COVID-19 Screening by Anti-SARS-CoV-2 Antibody Seropositivity: Clinical and Epidemiological Characteristics, Comorbidities, and Food Intake Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
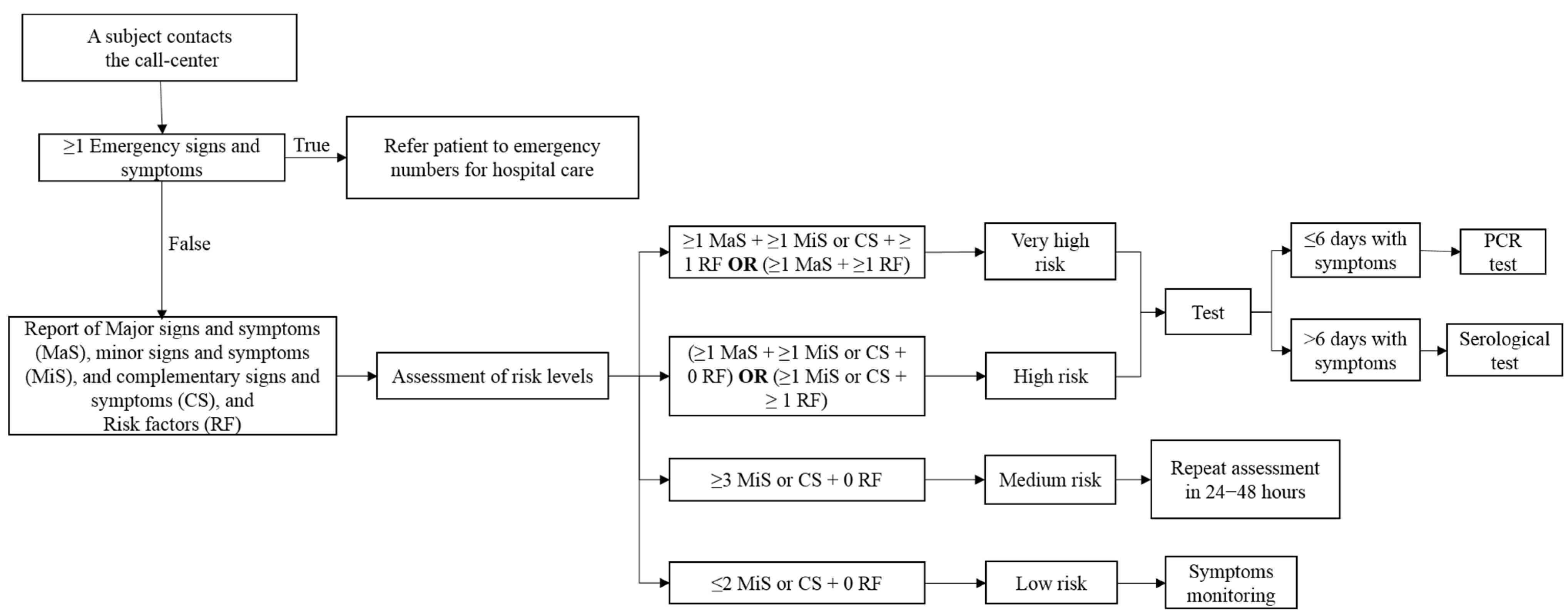
2.2. The Setting of the Study, Materials, and Process
2.3. Statistical Analysis
3. Results
3.1. Clinical and Epidemiological Characteristics of the Study Participants
3.2. Symptoms of the Study Participants
3.3. Symptoms According to Seropositivity Pattern to Anti-SARS-CoV-2 Antibodies
3.4. Food Intake Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A Familial Cluster of Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-Person Transmission: A Study of a Family Cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Rai, P.; Kumar, B.K.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I. Detection Technologies and Recent Developments in the Diagnosis of COVID-19 Infection. Appl. Microbiol. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Paradiso, A.V.; De Summa, S.; Loconsole, D.; Procacci, V.; Sallustio, A.; Centrone, F.; Silvestris, N.; Cafagna, V.; De Palma, G.; Tufaro, A.; et al. Rapid Serological Assays and SARS-CoV-2 Real-Time Polymerase Chain Reaction Assays for the Detection of SARS-CoV-2: Comparative Study. J. Med. Internet Res. 2020, 22, e19152. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Antibody Tests for Identification of Current and Past Infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Diaz, E.; Llopis, J.; Parra, R.; Roig, I.; Ferrer, G.; Grifols, J.; Millán, A.; Ene, G.; Ramiro, L.; Maglio, L.; et al. Relationship between the ABO Blood Group and COVID-19 Susceptibility, Severity and Mortality in Two Cohorts of Patients. Blood Transfus. 2020. [Google Scholar] [CrossRef]
- Severe COVID-19 GWAS Group; Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; et al. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Zietz, M.; Zucker, J.; Tatonetti, N.P. Associations between Blood Type and COVID-19 Infection, Intubation, and Death. Nat. Commun. 2020, 11, 5761. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zhang, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship Between the ABO Blood Group and the Coronavirus Disease 2019 (COVID-19) Susceptibility. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; El-Hennawy, D. The Possible Beneficial Adjuvant Effect of Influenza Vaccine to Minimize the Severity of COVID-19. Med. Hypotheses 2020, 140, 109752. [Google Scholar] [CrossRef]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the Influenza Vaccine on COVID-19 Infection Rates and Severity. Am. J. Infect. Control 2021. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O’Horo, J.C.; Gores, G.J.; Williams, A.W.; Halamka, J.; et al. Exploratory Analysis of Immunization Records Highlights Decreased SARS-CoV-2 Rates in Individuals with Recent Non-COVID-19 Vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Hamadeh, R. Nutrition amid the COVID-19 Pandemic: A Multi-Level Framework for Action. Eur. J. Clin. Nutr. 2020, 74, 1117–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 Detection: A Comprehensive Review of the FDA-EUA COVID-19 Testing Landscape. Biosens. Bioelectron. 