Integrative Review of Exercise at Altitude during Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search and Eligibility Criteria
2.2. Study Selection
2.3. Risk of Bias in Individual Studies
2.4. Publication Bias
3. Results
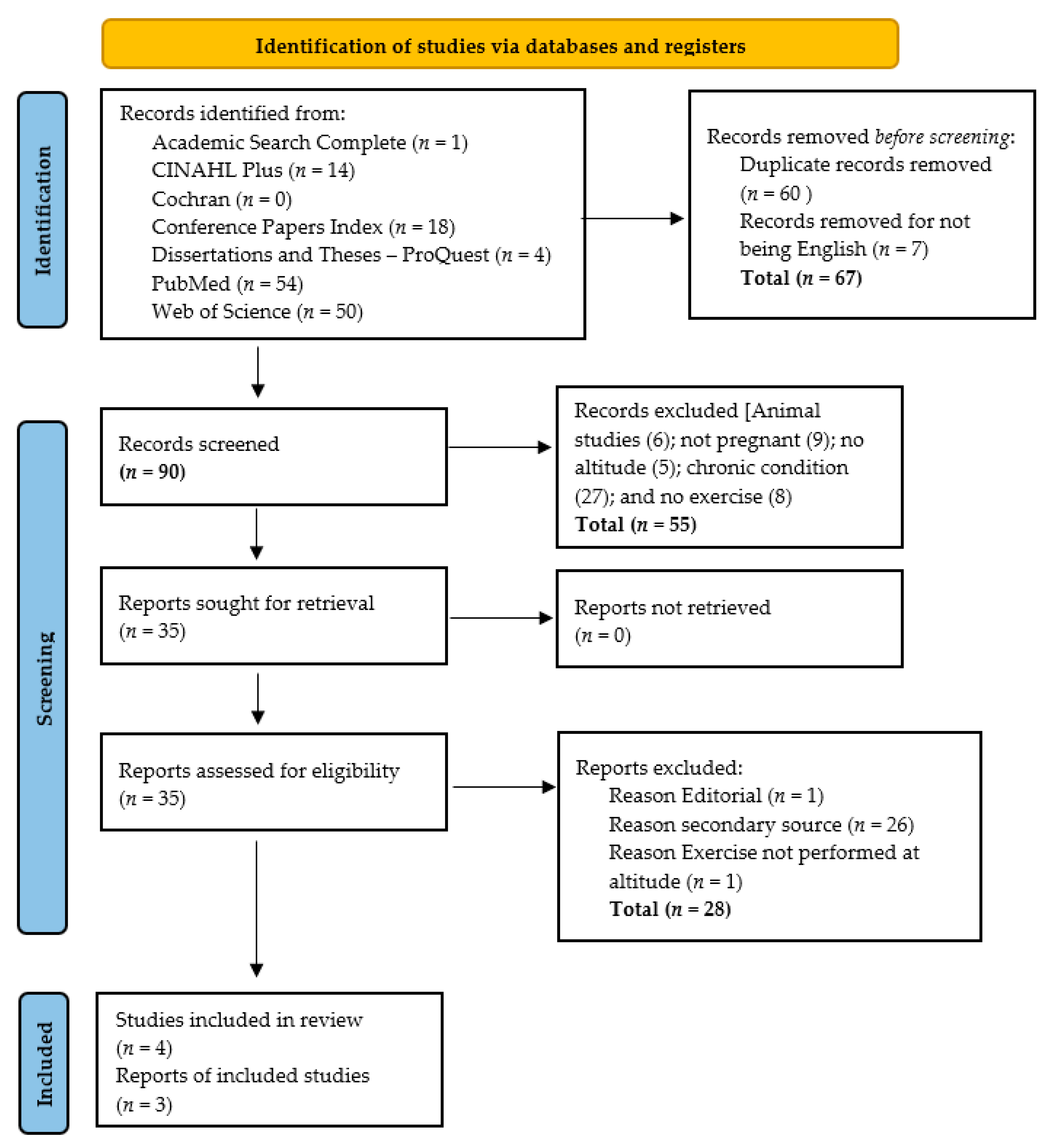
3.1. Study Selection
3.2. Description of Included Studies
3.3. Physical Activities, Work Loads and Aerobic Capacity at Altitude
3.4. Cardiopulmonary Effects of Exercise at Altitude
3.4.1. Cardiovascular Effects
3.4.2. Hemoglobin Effects
3.4.3. Pulmonary Effects
3.5. Perinatal and Fetal Effects
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| First Author, Year, Country | Purpose | Methods | Study Sample |
|---|---|---|---|
| Artal 1995 California, United States | Determine if the cardiopulmonary responses, aerobic work capacity, work efficiency and FHR response while pregnant differ between altitude (6000 ft/1829 m) and sea level. | Quantitative study. Quasi experimental, repeated-measures design. | Seven pregnant, lowlander, females in third trimester, 5 Hispanic and 2 White, sedentary, completed the study. Two subjects dropped out due to personal reasons after the first test and the third had persistent uterine activity after the first test. The mean age was 24.86 ± 2.18 SE and gestational age was 33.86 ± 1.46 SE. |
| Ballew 1984 La Paz, Bolivia | One of the purposes of this dissertation was to investigate the relationship between maternal exercise performance at high altitude and body composition of the newborn. | Quantitative study. Non-experimental, correlational design. | Twenty pregnant, females of Indian ancestry, born and living in La Paz, Bolivia (3000 m or more), in the seventh or eighth month of pregnancy, 18 to 35 years of age, participated in the exercise portion of the study. Potential subjects were excluded for infections, hypertension, anemia, and respiratory diseases. |
| Davenport, 2018 Everest Base Camp, Nepal | Assess the physical activity, sleep and cardiovascular function in a female, native, trekking Sherpa guide during a trek from 3400 to 5300 min Nepal Himalaya. | Quantitative study. Case study. | A 28-year-old primiparous female at ~31 weeks who lived most of her life above 3450 m, with grandparents and mother who were also born and lived at high altitude. The participant was the only female Sherpa who trekked and guided tourists from the Everest Base Camp. She was also an elite competitive endurance athlete at the time. |
| Huch, 1996 (Baumann 1985 and Bung, 1987 as reported by Huch) Swiss Mountains and lab, Switzerland | Describes two studies that investigated the effects of exercise in high altitude on sedentary pregnant individuals. The first study took place during a short-term trip to altitude and the second study was in a chamber that simulated moderately high altitude. | Quantitative study. Quasi-experimental, repeated-measures design. | First study: Twelve sedentary pregnant lowlander females with uncomplicated pregnancies. Ten were primipara and two were multipara. Gestational ages ranged from 30 to 39, with a mean of 36 weeks. Mean age of participants was not reported. Second study: Six pregnant females with gestational ages ranging from 31 to 38 weeks. Four were primipara and two were multipara. Second Study: Due to the bradycardia in the first study, the second study was conducted in a in low pressure chamber. Four primiparas and two multiparas between 31- and 38-weeks gestational age participated. Mean age of participants was not reported. |
| Niermeyer 1999 Colorado, United States | Determine the observed pregnancy complications and risk factors for pregnant individuals who visit high altitude. | Quantitative study. Survey design. | Respondents included 12 Colorado obstetrical care providers who worked in mountain communities and Denver referral lefts. |
