Risk Characterization and Benefit–Risk Assessment of Brominated Flame Retardant in Commercially Exploited Freshwater Fishes and Crayfish of Lake Trasimeno, Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemical Analysis
2.3. Dietary Exposure Assessment
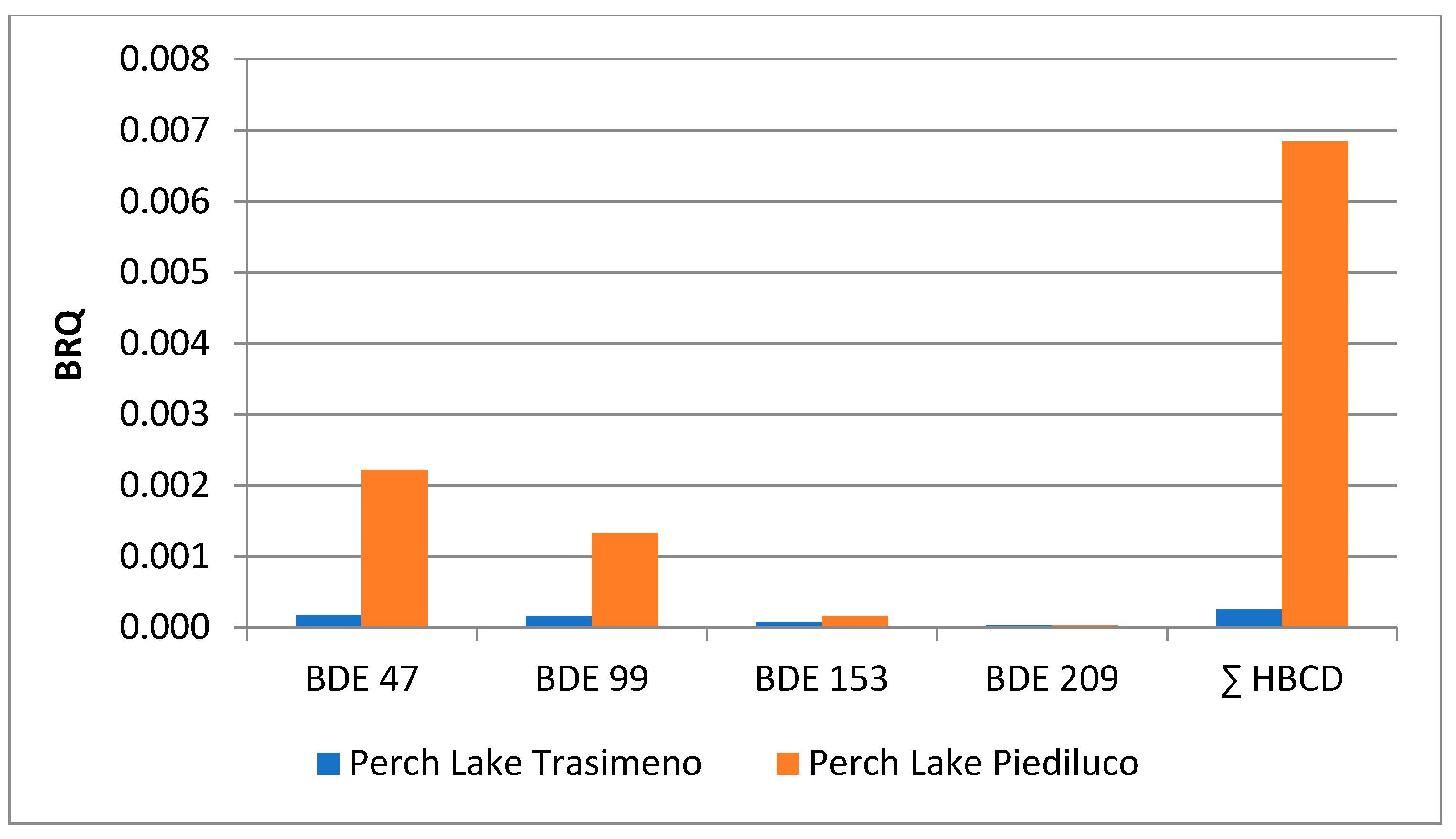
2.4. Risk Characterization
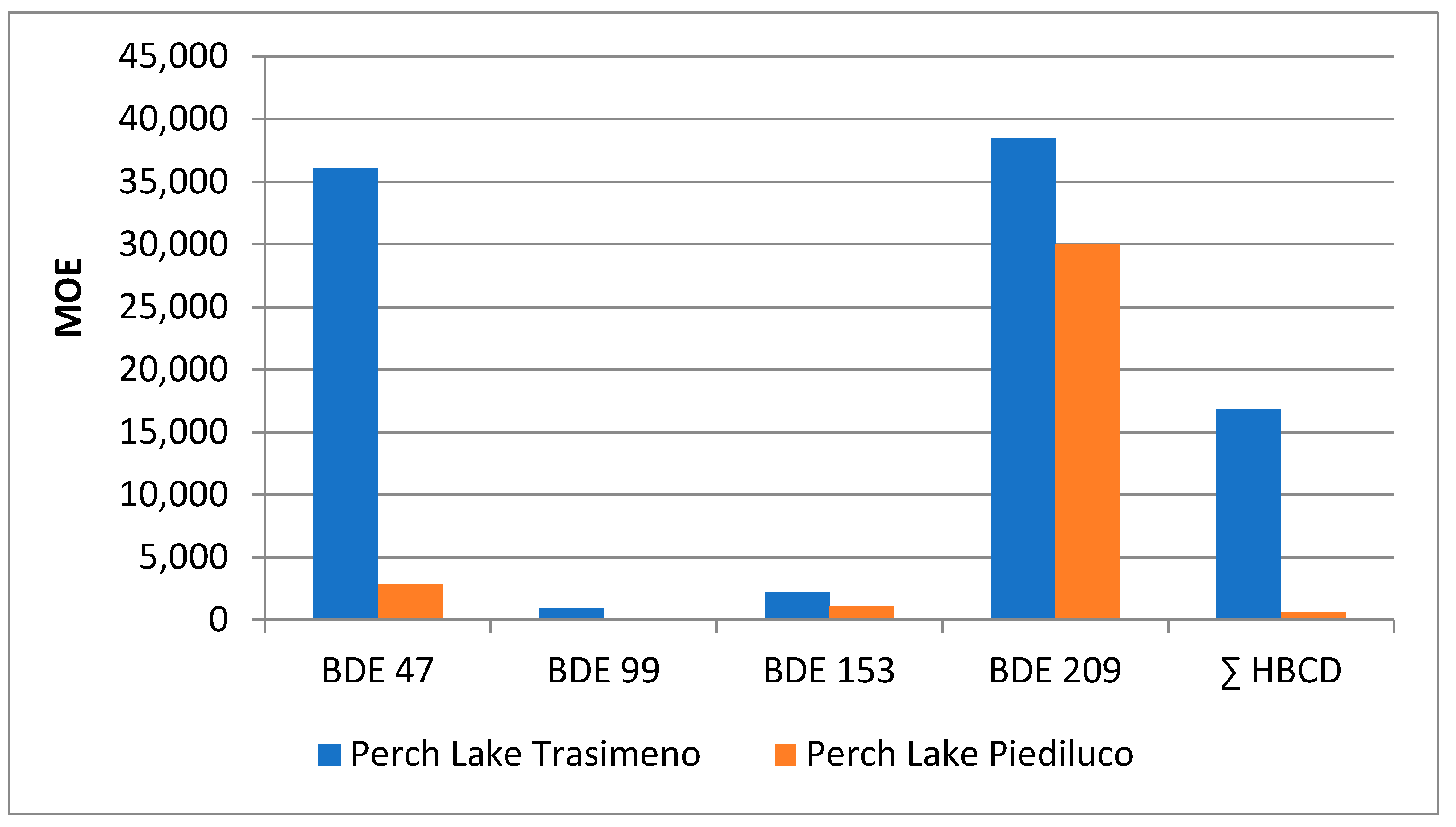
2.5. Benefit–Risk Assessment
2.6. Comparative Assessment of Different Contamination Patterns
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuiderveen, E.A.R.; Slootweg, J.C.; de Boer, J. Novel brominated flame retardants—A review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere 2020, 255, 126816. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA J. 2011, 9, 2156. [Google Scholar] [CrossRef]
- Tavoloni, T.; Stramenga, A.; Stecconi, T.; Siracusa, M.; Bacchiocchi, S.; Piersanti, A. Single sample preparation for brominated flame retardants in fish and shellfish with dual detection: GC-MS/MS (PBDEs) and LC-MS/MS (HBCDs). Anal. Bioanal. Chem. 2019, 412, 397–411. [Google Scholar] [CrossRef]
- Weidemann, E.; Andersson, P.L.; Bidleman, T.; Boman, C.; Carlin, D.J.; Collina, E.M.; Cormier, S.A.; Gouveia-Figueira, S.C.; Gullett, B.K.; Johansson, C.; et al. 14th congress of combustion by-products and their health effects—origin, fate, and health effects of combustion-related air pollutants in the coming era of bio-based