2020, 165, 112454. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Orozco, M.F.; Salmeron-Curiel, P.B.; Prado-Arriaga, R.J.; Orozco-Gutiérrez, J.F.; Badillo-Camacho, N.; Márquez-Sandoval, F.; Altamirano-Martínez, M.B.; González-Gómez, M.; Gutiérrez-González, P.; Vizmanos, B.; et al. Second Version of a Mini-Survey to Evaluate Food Intake Quality (Mini-ECCA v.2): Reproducibility and Ability to Identify Dietary Patterns in University Students. Nutrients 2020, 12, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal-Orozco, M.F.; Badillo-Camacho, N.; Macedo-Ojeda, G.; González-Gómez, M.; Orozco-Gutiérrez, J.F.; Prado-Arriaga, R.J.; Márquez-Sandoval, F.; Altamirano-Martínez, M.B.; Vizmanos, B. Design and Reproducibility of a Mini-Survey to Evaluate the Quality of Food Intake (Mini-ECCA) in a Mexican Population. Nutrients 2018, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Bermingham, W.H.; Wilding, T.; Beck, S.; Huissoon, A. SARS-CoV-2 Serology: Test, Test, Test, but Interpret with Caution! Clin. Med. 2020, 20, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male Sex Identified by Global COVID-19 Meta-Analysis as a Risk Factor for Death and ITU Admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Ahrenfeldt, L.J.; Otavova, M.; Christensen, K.; Lindahl-Jacobsen, R. Sex and Age Differences in COVID-19 Mortality in Europe. Wien. Klin. Wochenschr. 2021, 133, 393–398. [Google Scholar] [CrossRef]
- CDCMMWR. COVID-19 Stats: COVID-19 Incidence, by Age Group—United States, March 1–November 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 69. [Google Scholar] [CrossRef]
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T.; Li, P.; Zhou, Y.; Lin, Y.; Duan, Q.; et al. Clinical Characteristics of Coronavirus Disease 2019 (COVID-19) in China: A Systematic Review and Meta-Analysis. J. Infect. 2020, 80, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Traugott, M.T.; Graninger, M.; Hoepler, W.; Seitz, T.; Kelani, H.; Karolyi, M.; Pawelka, E.; Cruz, S.A.d.L.; Puchhammer-Stöckl, E.; et al. Assessment of S1-, S2-, and NCP-Specific IgM, IgA, and IgG Antibody Kinetics in Acute SARS-CoV-2 Infection by a Microarray and Twelve Other Immunoassays. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef] [PubMed]
- Bajgain, K.T.; Badal, S.; Bajgain, B.B.; Santana, M.J. Prevalence of Comorbidities among Individuals with COVID-19: A Rapid Review of Current Literature. Am. J. Infect. Control 2020. [Google Scholar] [CrossRef]
- Villalpando, S.; Rodrigo, J.R. The Status of Non-Transmissible Chronic Disease in Mexico Based on the National Health and Nutrition Survey 2006. Salud Públ. México 2010, 52, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 September 2020).