| Pivarnik 1992 Utah, United States | Determine the alterations in maternal respiration and blood gases in pregnant individuals while performing aerobic exercise at moderate altitude during the late third trimester and postpartum. | Quantitative study. Quasi-experimental, repeated-measures design. | Seven, healthy, primigravid, untrained individuals at 36.9 ± 0.9 weeks of gestation. Average age was 21.0 ± 1.9 years. All participants lived at 1388 m elevation and fully acclimatized. |
| First Author, Year and Country | Data Collection/Type of Intervention | Key Findings |
|---|---|---|
| Artal 1995 California United States | Symptom-limited maximal exercise tests on a bicycle ergometer at sea level (180 feet) and at 6000 feet 2 to 4 days later. Brief medical exam, including ECG, BP assessment and 30 min of fetal monitoring prior to exercise test and CO assessments. Exercise protocol: Rest on bicycle ergometer 5 min. Pedal 50 revolutions/min for 5 min at 25, 50, and 75 W until volitional fatigue. Peak O2, oxygen consumption, ventilation, HR, CO, SV, TV, and RR measured at rest, at each work level, maximal exercise, and recovery. BP, uterine activity, and FHR measured before and after each test. | Sedentary, lowlander women performed less work (p = 0.03), had a lower peak O2, (L/min: p = 0.03; mL/kg/min: p = 0.04), and CO2max (p = 0.03) at altitude compared to sea level. The oxygen consumption was significantly lower at altitude than sea level but was similar at rest and each workload. There were no significant differences between sea level and altitude for HR, TV, RR, and plasma lactate. Ventilation was significantly higher at altitude at 75 W, suggesting hyperventilation occurred at this workload. CO and SV only differed at the two locations during rest. The pregnant women did not experience the typical hyperventilation observed at altitude. The FHR tracings were reactive at both sea level and altitude and the were no significant differences between the two locations. After exercise altitude, one subject had a fetal bradycardia to 105 beats/min for 2 min during which the FHR remained reactive. One subject had uterine activity, which required observation, which resolved without sequela. The ability of pregnant women to perform submaximal exercise at mild or moderate intensity at altitude does not seem to be adversely affected, but it does appear that maximal aerobic capacity is limited in sedentary pregnant women in their third trimester. |
| Ballew 1984 La Paz, Bolivia | Modified Harvard step test performed at steady-state submaximal level was performed at a clinic in La Paz (3200–4000 m). Step height was 20 cm to compensate for height of the subjects and stage of pregnancy. Subjects stepped at a pace of 76 beats per minute for 6 min. Some results excluded if a subject was unwilling or unable to maintain the stepping pace. Expired air was collected in the last 2 min of the test and HR measured before and after the test. HGB and HCT were measured in the subjects at 8 months. The subjects’ HT, WT, and skinfolds were measured, but timing not indicated. Newborn birth WT, crown-heel length, and skinfolds were measured within 30 h of birth. | Exercise intensity during the stepping test was equivalent to mild to moderate intensity although the range of intensity varied greatly. Subject WT was correlated with exercise test performance and sum of the newborn skinfolds. Ventilation equivalent and maximal HR tended to be negatively correlated with HGB and HCT, but not significantly. When controlled for