energy sources. Environ. Sci. Pollut. Res. 2016, 23, 8141–8159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Directive 2003/11/ECDirective 2003/11/EC of the European Parliament and of the Council of 6 February 2003 Amending for the 24th Time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Pentabromodiphenyl Ether, Octabromodiphenyl Ether). OJEU 2003, 42, 45–46. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003L0011:EN:HTML (accessed on 27 May 2021).
- European Court of Justice Joined Cases C-14/06 and C-295/06 Addressing Directive 2002/95/EC and Commission Decision 2005/717/EC—Exemption of decaBDE from the Prohibition on Use—Actions for Annulment. Available online: https://curia.europa.eu/jcms/jcms/j_6/en/ (accessed on 25 June 2021).
- UNEP. Listing of Decabromodiphenyl Ether, Decis. SC-8/10. Available online: http://chm.pops.int/Convention/ConferenceofthePartiesCOP/COPDecisions/tabid/208/Default.aspx (accessed on 25 May 2021).
- UNEP. Listing of Tetrabromodiphenyl Ether and Pentabromodiphenyl Ether, Decis.SC-4/18. Available online: http://chm.pops.int/Convention/ConferenceofthePartiesCOP/COPDecisions/tabid/208/Default.aspx (accessed on 25 May 2021).
- UNEP. Listing of Hexabromodiphenyl Ether and Heptabromodiphenyl Ether, Decis.SC-4/14. Available online: http://chm.pops.int/Convention/ConferenceofthePartiesCOP/COPDecisions/tabid/208/Default.aspx (accessed on 25 May 2021).
