Interactions of Comorbidity and Five Simple Environmental Unhealthy Habits Concerning Physical and Mental Quality of Life in the Clinical Setting
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Health Organization. Quality of Life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Mujica-Mota, R.E.; Roberts, M.; Abel, G.; Elliott, M.; Lyratzopoulos, G.; Roland, M.; Campbell, J. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: Evidence from a national survey. Qual. Life Res. 2015, 24, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Bolge, S.C.; Doan, J.F.; Kannan, H.; Baran, R.W. Association of insomnia with quality of life, work productivity, and activity impairment. Qual. Life Res. 2009, 18, 415–422. [Google Scholar] [CrossRef]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vázquez-Ruiz, Z.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; et al. PREDIMED-PLUS Study Investigators. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef] [Green Version]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- The World Health Organization. Quality of Life Assessment (WHOQOL): Development and general psychometric properties. Soc. Sci. Med. 1998, 46, 1569–1585. [Google Scholar] [CrossRef]
- Phyo, A.Z.Z.; Freak-Poli, R.; Craig, H.; Gasevic, D.; Stocks, N.P.; Gonzalez-Chica, D.A.; Ryan, J. Quality of life and mortality in the general population: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1596. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Njai, R.; Barker, L.; Siegel, P.Z.; Liao, Y. Summarizing health-related quality of life (HRQOL): Development and testing of a one-factor model. Popul. Health Metr. 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Cuevillas, B.; Álvarez Álvarez, I.; Cuervo, M.; Fernández Montero, A.; Navas Carretero, S.; Martínez, J.A. Definition of nutritionally qualitative categorizing (proto)nutritypes and a pilot quantitative nutrimeter for mirroring nutritional well-being based on a quality of life health related questionnaire. Nutr. Hosp. 2019, 36, 862–874. [Google Scholar] [PubMed] [Green Version]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Bjorner, J.B.; Lyng Wolden, M.; Gundgaard, J.; Miller, K.A. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health 2013, 16, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Daneshi, F.; Behzadmehr, R.; Rafiemanesh, H.; Bouya, S.; Raeisi, M. Quality of life of chronic heart failure patients: A systematic review and meta-analysis. Heart Fail. Rev. 2020, 25, 993–1006. [Google Scholar] [CrossRef]