subject’s WT, O2, ventilation equivalent and RQ were not related to the newborns’ WT, length, or skinfolds. The maximum HR reached at the end of the exercise test tended to be positively related to the newborn brachial skinfold but did not reach significance. Some limitations of the study were the small sample size, the submaximal exercise test, and that the subjects were native and thus, acclimatized to the high altitude. They hypothesis that women who performed better on the submaximal exercise test at high altitude would have larger babies was not supported. In addition, the evidence did not support the hypothesis that there would be a positive correlation between newborn composition and ventilation equivalent from pregnant individuals experiencing hyperventilation and a resulting increase in arterial saturation because of being at altitude. More research on pregnant individuals who recently migrated or are acutely novel to high altitude is needed. |
| Davenport, 2018 Everest Base Camp, Nepal | Physical and sleep activity was measured daily with an ActiGraph triaxial accelerometer. Weight, HGB and HCT were measured each morning in the fasting state. RR, HR, pressure of end tidal CO2, SpO2, and BP were then measured in a quiet room. The BP was used to calculate pulse pressure and mean arterial pressure. The participant completed the Lake Louise acute mountain sickness questionnaire each morning. | Participant’s BMI was 26.6–27.3 kg/m2. The participant performed MVPA ~270 min/day, which was approximately 20–30% of waking hours. A slightly lower level of activity was performed by the participant 10 months later during PP on a subsequent trip. Compared to PP, the participant’s RR was 16–21 breaths/min, indicating hyperventilation. The mean arterial pressure was unchanged during ascent and descent and was between 76 to 86 mmHg. The participant’s HR of 69 to 77 beats/min was lower than lowlander’s typical tachycardia seen in the third trimester and was similar to the subsequent PP values. Unknown if this is particular to Sherpas or because the participant was a trained athlete. Sherpas typically have physiological and pregnancy-induced hemodilution and the participant was no different. The lowest SpO2 of 83% was observed after spending the night at 5160 m and after one of the participant’s most active days. The SpO2 values were similar prenatally and PP. There was reduced fetal movement at 5300 m but normalized upon the return to 4370 m. Participant reported no mountain sickness. Participant delivered at 42 weeks via emergency caesarean section secondary to nuchal cord and associated fetal distress. Fetal birth weight was 3.2 kg. A year later, the baby was developing normally and did not have any health concerns. |
| Huch 1996 (Baumann 1985 and Bung, 1987 as reported in Huch) Swiss Mountains and lab, Switzerland | First Study: Participants were driven 130 km from ~408 m (1339 ft) to valley cable car station located at 1080 m (3500 ft). They rode up the cable car in a standing position to 2228 m (7250 ft) in 10 min. After 5 min rest, they rode a bicycle ergometer for 3 min at 25 W, followed by 5 min of rest. The car descended back to the valley and the 3- minute session was repeated. TcPO2 and PCO2, RR, HR, FHR, and uterine contractions were measured continuously. BP was measured intermittently. Second Study: Participants complete the same protocol at 50 W in a low-pressure chamber simulating altitudes of 1000 m (3500 ft) and 2200 m (7250 ft). | First Study: TcPO2 was 71 and 58 mm Hg at the valley and mountain stations, respectively. PCO2 was relatively unchanged between the two altitudes or exercise sessions. It did not decrease