- Poma, G.; Volta, P.; Roscioli, C.; Bettinetti, R.; Guzzella, L. Concentrations and trophic interactions of novel brominated flame retardants, HBCD, and PBDEs in zooplankton and fish from Lake Maggiore (Northern Italy). Sci. Total Environ. 2014, 481, 401–408. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM). Update of the risk assess-ment of hexabromocyclododecanes (HBCDD s) in food. EFSA J. 2021, 19, 06421. [Google Scholar]
- D’Silva, K.; Fernandes, A.; Rose, M. Brominated Organic Micropollutants—Igniting the Flame Retardant Issue. Crit. Rev. Environ. Sci. Technol. 2004, 34, 141–207. [Google Scholar] [CrossRef]
- European Union Risk Assessment Draft Report for Hexabromocyclododecane, European Chemicals Bureau. Available online: https://echa.europa.eu/documents/10162/661bff17-dc0a-4475-9758-40bdd6198f82 (accessed on 24 August 2020).
- UNEP. Listing of Hexabromocyclododecane, Decis. SC-6/13. Available online: http://chm.pops.int/Convention/ConferenceofthePartiesCOP/COPDecisions/tabid/208/Default.aspx (accessed on 25 May 2021).
- Shi, Z.; Zhang, L.; Li, J.; Wu, Y. Legacy and emerging brominated flame retardants in China: A review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere 2018, 198, 522–536. [Google Scholar] [CrossRef]
- Zacs, D.; Perkons, I.; Abdulajeva, E.; Pasecnaja, E.; Bartkiene, E.; Bartkevics, V. Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecanes (HBCDD), dechlorane-related compounds (DRCs), and emerging brominated flame retardants (EBFRs) in foods: The levels, profiles, and dietary intake in Latvia. Sci. Total Environ. 2021, 752, 141996. [Google Scholar] [CrossRef]
- Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef]
- Covaci, A.; Harrad, S.; Abdallah, M.A.-E.; Ali, N.; Law, R.J.; Herzke, D.; de Wit, C. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int. 2011, 37, 532–556. [Google Scholar] [CrossRef]
- Domingo, J.L. Polybrominated diphenyl ethers in food and human dietary exposure: A review of the recent scientific literature. Food Chem. Toxicol. 2012, 50, 238–249. [Google Scholar] [CrossRef]
- Commission Recommendation 2014/118/EU of the European Commission on the Monitoring of Traces of Brominated Flame Retardants in Food, 2014 OJEU 2014, L 65/39. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32014H0118 (accessed on 28 May 2021).
- Branciari, R.; Franceschini, R.; Roila, R.; Valiani, A.; Pecorelli, I.; Piersanti, A.; Haouet, N.; Framboas, M.; Ranucci, D. Nutri-tional value and contaminant risk assessment of some commercially important fishes and crawfish of Lake Trasimeno, Italy. Int. J. Environ. Res. Pub Health 2020, 17, 2545. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EU) EC Regulation 2017/Laying down Methods of Sampling and Analysis for the Control of Levels of Dioxins, Dioxin-Like PCBs and Non-Dioxin-Like PCBs in Certain Foodstuffs and Repealing Regulation (EU) No 589/2014 OJEU L 92 (2017/644), 9–34 of 5 April 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0644 (accessed on 25 May 2021).
- European Union Reference Laboratory for Halogenated POPs in Feed and Food. Available online: http://www.crl-freiburg.eu/dioxin/foodfeed.html (accessed on 10 March 2021).
- EFSA (European Food Safety Authority). Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1557. [Google Scholar] [CrossRef] [Green Version]
- Roila, R.; Branciari, R.; Pecorelli, I.; Cristofani, E.; Carloni, C.; Ranucci, D.; Fioroni, L. Occurrence and Residue Concentration of Coccidiostats in Feed and Food of Animal Origin; Human Exposure Assessment. Foods 2019, 8, 477. [Google Scholar] [CrossRef] [Green Version]
- Branciari, R.; Ranucci, D.; Urbani, E.; Valiani, A.; Trabalza-Marinucci, M.; Bosco, A.D.; Franceschini, R. Freshwater Fish Burgers Made from Four Different Fish Species as a Valuable Strategy Appreciated by Consumers for Introducing EPA and DHA into a Human Diet. J. Aquat. Food Prod. Technol. 2017, 26, 686–694. [Google Scholar] [CrossRef]
- SINU-Società Italiana di Nutrizione Umana. Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana IV Revisione (LARN); SICS: Milan, Italy, 2014; Available online: https://sinu.it/tabelle-larn-2014/ (accessed on 17 April 2021).
- Altissimi, M.S.; Roila, R.; Branciari, R.; Miraglia, D.; Ranucci, D.; Framboas, M.; Haouet, N. Contribution of street food on dietary acrylamide exposure by youth aged nineteen to thirty in Perugia, Italy. Ital. J. Food Saf. 2017, 6, 6881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branciari, R.; Roila, R.; Ranucci, D.; Altissimi, M.S.; Mercuri, M.L.; Haouet, N.M. Estimation of acrylamide exposure in Italian schoolchildren consuming a canteen menu: Health concern in three age groups. Int. J. Food Sci. Nutr. 2019, 71, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Tijhuis, M.; de Jong, N.; Pohjola, M.; Gunnlaugsdóttir, H.; Hendriksen, M.; Hoekstra, J.; Holm, F.; Kalogeras, N.; Leino, O.; van Leeuwen, F.; et al. State of the art in benefit–risk analysis: Food and nutrition. Food Chem. Toxicol. 2012, 50, 5–25. [Google Scholar] [CrossRef]
- EFSA(European Food Safety Authority). Scientific Opinion on the Tolerable Upper Intake Level of Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA) and Docosapentaenoic Acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar]
- Barchiesi, F.; Branciari, R.; Latini, M.; Roila, R.; Lediani, G.; Filippini, G.; Scortichini, G.; Piersanti, A.; Rocchegiani, E.; Ranucci, D. Heavy Metals Contamination in Shellfish: Benefit-Risk Evaluation in Central Italy. Foods 2020, 9, 1720. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.-J.; Li, H.; Liu, J.-P.; Yang, Y.; Jin, Z.-L.; Zhang, Y.-N.; Zhang, M.-L.; Chen, L.-Q.; Du, Z.-Y. Nutrients and contaminants in tissues of five fish species obtained from Shanghai markets: Risk–benefit evaluation from human health perspectives. Sci. Total Environ. 2015, 536, 933–945. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency IRIS, U., 2008. 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) (CASRN 5436-43-1), in: Washington, D.U.E.P.A. Available online: https://cfpub.epa.gov/ncea/iris2/chemicallanding.cfm?substance_nmbr=1010 (accessed on 25 June 2021).