- Sosa-Rubí, S.G.; Seiglie, J.A.; Chivardi, C.; Manne-Goehler, J.; Meigs, J.B.; Wexler, D.J.; Wirtz, V.J.; Gómez-Dantés, O.; Serván-Mori, E. Incremental Risk of Developing Severe COVID-19 Among Mexican Patients With Diabetes Attributed to Social and Health Care Access Disadvantages. Diabetes Care 2020. [Google Scholar] [CrossRef]
- Jones, G.J.B.; Roper, R.L. The Effects of Diets Enriched in Omega-3 Polyunsaturated Fatty Acids on Systemic Vaccinia Virus Infection. Sci. Rep. 2017, 7, 15999. [Google Scholar] [CrossRef] [Green Version]
- Husson, M.-O.; Ley, D.; Portal, C.; Gottrand, M.; Hueso, T.; Desseyn, J.-L.; Gottrand, F. Modulation of Host Defence against Bacterial and Viral Infections by Omega-3 Polyunsaturated Fatty Acids. J. Infect. 2016, 73, 523–535. [Google Scholar] [CrossRef]
- Schwerbrock, N.M.J.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish Oil-Fed Mice Have Impaired Resistance to Influenza Infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Lian, M.; Luo, W.; Sui, Y.; Li, Z.; Hua, J. Dietary N-3 PUFA Protects Mice from Con A Induced Liver Injury by Modulating Regulatory T Cells and PPAR-γ Expression. PLoS ONE 2015, 10, e0132741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi Saik, I.; Guessous, F.; Akarid, K. The Pro-Inflammatory Cytokines in COVID-19 Pathogenesis: What Goes Wrong? Microbial. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef] [PubMed]
- Ramon, S.; Baker, S.F.; Sahler, J.M.; Kim, N.; Feldsott, E.A.; Serhan, C.N.; Martínez-Sobrido, L.; Topham, D.J.; Phipps, R.P. The Specialized Proresolving Mediator 17-HDHA Enhances the Antibody-Mediated Immune Response against Influenza Virus: Anew Class of Adjuvant? J. Immunol. 2014, 193, 6031–6040. [Google Scholar] [CrossRef] [PubMed]
| Variables | Positive for Anti-SARS-CoV-2 Antibodies * | Negative for Anti-SARS-CoV-2 Antibodies | p-Value a |
|---|---|---|---|
| n= 756 | n = 1043 | ||
| Age (years) Median (25th–75th percentiles) | 37 (28–49) | 34 (25–46) | 0.0001 b |
| 0–20 | 40 (5.69) | 89 (9.09) | 0.002 |
| 21–30 | 190 (27.03) | 325 (33.20) | |
| 31–40 | 188 (26.74) | 236 (24.11) | |
| 41–50 | 126 (17.92) | 153 (15.63) | |
| 51–60 | 100 (14.22) | 121 (12.36) | |
| >60 | 59 (8.39) | 55 (5.62) | |
| Sex: Female/Male n (%) | 446 (58.99)/310 (41.01) | 590(56.57)/453(43.43) | 0.304 |
| Vaccinated against influenza n (%) | 385 (50.93) | 541 (51.87) | 0.693 |
| Comorbidities n (%) | |||
| Overweight | 150 (19.84) | 181 (17.35) | 0.179 |
| Obesity | 69 (9.13) | 83 (7.96) | 0.379 |
| Hypertension | 87 (11.51) | 109 (10.45) | 0.477 |
| Diabetes | 71 (9.39) | 58 (5.56) | 0.002 |
| Immunodeficiency | 8 (1.06) | 10 (0.96) | 0.834 c |
| Number of comorbidities | 4.489 ± 2.409 | 3.810 ± 2.423 | 0.008 d |
| Blood group n (%) | |||
| A (Rh+ or Rh-) | 215 (28.44) | 300 (28.76) | 0.403 |
| B (Rh+ or Rh-) | 61 (8.07) | 94 (9.01) | |
| AB (Rh+ or Rh-) | 19 (2.51) | 32 (3.07) | |
| O (Rh+ or Rh-) | 377 (49.87) | 528 (50.62) | |