during exercise ruling out significant hyperventilation. RR was unchanged between the two stations. RR increased from 11 to 20 breaths/min during exercise. The mean resting HR was 101 beats/min. At both stations, mean HR increased from 103 to 128 beats/min during exercise. Systolic BP increased 13 and 27 mm Hg during the valley and mountain exercise sessions, respectively, with diastolic increasing in a similar fashion. Mild uterine contractions were felt by six of the subjects and were unchanged during exercise. The mean FHR increased from 138 to 142 beats/min. during the ascent and was 144 beats/min after exercise. One subject, who smoked at altitude, had a slight fetal bradycardia, and reduced FHR variability after the mountain exercise session, with the FHR returning to normal on the descent. Second Study: Results were similar to the first study except the RR and HRs were slightly higher during exercise. FHR was normal in five of the six pregnant individuals. The sixth pregnant individual, who smoked throughout pregnancy, had a 2 min bradycardia after completing the 2200 m simulated portion of the protocol. Recommendations included lowlanders avoiding altitudes greater than 2500 m for first 4 to 5 days and to exercise at lower altitude levels after initial exposure to altitude. |
| Niermeyer 1999 Colorado, United States | A Delphi survey was distributed to obstetrical care providers who worked in mountain communities and a Denver referral services inquiring about observed pregnancy complications and risk factors for pregnant individual who visit high altitude. | The rate of acute mountain sickness was the same, if not lower, than the general population. Risk factors for complications for altitude visitors included dehydration and vigorous exercise. The change in left of gravity and changes in body mass may adversely affect balance and coordination leading to increased risk of falling and injury. The respondents recommended a 3 to 4 day acclimatization period before beginning any exercise and short-term visitors should reduce or not exceed their usual level of intensity. Some providers recommended full acclimatization before exercising above 3000 m. |
| Pivarnik 1992 Utah, United States | A radial artery cannulation was used to obtain arterial blood gases at rest and during exercise. Minute ventilation, alveolar ventilation, dead space ventilation, oxygen uptake, carbon dioxide output, TV, and RR, were also obtained at rest and exercise. After the cannulation was placed and the participants rested, they performed two cycle ergometer (C1 = 50 W; C2 = 75 W) and two treadmill (T1 = 67 m·min−1; 2.5% grade, T2 = 67 m·min−1; 12% grade) tests. A 10 min rest period was provided between each 6 min test. Metabolic and respiratory values were obtained in the fifth and sixth minute of each test. Blood samples were obtained during minute 5. The entire test protocol was repeated at 12 weeks PP. Test took place at 1388 m altitude. | During pregnancy, minute ventilation, alveolar ventilation, TV and ventilatory equivalent for carbon dioxide were greater during exercise compared to PP. Minute ventilation was increased by increases in TV instead of RR. Ventilatory dead space did not differ between exercise and rest or between pregnancy and PP. The increased minute ventilation would not be beneficial to gas exchange unless alveolar ventilation increased, which was observed. The average arterial pH was significantly higher (0.04 units) in pregnancy compared to PP. The arterial pH decreased from rest to exercise during both pregnancy and PP with the greatest decrease occurring during the 75 W cycling test. It appears that pregnancy status did not influence the pH changes during exercise. PaCO2 was significantly lower during pregnancy. However, it was unchanged from rest to exercise, unlike PP for which PaCO2 decreased. Smaller decreases in arterial bicarbonate were observed during pregnancy compared to PP during exercise. The researchers concluded that the acid–base response to cycling and treadmill walking were not compromised in late pregnancy and gestational hyperventilation helped maintain PaCO2 and arterial bicarbonate during pregnancy. |
