1.5) | 252 (17.4) | 274 (18.9) |
| SLT, n% | |||
| User | 3 (0.2) | 89 (6.1) | 92 (6.4) |
| Former user | 2 (0.1) | 13 (0.9) | 15 (1.0) |
| BQ, n% | |||
| Chewer | 25 (1.7) | 501 (34.6) | 526 (36.3) |
| Former chewer | 4 (0.3) | 31 (2.1) | 35 (2.4) |
| Alcohol, n% | |||
| Drinker | 143 (9.9) | 93 (6.4) | 236 (16.3) |
| Former drinker | 69 (4.8) | 30 (2.1) | 99 (6.8) |
| Categories | Odds Ratio | (95% CI) | p-Value |
|---|---|---|---|
| Gender | |||
| Male | 1.00 | ||
| Female | 2.17 | 1.65, 2.85 | <0.001 * |
| Age | |||
| <60 years | 1.00 | ||
| ≥60 years | 1.79 | 1.38, 2.32 | <0.001 * |
| Risk Factors | |||
| Number of exposed risk factor(s) | |||
| None | 1.00 | ||
| 1 risk factor | 3.04 | 2.31, 4.00 | <0.001 * |
| 2 risk factors | 5.40 | 3.91, 7.44 | <0.001 * |
| 3 risk factors | 24.82 | 10.16, 60.61 | <0.001 * |
| Risk Factors | Odds Ratio | (95% CI) | p-Value |
|---|---|---|---|
| Tobacco | |||
| Current | 0.89 | 0.66, 1.21 | 0.467 |
| Timing of tobacco smoking | |||
| <30 years | 1.00 | ||
| ≥30 years | 1.41 | 0.78, 2.53 | 0.258 |
| Smoking frequency per week | |||
| <35 cigarettes | 1.00 | ||
| ≥35 cigarettes | 1.27 | 0.72, 2.25 | 0.406 |
| Former | 0.26 | 0.14, 0.49 | <0.001 * |
| Period of quit smoking | |||
| <10 years | 1.00 | ||
| ≥10 years | 0.41 | 0.11, 1.51 | 0.179 |
| Period of past smoking | |||
| <20 years | 1.00 | ||
| ≥20 years | 0.56 | 0.16, 2.03 | 0.380 |
| SS | 0.99 | 0.76, 1.30 | 0.962 |
| SLT | |||
| Current | 3.98 | 2.52, 6.30 | < 0.001 * |
| Timing of using | |||
| <25 years | 1.00 | ||
| ≥25 years | 1.07 | 0.44, 2.60 | 0.887 |
| Former | 2.61 | 0.93, 7.39 | 0.070 |
| Period of quit using | |||
| <10 years | 1.00 | ||
| ≥10 years | 0.40 | 0.05, 3.42 | 0.403 |
| Period of past using | |||
| <20 years | 1.00 | ||
| ≥20 years | 0.63 | 0.07, 5.35 | 0.668 |
| BQ | |||
| Current | 6.91 | 5.43, 8.79 | < 0.001 * |
| Timing of chewing | |||
| <30 years | 1.00 | ||
| ≥30 years | 1.88 | 1.31, 2.72 | 0.001 * |
| Former | 6.89 | 3.37, 14.10 | <0.001 * |
| Period of quit chewing | |||
| <5 years | 1.00 | ||
| ≥5 years | 0.94 | 0.22, 3.92 | 0.930 |
| Period of past chewing | |||
| <20 years | 1.00 | ||
| ≥20 years | 1.16 | 0.27, 4.93 | 0.840 |
| Alcohol | |||
| Current | 1.48 | 1.12, 1.96 | 0.007 * |
| Timing of drinking | |||
| <20 years | 1.00 | ||
| ≥20 years | 0.82 | 0.48, 1.38 | 0.452 |
| Drinking frequency per week | |||
| <5 times | 1.00 | ||
| ≥5 times | 1.19 | 0.70, 2.03 | 0.518 |
| Former | 1.10 | 0.72, 1.68 | 0.652 |
| Period of quit drinking | |||
| <10 years | 1.00 | ||
| ≥10 years | 1.67 | 0.76, 3.68 | 0.206 |
| Period of past drinking | |||
| <10 years | 1.00 | ||
| ≥10 years | 0.76 | 0.33, 1.78 | 0.529 |
| Adjusted Odds Ratio | (95% CI) | p-Value | |
|---|---|---|---|
| Gender | |||
| Male | 1.00 | ||
| Female | 1.64 | 1.10, 2.43 | 0.015 * |
| BQ | |||
| Chewer/former chewer | 4.65 | 3.29, 6.58 | <0.001 * |
| Non-chewer | 1.00 | ||
| Alcohol | |||
| Drinker/former drinker | 3.40 | 2.23, 5.18 | <0.001 * |
| Non-drinker | 1.00 | ||
| Risk Factors | PMD (N = 562) | No PMD (N = 886) | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| 1 risk factor | |||||