- Bunevicius, A. Reliability and validity of the SF-36 Health Survey Questionnaire in patients with brain tumors: A cross-sectional study. Health Qual. Life Outcomes 2017, 15, 92. [Google Scholar] [CrossRef]
- Boussoffara, L.; Keskes Boudawara, N.; Loukil, M.; Touil, I.; Knani, J. Contrôle de l’asthme et qualité de vie [Asthma control and quality of life]. Rev. Pneumol. Clin. 2017, 73, 225–230. [Google Scholar] [CrossRef]
- Rogan, A.; McCarthy, K.; McGregor, G.; Hamborg, T.; Evans, G.; Hewins, S.; Aldridge, N.; Fletcher, S.; Krishnan, N.; Higgins, R.; et al. Quality of life measures predict cardiovascular health and physical performance in chronic renal failure patients. PLoS ONE 2017, 12, e0183926. [Google Scholar]
- Makovski, T.T.; Schmitz, S.; Zeegers, M.P.; Stranges, S.; van den Akker, M. Multimorbidity and quality of life: Systematic literature review and meta-analysis. Ageing Res. Rev. 2019, 53, 100903. [Google Scholar] [CrossRef] [PubMed]
- González-Chica, D.A.; Hill, C.L.; Gill, T.K.; Hay, P.; Haag, D.; Stocks, N. Individual diseases or clustering of health conditions? Association between multiple chronic diseases and health-related quality of life in adults. Health Qual. Life Outcomes 2017, 15, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itani, L.; Calugi, S.; Kreidieh, D.; El Kassas, G.; El Masri, D.; Tannir, H.; Dalle Grave, R.; Harfoush, A.; El Ghoch, M. Validation of an Arabic Version of the Obesity-Related Wellbeing (ORWELL 97) Questionnaire in Adults with Obesity. Curr. Diabetes Rev. 2019, 15, 127–132. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Khalesi, S.; Irwin, C.; Sun, J. Dietary Patterns, Nutrition Knowledge, Lifestyle, and Health-Related Quality of Life: Associations with Anti-Hypertension Medication Adherence in a Sample of Australian Adults. High Blood Press Cardiovasc. Prev. 2017, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Daimiel, L.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Schröder, H.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; Lopez-Miranda, J.; et al. Physical fitness and physical activity association with cognitive function and quality of life: Baseline cross-sectional analysis of the PREDIMED-Plus trial. Sci. Rep. 2020, 10, 3472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L.; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [PubMed] [Green Version]