Appendix B
| Yes | No | Unclear | Not Applicable | |
|---|---|---|---|---|
| x | □ | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| Overall appraisal: Include x Exclude □ Seek further info □. | ||||
| Yes | No | Unclear | Not Applicable | |
|---|---|---|---|---|
| x | □ | □ | □ |
| □ | □ | □ | x |
| □ | x | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| □ | □ | x | □ |
| x | □ | □ | □ |
| □ | x | □ | □ |
| x | □ | □ | □ |
| Overall appraisal: Include X Exclude □ Seek further info □. | ||||
| Yes | No | Unclear | Not Applicable | |
|---|---|---|---|---|
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
Overall appraisal: Include X Exclude □ Seek further info □. | x | □ | □ | □ |
| Yes | No | Unclear | Not Applicable | |
|---|---|---|---|---|
| x | □ | □ | □ |
| □ | □ | □ | x |
| □ | x | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| □ | □ | x | □ |
Overall appraisal: Include X Exclude □ Seek further info □. | □ | □ | x | □ |
| Yes | No | Unclear | Not Applicable | |
|---|---|---|---|---|
| x | □ | □ | □ |
| □ | □ | □ | x |
| □ | x | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| □ | □ | x | □ |
Overall appraisal: Include x Exclude □ Seek further info □. | □ | □ | x | □ |
| Validity |
| Are the results of the study valid? |
|
| Reliability What are the results? |
|
| Applicability |
| Will the results help me in caring for my patients? |
|
| Would you use the study results in your practice to make a difference in patient outcomes? |
|
| Additional Comments/Reflections: The results from a Delphi survey of 12 obstetrical providers were provided in a narrative literature review. Methodology for the survey was not reported. This study discussed reports of performing exercise at altitude and the possible complications observed in the past. |
| Recommendations for article use within a body of evidence: © Fineout-Overholt and Gallagher-Ford, 2012. This form may be used for educational, practice change and research purposes without permission. |
| Yes | No | Unclear | Not Applicable | |
|---|---|---|---|---|
| x | □ | □ | □ |
| x | □ | □ | □ |
| □ | x | □ | □ |
| □ | x | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
| x | □ | □ | □ |
Overall appraisal: Include x Exclude □ Seek further info □. | x | □ | □ | □ |
References
- Entin, P.L.; Coffin, L. Physiological Basis for Recommendations Regarding Exercise during Pregnancy at High Altitude. High Alt. Med. Biol. 2004, 5, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Julian, C.G. High Altitude during Pregnancy. Clin. Chest Med. 2011, 32, 21–31. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologist. Physical Activity and Exercise during Pregnancy and the Postpartum Period: ACOG Committee Opinion Summary, Number 804. Obstet. Gynecol. 2020, 135, 991–993. [Google Scholar] [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.-M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian Guideline for Physical Activity throughout Pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Kasica, S.W. Top 10 Towns for High-Altitude Running. Available online: https://www.outsideonline.com/1926461/top-10-towns-high-altitude-running (accessed on 5 June 2021).