| Unknown | 84 (11.11) | 89 (8.53) | |
| Risk contact | |||
| Contact with COVID-19 patient n (%) | 508 (67.20) | 768 (73.63) | 0.003 |
| Days of contact with COVID-19 patients pre-test Median (25th–75th percentiles) | 12 (7–17) | 10 (6–15) | 0.0023 b |
| Variables | Positive for Anti-SARS-CoV-2 Antibodies * | Negative for Anti-SARS-CoV-2 Antibodies | p-Value a |
|---|---|---|---|
| n = 756 | n = 1043 | ||
| Days post symptom onset Median (25th–75th percentiles) | 10 (7–14) | 8 (5–11) | <0.0001 |
| Number of symptoms Median (25th–75th percentiles) | 5 (3–6) | 4 (2–6) | <0.0001 |
| Asymptomatic n (%) | 47 (6.22) | 142 (13.61) | <0.0001 |
| Headache n (%) | 508 (67.20) | 688 (65.96) | 0.585 |
| Cough n (%) | 437 (57.80) | 481 (46.12) | <0.0001 |
| Fever n (%) | 246 (32.54) | 307 (29.43) | 0.159 |
| Respiratory distress n (%) | 194 (25.66) | 176 (16.87) | <0.0001 |
| Runny nose, nasal congestion, sneezing n (%) | 384 (50.79) | 559 (53.60) | 0.240 |
| Muscle pain n (%) | 418 (55.29) | 481 (46.12) | <0.0001 |
| Joint pain n (%) | 285 (37.70) | 306 (29.34) | <0.0001 |
| Sore throat n (%) | 374 (49.47) | 546 (52.35) | 0.228 |
| Anosmia n (%) | 453 (59.92) | 305 (29.24) | <0.0001 |
| Hypogeusia n (%) | 39 (5.16) | 27 (2.59) | 0.004 |
| Diarrhea, vomiting, upset stomach n (%) | 37 (4.89) | 63 (6.04) | 0.295 |
| Chest pain n (%) | 5 (0.66) | 12 (1.15) | 0.290 b |
| Fatigue n (%) | 9 (1.19) | 11 (1.05) | 0.786 b |
| Dizziness n (%) | 5 (0.66) | 12 (1.15) | 0.290 b |
| Variables | Anti-SARS-CoV-2 IgM Seropositivity | Anti-SARS-CoV-2 IgG Seropositivity | Anti-SARS-CoV-2 IgM + IgG Seropositivity | p-Value a |
|---|---|---|---|---|
| Day’s post symptom onset Median (25th–75th percentiles) | n = 69 | n = 66 | n = 270 | |
| 9.00 (7.00–11.00) | 10 (7.75–13.25) | 10.00 (7.00–15.00) | 0.086 b | |
| n = 118 | n = 125 | n = 513 | ||
| Number of symptoms Median (25th–75th percentiles) | 4.50 (3.00–6.00) | 4.00 (2.00–6.00) | 5.00 (3.00–6.50) | 0.088 b |
| Headache n (%) | 82 (69.5) | 81 (64.8) | 345 (67.3) | 0.750 |
| Cough n (%) | 56 (47.5) | 65 (52.0) | 316 (61.6) | 0.007 |
| Fever n (%) | 34 (28.8) | 33 (26.4) | 179 (34.9) | 0.122 |
| Respiratory distress n (%) | 24 (20.3) | 23 (18.4) | 147 (28.7) | 0.022 |
| Runny nose, nasal congestion, sneezing n (%) | 65 (55.1) | 61 (48.8) | 258 (50.3) | 0.576 |
| Muscle pain n (%) | 69 (58.5) | 66 (52.8) | 283 (55.2) | 0.672 |
| Joint pain n (%) | 48 (40.7) | 40 (32.0) | 197 (38.4) | 0.324 |
| Sore throat n (%) | 57 (48.3) | 62 (49.6) | 255 (49.7) | 0.969 |
| Anosmia n (%) | 75 (63.6) | 62 (49.6) | 316 (61.6) | 0.034 |
| Hypogeusia n (%) | 11 (9.3) | 6 (4.8) | 22 (4.3) | 0.082 |
| Diarrhea, vomiting, upset stomach n (%) | 7 (5.9) | 2 (1.6) | 28 (5.5) | 0.174 |
| Chest pain n (%) | 1 (0.8) | 1 (0.8) | 3 (0.6) | 0.659 c |
| Fatigue n (%) | 1 (0.8) | 4 (3.2) | 4 (0.8) | 0.092 c |
| Dizziness n (%) | 1 (0.8) | 1 (0.8) | 3 (0.6) | 0.659 c |
| Question | Answer Options | Positive for Anti-SARS-CoV-2 Antibodies * n = 756 | Negative for Anti-SARS-CoV-2 Antibodies n = 1043 | p-Value a |