- Llewellyn, A.M.; Skevington, S.M. Using guided individualised feedback to review self-reported quality of life in health and its importance. Psychol. Health 2015, 30, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Espallargues, M.; Valderas, J.M.; Alonso, J. Provision of feedback on perceived health status to health care professionals: A systematic review of its impact. Med. Care 2000, 38, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Bullinger, M.; Alonso, J.; Apolone, G.; Leplège, A.; Sullivan, M.; Wood-Dauphinee, S.; Gandek, B.; Wagner, A.; Aaronson, N.; Bech, P.; et al. Translating health status questionnaires and evaluating their quality: The IQOLA Project approach. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 913–923. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. PREDIMED Study Investigators. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Jomaa, L.; Hwalla, N.; Itani, L.; Chamieh, M.C.; Mehio-Sibai, A.; Naja, F. A Lebanese dietary pattern promotes better diet quality among older adults: Findings from a national cross-sectional study. BMC Geriatr. 2016, 16, 85. [Google Scholar] [CrossRef] [Green Version]
- Greenhalgh, J.; Meadows, K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: A literature review. J. Eval. Clin. Pract. 1999, 5, 401–416. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef] [Green Version]
- Fortin, M.; Lapointe, L.; Hudon, C.; Vanasse, A.; Ntetu, A.L.; Maltais, D. Multimorbidity and quality of life in primary care: A systematic review. Health Qual. Life Outcomes 2004, 2, 51. [Google Scholar] [CrossRef] [Green Version]
- Vilagut, G.; Ferrer, M.; Rajmil, L.; Rebollo, P.; Permanyer-Miralda, G.; Quintana, J.M.; Santed, R.; Valderas, J.M.; Ribera, A.; Domingo-Salvany, A.; et al. El Cuestionario de Salud SF-36 español: Una década de experiencia y nuevos desarrollos (The Spanish version of the Short Form 36 Health Survey: A decade of experience and new developments). Gac. Sanit. 2005, 19, 135–150. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Villar, R.; Martinez-Urbistondo, D.; Fernandez, M.A.; Lopez-Cano, M.; Fernandez, E.; Dominguez, A.; Prosper, L.; Rodriguez-Cobo, A.; Tinoco, M.E.C.; Nadal, P.; et al. Cross-sectional evaluation of the interaction between activity relative-time expenditure and comorbidity concerning physical quality of life. Medicine 2020, 99, e22552. [Google Scholar] [CrossRef]