- Map Boulder -Colorado Longitude, Altitude–Sunset. Available online: https://www.usclimatedata.com/map/USCO0456 (accessed on 5 June 2021).
- Weather Averages Flagstaff, Arizona. Available online: https://www.usclimatedata.com/climate/flagstaff/arizona/united-states/usaz0068 (accessed on 5 June 2021).
- Ysbrand Visser Training at Altitude. Available online: https://www.runnersworld.com/advanced/a20826689/training-at-altitude/ (accessed on 5 June 2021).
- Huch, R. Physical Activity at Altitude in Pregnancy. Semin. Perinatol. 1996, 20, 303–314. [Google Scholar] [CrossRef]
- Evans, D.; Pearson, A. Systematic Reviews: Gatekeepers of Nursing Knowledge. J. Clin. Nurs. 2001, 10, 593–599. [Google Scholar] [CrossRef]
- Whittemore, R.; Knafl, K. The Integrative Review: Updated Methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef]
- Beyea, S.C.; Nicholl, L.H. Writing an Integrative Review. AORN J. 1998, 67, 877–880. [Google Scholar] [CrossRef]
- Toronto, C.E.; Remington, R. (Eds.) A Step-by-Step Guide to Conducting an Integrative Review; Springer Nature Switzerland AG: Cham, Switzerland, 2020; ISBN 978-3-030-37503-4. [Google Scholar]
- Zotero. Available online: https://www.zotero.org/ (accessed on 5 May 2021).
- Melnyk, B.M. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice, 4th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2019; ISBN 978-1-4963-8453-9. [Google Scholar]
- Joanna Briggs Institute Joanna Briggs Institute Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools (accessed on 16 August 2021).
- Weaving, C. Prenatal Paranoia: An Analysis of the Bumpy Landscape for the Pregnant Athlete. Sport Ethics Philos. 2020, 14, 176–191. [Google Scholar] [CrossRef]
- Payne, P. Including Pregnant Women in Clinical Research: Practical Guidance for Institutional Review Boards. Ethics Hum. Res. 2019, 41, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Vulnerable Subjects–Pregnant Women Research. Available online: https://research.virginia.edu/irb-hsr/vulnerable-subjects-pregnant-women (accessed on 5 June 2021).