|---|---|---|---|---|
| n (%) | n (%) | |||
| 1. Do you drink at least 1.5 L of water per day? | A. Never | 16 (2.1) | 23 (2.2) | 0.141 |
| B. Sometimes | 237 (31.3) | 285 (27.3) | ||
| C. Almost always | 276 (36.5) | 373 (35.8) | ||
| D. Always | 227 (30.0) | 362 (34.7) | ||
| 2. Do you consume at least 200 g of cooked or raw vegetables per day? | A. Never | 17 (2.2) | 35 (3.4) | 0.104 |
| B. Sometimes | 376 (49.7) | 466 (44.7) | ||
| C. Almost always | 257 (34.0) | 396 (38.0) | ||
| D. Always | 106 (14.0) | 146 (14.0) | ||
| 3. Do you eat fresh or frozen fish (100 g) at least one day per week? | A. Never | 122 (16.1) | 146 (14.0) | 0.351 |
| B. Sometimes | 452 (59.8) | 641 (61.5) | ||
| C. Almost always | 118 (15.6) | 181 (17.4) | ||
| D. Always | 64 (8.5) | 75 (7.2) | ||
| 4. Do you consume one or more glass (can) of sweetened beverages per week? | A. Never | 116 (15.3) | 193 (18.5) | 0.319 |
| B. 1 to 3 times | 433 (57.3) | 570 (54.7) | ||
| C. 4 to 6 times | 114 (15.1) | 162 (15.5) | ||
| D. Daily | 93 (12.3) | 118 (11.3) | ||
| 5. Do you consume at least 200 g of fruit per day? | A. Never | 15 (2.0) | 23 (2.2) | 0.880 |
| B. Sometimes | 286 (37.8) | 405 (38.8) | ||
| C. Almost always | 294 (38.9) | 408 (39.1) | ||
| D. Always | 161 (21.3) | 207 (19.1) | ||
| 6. What type of fat do you consume most frequently on a weekly basis? | A. Monounsaturated | 151 (20.0) | 281 (26.9) | 0.006 |
| B. Polyunsaturated | 565 (74.7) | 720 (69.0) | ||
| C. Saturated | 23 (3.0) | 23 (2.2) | ||
| D. Do not know | 17 (2.2) | 19 (1.8) | ||
| 7. Do you consume at least 30 g of oilseeds or 1/2 of an avocado per day? | A. Never | 54 (7.1) | 61 (5.8) | 0.351 |
| B. Sometimes | 423 (56.0) | 566 (54.3) | ||
| C. Almost always | 218 (28.8) | 312 (29.9) | ||
| D. Always | 61 (8.1) | 104 (10.0) | ||
| 8. Do you eat foods not prepared at home three or more days per week? | A. Never | 71 (9.4) | 118 (11.3) | 0.576 |
| B. Sometimes | 465 (61.5) | 624 (59.8) | ||
| C. Almost always | 152 (20.1) | 202 (19.4) | ||
| D. Always | 68 (9.0) | 99 (9.5) | ||
| 9. What type of meat do you consume most often? | A. Red meat | 399 (52.8) | 516 (49.5) | 0.361 |
| B. Chicken | 315 (41.7) | 469 (45.0) | ||
| C. Fish | 42 (5.6) | 58 (5.6) | ||
| 10. Do you eat processed foods two or more days per week? | A. Never | 112 (14.8) | 129 (12.4) | 0.398 |
| B. Sometimes | 567 (75.0) | 792 (75.9) | ||
| C. Almost always | 70 (9.3) | 112 (10.7) | ||
| D. Always | 7 (0.9) | 10 (1.0) | ||
| 11. Do you consume sweets or commercially produced desserts two or more days per week? | A. Never | 64 (8.5) | 105 (10.1) | 0.470 |
| B. Sometimes | 542 (71.7) | 719 (68.9) | ||
| C. Almost always | 120 (15.9) | 182 (17.4) | ||
| D. Always | 30 (4.0) | 37 (3.5) | ||
| 12. Do you eat legumes at least three days per week (300 g per week)? | A. Never | 20 (2.6) | 37 (3.5) | 0.554 |
| B. Sometimes | 344 (45.5) | 455 (43.6) | ||