- United States Environmental Protection Agency IRIS, U., 2008. 2,2′,4,4′,5-Pentabromodiphenyl Ether (BDE-99) (CASRN 60348-60-9), in: Washington, D.U.E.P.A. Available online: https://cfpub.epa.gov/ncea/iris2/chemicallanding.cfm?substance_nmbr=1008 (accessed on 25 June 2021).
- United States Environmental Protection Agency IRIS, U., 2008. 2,2′,4,4′,5,5′-Hexabromodiphenyl Ether (BDE-153) (CASRN 68631-49-2), in: Washington, D.U.E.P.A. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=1009 (accessed on 25 June 2021).
- United States Environmental Protection Agency IRIS, U., 2008. 2,2′,3,3′,4,4′,5,5′,6,6′-Decabromodiphenyl Ether (BDE-209) (CASRN 1163-19-5), in: Washington, D.U.E.P.A. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=35 (accessed on 25 June 2021).
- Besis, A.; Christia, C.; Poma, G.; Covaci, A.; Samara, C. Legacy and novel brominated flame retardants in interior car dust—Implications for human exposure. Environ. Pollut. 2017, 230, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Malarvannan, G.; Belpaire, C.; Geeraerts, C.; Eulaers, I.; Neels, H.; Covaci, A. Assessment of persistent brominated and chlorinated organic contaminants in the European eel (Anguilla anguilla) in Flanders, Belgium: Levels, profiles and health risk. Sci. Total Environ. 2014, 482–483, 222–233. [Google Scholar] [CrossRef]
- Law, R.J.; Herzke, D.; Harrad, S.; Morris, S.; Bersuder, P.; Allchin, C.R. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere 2008, 73, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Bragigand, V.; Amiard-Triquet, C.; Parlier, E.; Boury, P.; Marchand, P.; El Hourch, M. Influence of biological and ecological factors on the bioaccumulation of polybrominated diphenyl ethers in aquatic food webs from French estuaries. Sci. Total Environ. 2006, 368, 615–626. [Google Scholar] [CrossRef]
- Van Leeuwen, S.P.J.; De Boer, J. Brominated flame retardants in fish and shellfish—levels and contribution of fish consumption to dietary exposure of Dutch citizens to HBCD. Mol. Nutr. Food Res. 2008, 52, 194–203. [Google Scholar] [CrossRef]
- Roosens, L.; Geeraerts, C.; Belpaire, C.; Van Pelt, I.; Neels, H.; Covaci, A. Spatial variations in the levels and isomeric patterns of PBDEs and HBCDs in the European eel in Flanders. Environ. Int. 2010, 36, 415–423. [Google Scholar] [CrossRef]
- Freese, M.; Sühring, R.; Pohlmann, J.-D.; Wolschke, H.; Magath, V.; Ebinghaus, R.; Hanel, R. A question of origin: Dioxin-like PCBs and their relevance in stock management of European eels. Ecotoxicology 2015, 25, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Sühring, R.; Freese, M.; Schneider, M.; Schubert, S.; Pohlmann, J.-D.; Alaee, M.; Wolschke, H.; Hanel, R.; Ebinghaus, R.; Marohn, L. Maternal transfer of emerging brominated and chlorinated flame retardants in European eels. Sci. Total Environ. 2015, 530–531, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Saunders, D.; Yu, Y.; Yu, H.; Zhang, X.; Liu, H.; Giesy, J.P. Occurrence of additive brominated flame retardants in aquatic organisms from Tai Lake and Yangtze River in Eastern China, 2009–2012. Chemosphere 2014, 114, 340–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.