- Niermeyer, S. The Pregnant Altitude Visitor. Adv. Exp. Med. Biol. 1999, 474, 65–77. [Google Scholar] [CrossRef]
- Artal, R.; Fortunato, V.; Welton, A.; Constantino, N.; Khodiguian, N.; Villalobos, L.; Wiswell, R. A Comparison of Cardiopulmonary Adaptations to Exercise in Pregnancy at Sea Level and Altitude. Am. J. Obstet. Gynecol. 1995, 172, 1170–1178. [Google Scholar] [CrossRef]
- Pivarnik, J.M.; Lee, W.; Spillman, T.; Clark, S.L.; Cotton, D.B.; Miller, J.F. Maternal Respiration and Blood Gases during Aerobic Exercise Performed at Moderate Altitude. Med. Sci. Sports Exerc. 1992, 24, 868–872. [Google Scholar] [CrossRef]
- Ballew, C.C. The Effect of High Altitude Hypoxia on Newborn Body Composition in Two Populations in Bolivia. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 1984. [Google Scholar]
- Davenport, M.H.; Steinback, C.D.; Borle, K.J.; Matenchuk, B.A.; Vanden Berg, E.R.; de Freitas, E.M.; Linares, A.M.; O’Halloran, K.D.; Sherpa, M.T.; Day, T.A. Extreme Pregnancy: Maternal Physical Activity at Everest Base Camp. J. Appl. Physiol. 2018, 125, 580. [Google Scholar] [CrossRef] [PubMed]
- Meah, V.L.; Cockcroft, J.R.; Backx, K.; Shave, R.; Stöhr, E.J. Cardiac Output and Related Haemodynamics during Pregnancy: A Series of Meta-Analyses. Heart 2016, 102, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Skow, R.J.; Steinback, C.D. Maternal Responses to Aerobic Exercise in Pregnancy. Clin. Obstet. Gynecol. 2016, 59, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Prather, H.; Spitznagle, T.; Hunt, D. Benefits of Exercise during Pregnancy. PM R 2012, 4, 845–850. [Google Scholar] [CrossRef]
- Khodaee, M.; Grothe, H.L.; Seyfert, J.H.; VanBaak, K. Athletes at High Altitude. Sports Health Multidiscip. Approach 2016, 8, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Muckenthaler, M.U.; Mairbaeurl, H.; Gassmann, M. Iron Metabolism in High-Altitude Residents. J. Appl. Physiol. 2020, 129, 920–925. [Google Scholar] [CrossRef]
- Bobrowski, R.A. Pulmonary Physiology in Pregnancy. Clin. Obstet. Gynecol. 2010, 53, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, L.A.; Ohtake, P.J.; Mottola, M.F.; Mcgrath, M.J. Physiological Interactions between Pregnancy and Aerobic Exercise. Exerc. Sport Sci. Rev. 1989, 17, 295–352. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.K.; Carter, R.; Moran, F.; Banham, S.W. Use of a Combined Oxygen and Carbon Dioxide Transcutaneous Electrode in the Estimation of Gas Exchange during Exercise. Thorax 1993, 48, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, D.; Moore, L.G. Travel to High Altitude during Pregnancy: Frequently Asked Questions and Recommendations for Clinicians. High Alt. Med. Biol. 2012, 13, 73–81. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic Equivalents (METS) in Exercise Testing, Exercise Prescription, and Evaluation of Functional Capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Lotgering, F.; Vandoorn, M.; Struijk, P.; Pool, J.; Wallenburg, H. Maximal Aerobic Exercise in Pregnant-Women–Heart-Rate, O2 Consumption, CO2 Production, and Ventilation. J. Appl. Physiol. 1991, 70, 1016–1023. [Google Scholar] [CrossRef]
- Hesse, C.M.; Tinius, R.A.; Pitts, B.C.; Olenick, A.A.; Blankenship, M.M.; Hoover, D.L.; Maples, J.M. Assessment of Endpoint Criteria and Perceived Barriers during Maximal Cardiorespiratory Fitness Testing among Pregnant Women. J. Sports Med. Phys. Fit. 2017, 58, 1844–1851. [Google Scholar] [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Brun, C.R.; Inglis, S.D.; Charlesworth, S.; Sopper, M.M. o2peak Prediction and Exercise Prescription for Pregnant Women. Med. Sci. Sports Exerc. 2006, 38, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Kardel, K.R. Effects of Intense Training during and after Pregnancy in Top-Level Athletes. Scand. J. Med. Sci. Sports 2005, 15, 79–86. [Google Scholar] [CrossRef]