| C. Almost always | 295 (39.0) | 426 (40.8) | ||
| D. Always | 97 (12.8) | 125 (12.9) | ||
| 13. What type of cereals do you consume most often? | A. Whole grain | 260 (34.4) | 406 (38.9) | 0.145 |
| B. Minimally processed | 256 (33.9) | 329 (31.5) | ||
| C. Processed and ultra-processed | 240 (31.7) | 308 (29.5) | ||
| 14. If you are a man, do you consume more than 2 alcoholic beverages per day? If you are a woman, do you consume more than 1 alcoholic beverage per day? | A. Never | 519 (68.7) | 716 (68.6) | 0.077 |
| B. Sometimes | 228 (30.2) | 296 (28.4) | ||
| C. Almost always | 8 (1.1) | 26 (2.5) | ||
| D. Always | 1 (0.1) | 5 (0.5) | ||
| Dietary patterns | Healthy food intake | 260 (34.4) | 397 (38.1) | 0.221 |
| Habits in need of improvement | 357 (47.2) | 453 (43.4) | ||
| Unhealthy food intake | 139 (18.4) | 193 (18.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo-Ojeda, G.; Muñoz-Valle, J.F.; Yokogawa-Teraoka, P.; Machado-Sulbarán, A.C.; Loza-Rojas, M.G.; García-Arredondo, A.C.; Tejeda-Constantini, R.; Vega-Magaña, A.N.; González-Estevez, G.; García-Chagollán, M.; et al. COVID-19 Screening by Anti-SARS-CoV-2 Antibody Seropositivity: Clinical and Epidemiological Characteristics, Comorbidities, and Food Intake Quality. Int. J. Environ. Res. Public Health 2021, 18, 8995. https://doi.org/10.3390/ijerph18178995
Macedo-Ojeda G, Muñoz-Valle JF, Yokogawa-Teraoka P, Machado-Sulbarán AC, Loza-Rojas MG, García-Arredondo AC, Tejeda-Constantini R, Vega-Magaña AN, González-Estevez G, García-Chagollán M, et al. COVID-19 Screening by Anti-SARS-CoV-2 Antibody Seropositivity: Clinical and Epidemiological Characteristics, Comorbidities, and Food Intake Quality. International Journal of Environmental Research and Public Health. 2021; 18(17):8995. https://doi.org/10.3390/ijerph18178995
Chicago/Turabian StyleMacedo-Ojeda, Gabriela, José Francisco Muñoz-Valle, Patricia Yokogawa-Teraoka, Andrea Carolina Machado-Sulbarán, María Guadalupe Loza-Rojas, Atziri Citlally García-Arredondo, Rafael Tejeda-Constantini, Alejandra Natali Vega-Magaña, Guillermo González-Estevez, Mariel García-Chagollán, and et al. 2021. "COVID-19 Screening by Anti-SARS-CoV-2 Antibody Seropositivity: Clinical and Epidemiological Characteristics, Comorbidities, and Food Intake Quality" International Journal of Environmental Research and Public Health 18, no. 17: 8995. https://doi.org/10.3390/ijerph18178995
APA StyleMacedo-Ojeda, G., Muñoz-Valle, J. F., Yokogawa-Teraoka, P., Machado-Sulbarán, A. C., Loza-Rojas, M. G., García-Arredondo, A. C., Tejeda-Constantini, R., Vega-Magaña, A. N., González-Estevez, G., García-Chagollán, M., Zepeda-Nuño, J. S., & Hernández-Bello, J. (2021). COVID-19 Screening by Anti-SARS-CoV-2 Antibody Seropositivity: Clinical and Epidemiological Characteristics, Comorbidities, and Food Intake Quality. International Journal of Environmental Research and Public Health, 18(17), 8995. https://doi.org/10.3390/ijerph18178995