-P.; Luo, X.; Sun, Y.; Mo, L.; Chen, S.-J.; Mai, B.-X. Biota-sediment accumulation factors for Dechlorane Plus in bottom fish from an electronic waste recycling site, South China. Environ. Int. 2011, 37, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Covaci, A.; Gheorghe, A.; Hulea, O.; Schepens, P. Levels and distribution of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in sediments and biota from the Danube Delta, Romania. Environ. Pollut. 2006, 140, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Abdallah, M.A.-E.; Rose, N.L.; Turner, S.; Davidson, T. Current-Use Brominated Flame Retardants in Water, Sediment, and Fish from English Lakes. Environ. Sci. Technol. 2009, 43, 9077–9083. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, A.; Rüdel, H.; Lohmann, N.; Buchmeier, G.; Koschorreck, J. Biota monitoring under the Water Framework Directive: On tissue choice and fish species selection. Environ. Pollut. 2018, 235, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Svihlikova, V.; Lankova, D.; Poustka, J.; Tomaniova, M.; Hajslova, J.; Pulkrabova, J. Perfluoroalkyl substances (PFASs) and other halogenated compounds in fish from the upper Labe River basin. Chemosphere 2015, 129, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Strid, A.; Bignert, A.; Zhu, Z.; Zhao, J.; Athanasiadou, M.; Athanassiadis, I.; Bergman, Å. Chlorinated and brominated organic contaminants in fish from Shanghai markets: A case study of human exposure. Chemosphere 2012, 89, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Hajšlová, J.; Pulkrabova, J.; Poustka, J.; Cajka, T.; Randák, T. Brominated flame retardants and related chlorinated persistent organic pollutants in fish from river Elbe and its main tributary Vltava. Chemosphere 2007, 69, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Haglund, P.S.; Zook, D.S.; Buser, H.R.; Hu, J. Identification and quantification of polybrominatediphenyl ethers and meth-oxy-polybrominated diphenyl ethers in Baltic biota. Environ. Sci. Technol. 1997, 31, 3281–3287. [Google Scholar] [CrossRef]
- Meng, X.-Z.; Xiang, N.; Duan, Y.-P.; Chen, L.; Zeng, E.Y. Hexabromocyclododecane in consumer fish from South China: Implications for human exposure via dietary intake. Environ. Toxicol. Chem. 2012, 31, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- de Wit, C. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Burreau, S.; Broman, D.; Orn, U. Tissue distribution of 2,2′,4,4′-tetrabromo[C-14] diphenyl ether ([14C]-PBDE 47) in pike (Esox lucius) after dietary exposure—A time series study using whole body autoradiography. Chemosphere 2000, 40, 977–985. [Google Scholar] [CrossRef]