- Salvesen, K.Å.; Hem, E.; Sundgot-Borgen, J. Fetal Wellbeing May Be Compromised during Strenuous Exercise among Pregnant Elite Athletes. Br. J. Sports Med. 2012, 46, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Kametas, N.A.; Mcauliffe, F.; Krampl, E.; Chambers, J.; Nicolaides, K.H. Maternal Cardiac Function during Pregnancy at High Altitude. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 1051–1058. [Google Scholar] [CrossRef]
- Meah, V.L.; Backx, K.; Cockcroft, J.R.; Shave, R.E.; Stohr, E.J. Cardiac Responses to Submaximal Isometric Contraction and Aerobic Exercise in Healthy Pregnancy. Med. Sci. Sports Exerc. 2021, 53, 1010–1020. [Google Scholar] [CrossRef]
- Jensen, D.; Webb, K.A.; O’donnell, D.E. The Increased Ventilatory Response to Exercise in Pregnancy Reflects Alterations in the Respiratory Control Systems Ventilatory Recruitment Threshold for CO2. Respir. Physiol. Neurobiol. 2010, 171, 75–82. [Google Scholar] [CrossRef]
- Newton, E.R.; May, L. Adaptation of Maternal-Fetal Physiology to Exercise in Pregnancy: The Basis of Guidelines for Physical Activity in Pregnancy. Clin. Med. Insights Women’s Health 2017, 10, 1179562X17693224. [Google Scholar] [CrossRef]
- Moore, L.G.; Jahnigen, D.; Rounds, S.S.; Reeves, J.T.; Grover, R.F. Maternal Hyperventilation Helps Preserve Arterial Oxygenation during High-Altitude Pregnancy. J. Appl. Physiol. 1982, 52, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Cogo, A. The Lung at High Altitude. Multidiscip. Respir. Med. 2011, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Green, L.J.; Mackillop, L.H.; Salvi, D.; Pullon, R.; Loerup, L.; Tarassenko, L.; Mossop, J.; Edwards, C.; Gerry, S.; Birks, J.; et al. Gestation-Specific Vital Sign Reference Ranges in Pregnancy. Obstet. Gynecol. 2020, 135, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Wowdzia, J.B.; Mchugh, T.-L.; Thornton, J.; Sivak, A.; Mottola, M.F.; Davenport, M.H. Elite Athletes and Pregnancy Outcomes: A Systematic Review and Meta-Analysis. Med. Sci. Sports Exerc. 2021, 53, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Beetham, K.S.; Giles, C.; Noetel, M.; Clifton, V.; Jones, J.C.; Naughton, G. The Effects of Vigorous Intensity Exercise in the Third Trimester of Pregnancy: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2019, 19, 281. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Comte, C.; Desseauve, D. Impact of Maternal Physical Activity during an Uncomplicated Pregnancy on Fetal and Neonatal Well-Being Parameters: A Systematic Review of the Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 265–272. [Google Scholar] [CrossRef]
- Durak, E.P.; Jovanovic-Peterson, L.; Peterson, C.M. Comparative Evaluation of Uterine Response to Exercise on Five Aerobic Machines. Am. J. Obstet. Gynecol. 1990, 162, 754–756. [Google Scholar] [CrossRef]
- Juhl, M.; Andersen, P.K.; Olsen, J.; Madsen, M.; Jørgensen, T.; Nøhr, E.A.; Andersen, A.-M.N. Physical Exercise during Pregnancy and the Risk of Preterm Birth: A Study within the Danish National Birth Cohort. Am. J. Epidemiol. 2008, 167, 859–866. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McManis, B.G. Integrative Review of Exercise at Altitude during Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 9272. https://doi.org/10.3390/ijerph18179272
McManis BG. Integrative Review of Exercise at Altitude during Pregnancy. International Journal of Environmental Research and Public Health. 2021; 18(17):9272. https://doi.org/10.3390/ijerph18179272
Chicago/Turabian StyleMcManis, Beth G. 2021. "Integrative Review of Exercise at Altitude during Pregnancy" International Journal of Environmental Research and Public Health 18, no. 17: 9272. https://doi.org/10.3390/ijerph18179272