| Tobacco | 80 (14.2) | 131 (14.8) | 0.89 | 0.66, 1.21 | 0.467 |
| SLT | 64 (11.4) | 28 (3.2) | 3.98 | 2.52, 6.30 | <0.001 * |
| BQ | 346 (61.6) | 180 (20.3) | 6.91 | 5.46, 8.75 | <0.001 * |
| Alcohol | 110 (19.6) | 126 (14.2) | 1.48 | 1.12, 1.96 | 0.007 * |
| 2 risk factors | |||||
| Tobacco + SLT | 0 (0.0) | 0 (0.0) | NA | ||
| Tobacco + BQ | 9 (1.6) | 5 (0.6) | 2.87 | 0.96, 8.60 | 0.060 |
| Tobacco + Alcohol | 37 (6.6) | 51 (5.8) | 1.15 | 0.75, 1.79 | 0.521 |
| Tobacco + SS | 0 (0.0) | 0 (0.0) | NA | ||
| SLT + BQ | 57 (10.1) | 21 (2.4) | 4.65 | 2.79, 7.76 | <0.001 * |
| SLT + Alcohol | 17 (3.0) | 2 (0.2) | 13.79 | 3.17, 59.91 | <0.001 * |
| SLT + SS | 0 (0.0) | 2 (0.2) | NA | ||
| BQ + Alcohol | 59 (10.5) | 11 (1.2) | 9.33 | 4.86, 17.93 | <0.001 * |
| BQ + SS | 77 (13.7) | 45 (5.1) | 2.97 | 2.02, 4.36 | <0.001 * |
| Alcohol + SS | 19 (3.4) | 9 (1.0) | 3.41 | 1.53, 7.59 | 0.003 * |
| 3 risk factors | |||||
| Tobacco + BQ + Alcohol | 5 (0.9) | 0 (0.0) | NA | ||
| SLT + BQ + Alcohol | 13 (2.3) | 1 (0.1) | 20.96 | 2.73, 160.64 | 0.003 * |
| BQ + Alcohol + SS | 18 (3.2) | 4 (0.5) | 7.30 | 2.46, 21.67 | <0.001 * |
| CP (%) (95% CI) | Duration of Exposure | |||
|---|---|---|---|---|
| 10 Years | 30 Years | 50 Years | Median (95% CI) | |
| Tobacco | 7.5 (3.8, 11.2) | 28.2 (21.1, 35.3) | 57.9 (47.9, 67.9) | 50 (48.49, 51.51) |
| SLT | 16.1 (6.1, 26.1) | 50.6 (39.2, 62.0) | 82.9 (72.7, 93.1) | 30 (23.62, 36.38) |
| BQ | 12.3 (9.4, 15.2) | 44.7 (40.0, 49.4) | 83.6 (79.5, 87.7) | 40 (37.90, 42.09) |
| Alcohol | 20.3 (14.8, 25.8) | 49.8 (42.0, 57.6) | 78.5 (68.7, 88.3) | 36 (30.49, 41.52) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worakhajit, P.; Fuangtharnthip, P.; Khovidhunkit, S.-o.P.; Chiewwit, P.; Klongnoi, B. The Relationship of Tobacco, Alcohol, and Betel Quid with the Formation of Oral Potentially Malignant Disorders: A Community-Based Study from Northeastern Thailand. Int. J. Environ. Res. Public Health 2021, 18, 8738. https://doi.org/10.3390/ijerph18168738
Worakhajit P, Fuangtharnthip P, Khovidhunkit S-oP, Chiewwit P, Klongnoi B. The Relationship of Tobacco, Alcohol, and Betel Quid with the Formation of Oral Potentially Malignant Disorders: A Community-Based Study from Northeastern Thailand. International Journal of Environmental Research and Public Health. 2021; 18(16):8738. https://doi.org/10.3390/ijerph18168738
Chicago/Turabian StyleWorakhajit, Prangtip, Pornpoj Fuangtharnthip, Siribang-on Piboonniyom Khovidhunkit, Pim Chiewwit, and Boworn Klongnoi. 2021. "The Relationship of Tobacco, Alcohol, and Betel Quid with the Formation of Oral Potentially Malignant Disorders: A Community-Based Study from Northeastern Thailand" International Journal of Environmental Research and Public Health 18, no. 16: 8738. https://doi.org/10.3390/ijerph18168738
APA StyleWorakhajit, P., Fuangtharnthip, P., Khovidhunkit, S.-o. P., Chiewwit, P., & Klongnoi, B. (2021). The Relationship of Tobacco, Alcohol, and Betel Quid with the Formation of Oral Potentially Malignant Disorders: A Community-Based Study from Northeastern Thailand. International Journal of Environmental Research and Public Health, 18(16), 8738. https://doi.org/10.3390/ijerph18168738