- Ashley, J.T.F.; Libero, D.; Halscheid, E.; Zaoudeh, L.; Stapleton, H.M. Polybrominated Diphenyl Ethers in American Eels (Anguilla rostrata) from the Delaware River, USA. Bull. Environ. Contam. Toxicol. 2007, 79, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Letcher, R.J.; Li, J.; Baker, J.E. Dietary Accumulation And Metabolism Of Polybrominated Diphenyl Ethers By Juvenile Carp (Cyprinus Carpio). Environ. Toxicol. Chem. 2004, 23, 1939–1946. [Google Scholar] [CrossRef]
- Barghi, M.; Shin, E.-S.; Son, M.-H.; Choi, S.-D.; Pyo, H.; Chang, Y.-S. Hexabromocyclododecane (HBCD) in the Korean food basket and estimation of dietary exposure. Environ. Pollut. 2016, 213, 268–277. [Google Scholar] [CrossRef]
- Fernandes, A.; Mortimer, D.; Rose, M.; Smith, F.; Panton, S.; Garcia-Lopez, M. Bromine content and brominated flame retardants in food and animal feed from the UK. Chemosphere 2016, 150, 472–478. [Google Scholar] [CrossRef]
- Du, M.; Lin, L.; Yan, C.; Zhang, X. Diastereoisomer- and Enantiomer-Specific Accumulation, Depuration, and Bioisomerization of Hexabromocyclododecanes in Zebrafish (Danio rerio). Environ. Sci. Technol. 2012, 46, 11040–11046. [Google Scholar] [CrossRef]
- Szabo, D.T.; Pathmasiri, W.; Sumner, S.; Birnbaum, L.S. Serum Metabolomic Profiles in Neonatal Mice following Oral Brominated Flame Retardant Exposures to Hexabromocyclododecane (HBCD) Alpha, Gamma, and Commercial Mixture. Environ. Health Perspect. 2017, 125, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Zegers, B.N.; Mets, A.; Van Bommel, R.; Minkenberg, C.; Hamers, T.; Kamstra, J.; Pierce, G.; Boon, J.P. Levels of Hexabromocyclododecane in Harbor Porpoises and Common Dolphins from Western European Seas, with Evidence for Stereoisomer-Specific Biotransformation by Cytochrome P450. Environ. Sci. Technol. 2005, 39, 2095–2100. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.; Allchin, C.R.; Zegers, B.N.; Haftka, J.J.H.; Boon, J.P.; Belpaire, C.; Leonards, P.E.G.; van Leeuwen, S.; de Boer, J. Distribution and Fate of HBCD and TBBPA Brominated Flame Retardants in North Sea Estuaries and Aquatic Food Webs. Environ. Sci. Technol. 2004, 38, 5497–5504. [Google Scholar] [CrossRef]
- Trabalón, L.; Vilavert, L.; Domingo, J.L.; Pocurull, E.; Borrull, F.; Nadal, M. Human exposure to brominated flame retardants through the consumption of fish and shellfish in Tarragona County (Catalonia, Spain). Food Chem. Toxicol. 2017, 104, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Becher, G.; Hilger, B.; Völkel, W. Brominated flame retardants—Exposure and risk assessment for the general population. Int. J. Hyg. Environ. Health 2016, 219, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tay, J.-H.; Covaci, A.; Sánchez, J.A.P.; Papadopoulou, E.; Haug, L.S.; Neels, H.; Sellström, U.; De Wit, C.A. Assessment of dietary exposure to organohalogen contaminants, legacy and emerging flame retardants in a Norwegian cohort. Environ. Int. 2017, 102, 236–243. [Google Scholar] [CrossRef]
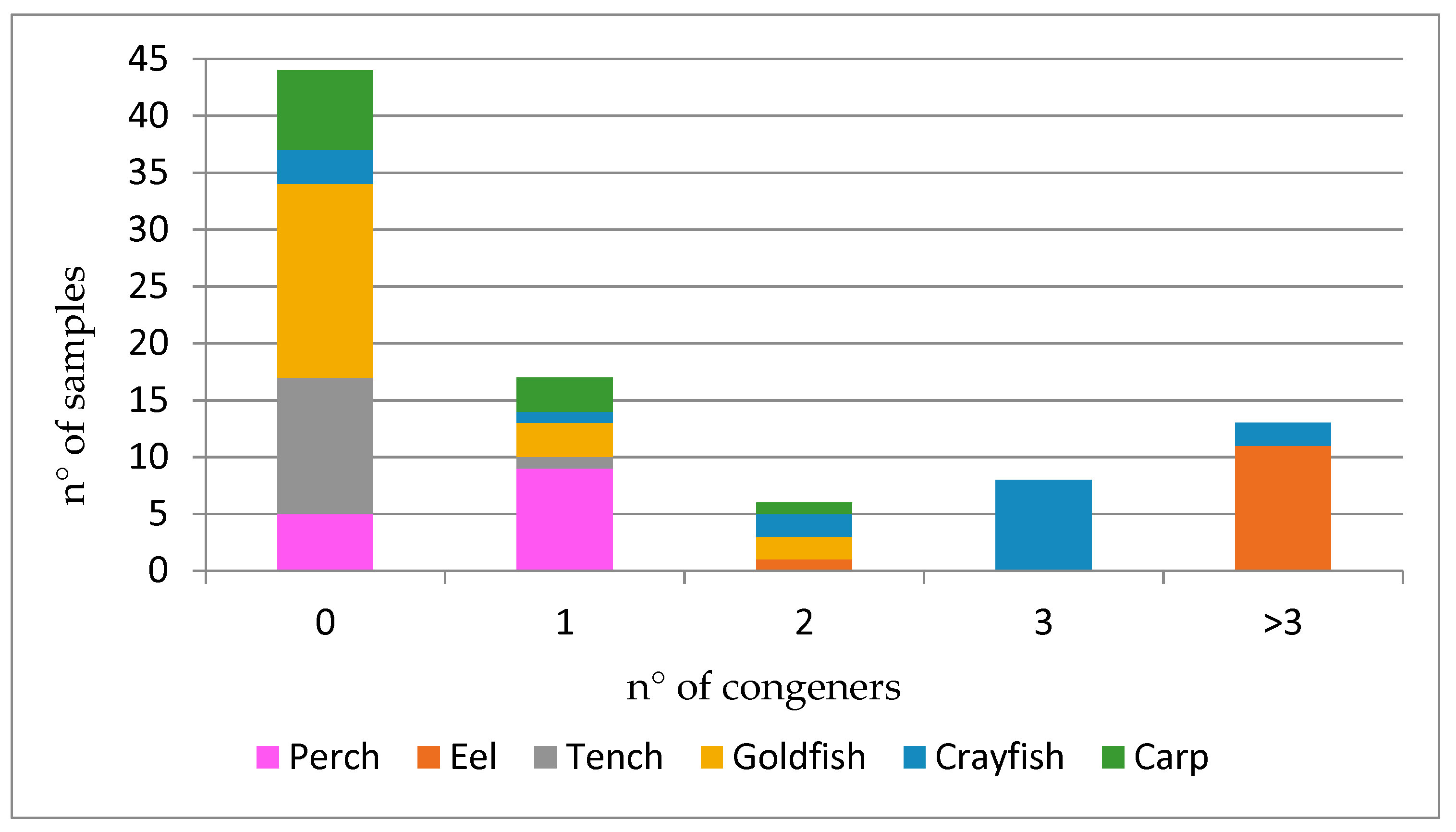
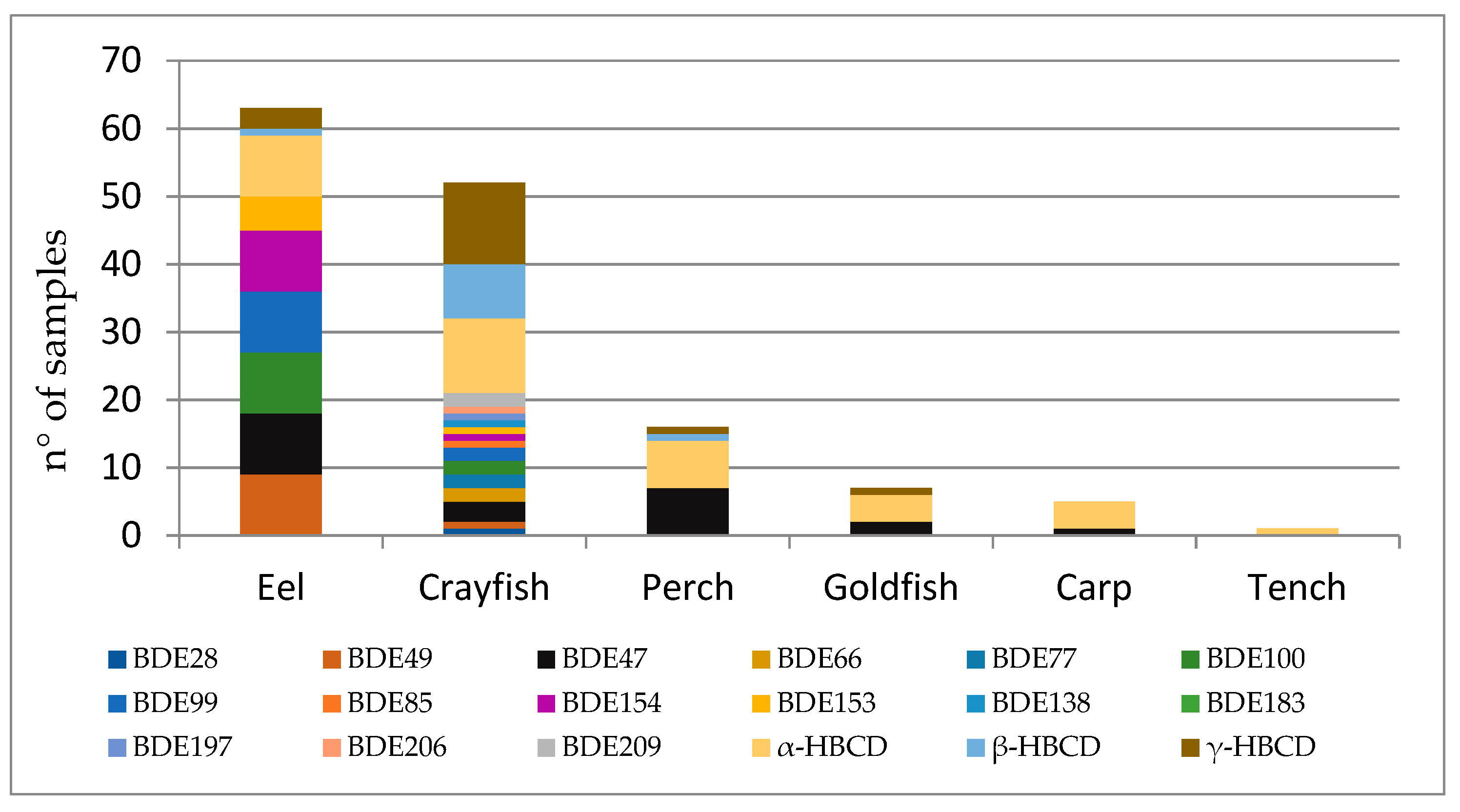
| Perch (n = 16) | Eel (n = 12) | Tench (n = 13) | Goldfish (n = 22) | Crayfish (n = 16) | Carp (n = 11) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | |
| BDE-28 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.71 | 10.09 | 0.00 | 10.00 |
| BDE-49 | 0.00 | 10.00 | 27.68 | 29.34 | 0.00 | 10.00 | 0.00 | 10.00 | 0.66 | 10.03 | 0.00 | 10.00 |
| BDE-47 | 5.11 | 10.11 | 237.13 | 237.13 | 0.00 | 10.00 | 1.17 | 10.26 | 4.89 | 13.01 | 1.73 | 10.82 |
| BDE-66 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.94 | 9.69 | 0.00 | 10.00 |
| BDE-77 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 1.08 | 9.83 | 0.00 | 10.00 |
| BDE-100 | 0.00 | 10.00 | 106.92 | 107.75 | 0.00 | 10.00 | 0.00 | 10.00 | 1.33 | 10.08 | 0.00 | 10.00 |
| BDE-99 | 0.00 | 10.00 | 12.54 | 15.04 | 0.00 | 10.00 | 0.00 | 10.00 | 2.60 | 11.35 | 0.00 | 10.00 |
| BDE-85 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.67 | 10.05 | 0.00 | 10.00 |
| BDE-154 | 0.00 | 10.00 | 47.61 | 48.44 | 0.00 | 10.00 | 0.00 | 10.00 | 0.62 | 9.99 | 0.00 | 10.00 |
| BDE-153 | 0.00 | 10.00 | 5.28 | 11.12 | 0.00 | 10.00 | 0.00 | 10.00 | 0.65 | 10.02 | 0.00 | 10.00 |
| BDE-138 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 1.38 | 10.75 | 0.00 | 10.00 |
| BDE-183 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 |
| BDE-197 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.00 | 10.00 | 0.63 | 10.00 | 0.00 | 10.00 |
| BDE-206 | 0.00 | 100.00 | 0.00 | 100.00 | 0.00 | 100.00 | 0.00 | 100.00 | 1.85 | 95.60 | 0.00 | 100.00 |
| BDE-209 | 0.00 | 100.00 | 0.00 | 100.00 | 0.00 | 100.00 | 0.00 | 100.00 | 23.22 | 110.72 | 0.00 | 100.00 |
| ∑PBDE | 5.11 | 330.11 | 437.15 | 718.82 | 0.00 | 330.00 | 0.99 | 330.26 | 36.63 | 341.21 | 1.73 | 330.82 |
| ∑PBDE (l.w.) | 630.86 | 39,211.11 | 1797.49 | 2955.67 | 0.00 | 10,576.92 | 97.06 | 32,378.43 | 8721.43 | 81,240.48 | 37.94 | 7254.82 |
| α-HBCD | 5.32 | 10.94 | 714.81 | 714.81 | 1.33 | 10.56 | 2.42 | 10.60 | 189.93 | 193.06 | 11.56 | 17.92 |
| β-HBCD | 0.00 | 10.00 | 5.45 | 14.62 | 0.00 | 10.00 | 0.00 | 10.00 | 110.85 | 115.85 | 0.00 | 10.00 |
| γ-HBCD | 0.00 | 10.00 | 10.41 | 17.91 | 0.00 | 10.00 | 0.77 | 10.32 | 654.57 | 657.07 | 0.00 | 10.00 |
| ∑HBCD | 4.73 | 30.94 | 730.67 | 747.33 | 1.24 | 30.56 | 2.70 | 26.17 | 849.20 | 965.98 | 11.56 | 37.92 |
| ∑HBCD (l.w.) | 583.95 | 3665.43 | 3004.40 | 3072.90 | 39.74 | 979.49 | 264.71 | 2565.69 | 202,190.48 | 229,995.24 | 253.51 | 831.58 |
| Perch | Eel | Tench | Goldfish | Crayfish | Carp | ∑ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | |
| BDE-28 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.005 | 0.001 | 0.005 | 0.000 | 0.005 | 0.000 | 0.028 |
| BDE-49 | 0.000 | 0.004 | 0.013 | 0.014 | 0.000 | 0.005 | 0.000 | 0.005 | 0.001 | 0.005 | 0.000 | 0.005 | 0.013 | 0.037 |
| BDE-47 * | 0.002 | 0.005 | 0.112 | 0.112 | 0.000 | 0.005 | 0.001 | 0.005 | 0.002 | 0.006 | 0.001 | 0.005 | 0.118 | 0.137 |
| BDE-66 | 0.000 | 0.004 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.028 |
| BDE-77 | 0.000 | 0.004 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.001 | 0.005 | 0.000 | 0.005 | 0.000 | 0.028 |
| BDE-85 | 0.000 | 0.004 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.028 |
| BDE-99 * | 0.000 | 0.004 | 0.006 | 0.007 | 0.000 | 0.005 | 0.000 | 0.005 | 0.001 | 0.005 | 0.000 | 0.005 | 0.007 | 0.031 |
| BDE-100 | 0.000 | 0.004 | 0.050 | 0.051 | 0.000 | 0.005 | 0.000 | 0.005 | 0.001 | 0.005 | 0.000 | 0.005 | 0.051 | 0.074 |
| BDE-138 | 0.000 | 0.004 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.001 | 0.005 | 0.000 | 0.005 | 0.001 | 0.028 |
| BDE-153 * | 0.000 | 0.004 | 0.002 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.003 | 0.028 |
| BDE-154 | 0.000 | 0.004 | 0.022 | 0.023 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.023 | 0.046 |
| BDE-183 | 0.000 | 0.004 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.028 |
| BDE-197 | 0.000 | 0.004 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.005 | 0.000 | 0.028 |
| BDE-206 | 0.000 | 0.044 | 0.000 | 0.047 | 0.000 | 0.047 | 0.000 | 0.047 | 0.001 | 0.045 | 0.000 | 0.047 | 0.001 | 0.278 |
| BDE-209 * | 0.000 | 0.044 | 0.000 | 0.047 | 0.000 | 0.047 | 0.000 | 0.047 | 0.011 | 0.052 | 0.000 | 0.047 | 0.011 | 0.285 |
| ∑PBDE | 0.002 | 0.146 | 0.206 | 0.339 | 0.000 | 0.1556 | 0.001 | 0.156 | 0.019 | 0.161 | 0.001 | 0.156 | 0.229 | 1.113 |
| ∑PBDE * | 0.002 | 0.058 | 0.120 | 0.171 | 0.000 | 0.0613 | 0.001 | 0.061 | 0.015 | 0.068 | 0.001 | 0.062 | 0.138 | 0.482 |
| α-HBCD | 0.003 | 0.005 | 0.337 | 0.337 | 0.001 | 0.0050 | 0.0011 | 0.005 | 0.089 | 0.091 | 0.005 | 0.008 | 0.436 | 0.452 |
| β-HBCD | 0.000 | 0.004 | 0.003 | 0.007 | 0.000 | 0.0047 | 0.0000 | 0.005 | 0.052 | 0.055 | 0.000 | 0.005 | 0.055 | 0.080 |
| γ-HBCD | 0.000 | 0.004 | 0.005 | 0.008 | 0.000 | 0.0047 | 0.0004 | 0.005 | 0.309 | 0.310 | 0.000 | 0.005 | 0.314 | 0.337 |
| ∑HBCD * | 0.003 | 0.014 | 0.344 | 0.352 | 0.001 | 0.0144 | 0.0015 | 0.015 | 0.450 | 0.455 | 0.005 | 0.018 | 0.805 | 0.868 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roila, R.; Branciari, R.; Ranucci, D.; Stramenga, A.; Tavoloni, T.; Stecconi, T.; Franceschini, R.; Piersanti, A. Risk Characterization and Benefit–Risk Assessment of Brominated Flame Retardant in Commercially Exploited Freshwater Fishes and Crayfish of Lake Trasimeno, Italy. Int. J. Environ. Res. Public Health 2021, 18, 8763. https://doi.org/10.3390/ijerph18168763
Roila R, Branciari R, Ranucci D, Stramenga A, Tavoloni T, Stecconi T, Franceschini R, Piersanti A. Risk Characterization and Benefit–Risk Assessment of Brominated Flame Retardant in Commercially Exploited Freshwater Fishes and Crayfish of Lake Trasimeno, Italy. International Journal of Environmental Research and Public Health. 2021; 18(16):8763. https://doi.org/10.3390/ijerph18168763
Chicago/Turabian StyleRoila, Rossana, Raffaella Branciari, David Ranucci, Arianna Stramenga, Tamara Tavoloni, Tommaso Stecconi, Raffaella Franceschini, and Arianna Piersanti. 2021. "Risk Characterization and Benefit–Risk Assessment of Brominated Flame Retardant in Commercially Exploited Freshwater Fishes and Crayfish of Lake Trasimeno, Italy" International Journal of Environmental Research and Public Health 18, no. 16: 8763. https://doi.org/10.3390/ijerph18168763