- Puciato, D.; Borysiuk, Z.; Rozpara, M. Quality of life and physical activity in an older working-age population. Clin. Interv. Aging 2017, 12, 1627–1634. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, M.; Danovitch, I.; IsHak, W.W. Quality of life and smoking. Am. J. Addict. 2014, 23, 540–562. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bulló, M.; Martínez-González, M.Á.; Ros, E.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Wärnberg, J.; Fiol, M.; et al. PREDIMED study group. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Rico-Campà, A.; Martínez-González, M.A.; Alvarez-Alvarez, I.; Mendonça, R.D.; de la Fuente-Arrillaga, C.; Gómez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef] [Green Version]
- Fresán, U.; Gea, A.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Martínez-Gonzalez, M.A. Substitution Models of Water for Other Beverages, and the Incidence of Obesity and Weight Gain in the SUN Cohort. Nutrients 2016, 8, 688. [Google Scholar] [CrossRef] [Green Version]
- Hallit, S.; Hajj, A.; Sacre, H.; Al Karaki, G.; Malaeb, D.; Kheir, N.; Salameh, P.; Hallit, R. Impact of Sleep Disorders and Other Factors on the Quality of Life in General Population: A Cross-Sectional Study. J. Nerv. Ment. Dis. 2019, 207, 333–339. [Google Scholar] [CrossRef]
- Vogl, M.; Wenig, C.M.; Leidl, R.; Pokhrel, S. Smoking and health-related quality of life in English general population: Implications for economic evaluations. BMC Public Health 2012, 12, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pano, O.; Sayón-Orea, C.; Gea, A.; Bes-Rastrollo, M.; Martínez-González, M.Á.; Martínez, J.A. Nutritional Determinants of Quality of Life in a Mediterranean Cohort: The SUN Study. Int. J. Environ. Res. Public Health 2020, 17, 3897. [Google Scholar] [CrossRef] [PubMed]
- Barcones-Molero, M.F.; Sánchez-Villegas, A.; Martínez-González, M.A.; Bes-Rastrollo, M.; Martínez-Urbistondo, M.; Santabárbara, J.; Martínez, J.A. The influence of obesity and weight gain on quality of life according to the SF-36 for individuals of the dynamic follow-up cohort of the University of Navarra. Rev. Clin. Esp. 2018, 218, 408–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.Á.; Ordovas, J.M.; Martínez, J.A. Contribution of macronutrients to obesity: Implications for precision nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Pranata, S.; Wu, S.V.; Alizargar, J.; Liu, J.H.; Liang, S.Y.; Lu, Y.Y. Precision Health Care Elements, Definitions, and Strategies for Patients with Diabetes: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 6535. [Google Scholar] [CrossRef] [PubMed]
- Taberna, D.J.; Navas-Carretero, S.; Martinez, J.A. Current nutritional status assessment tools for metabolic care and clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 323–328. [Google Scholar] [CrossRef] [PubMed]
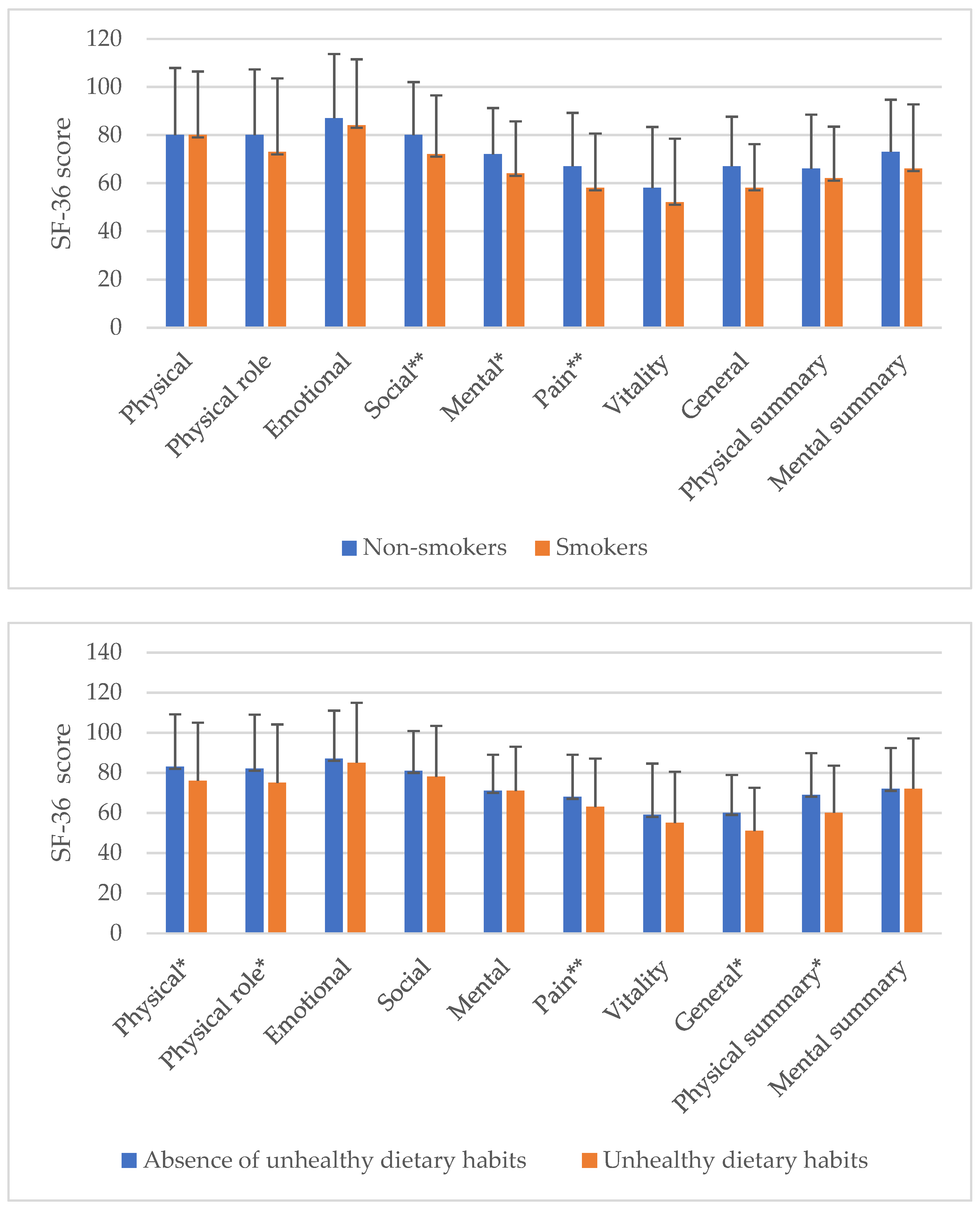
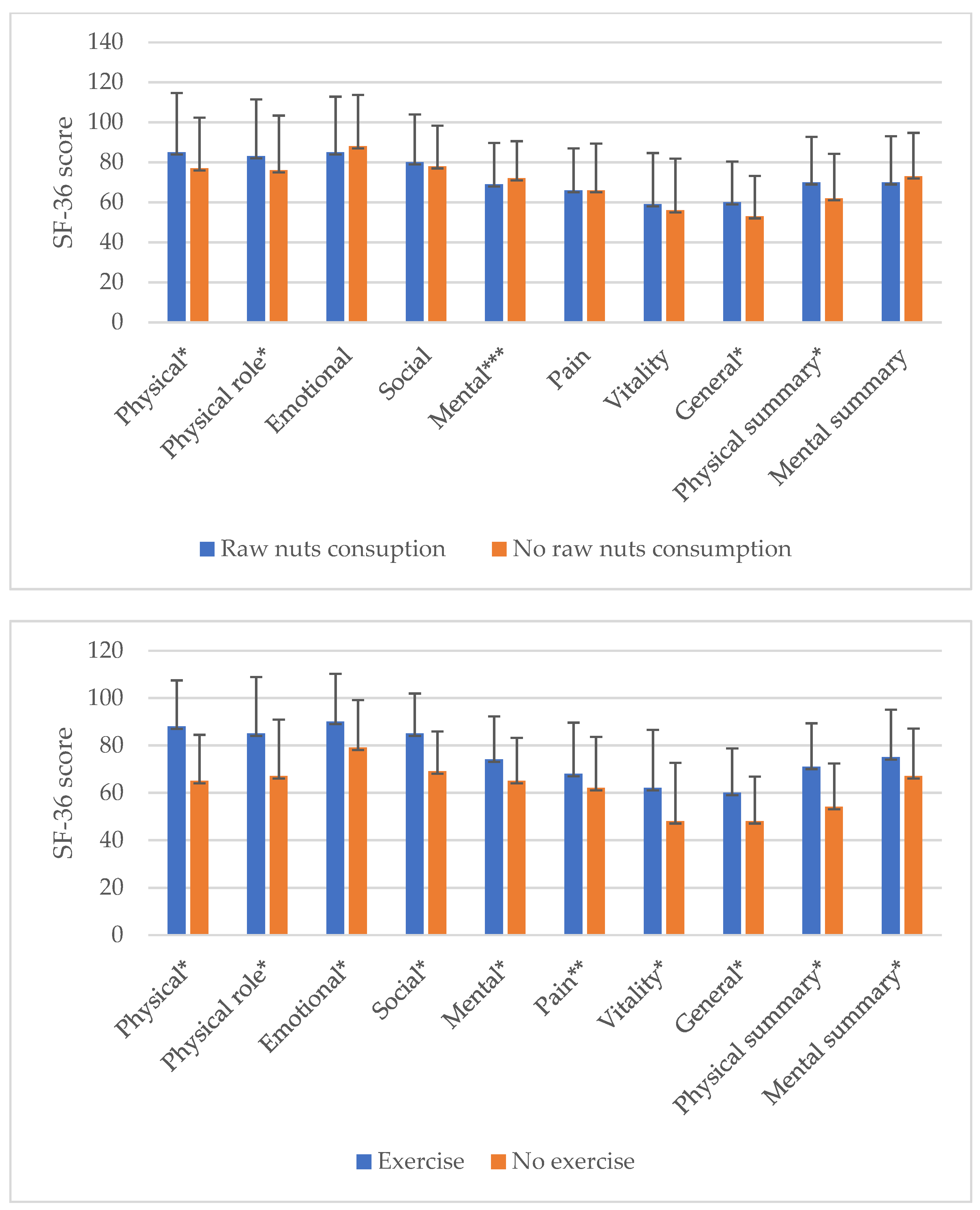
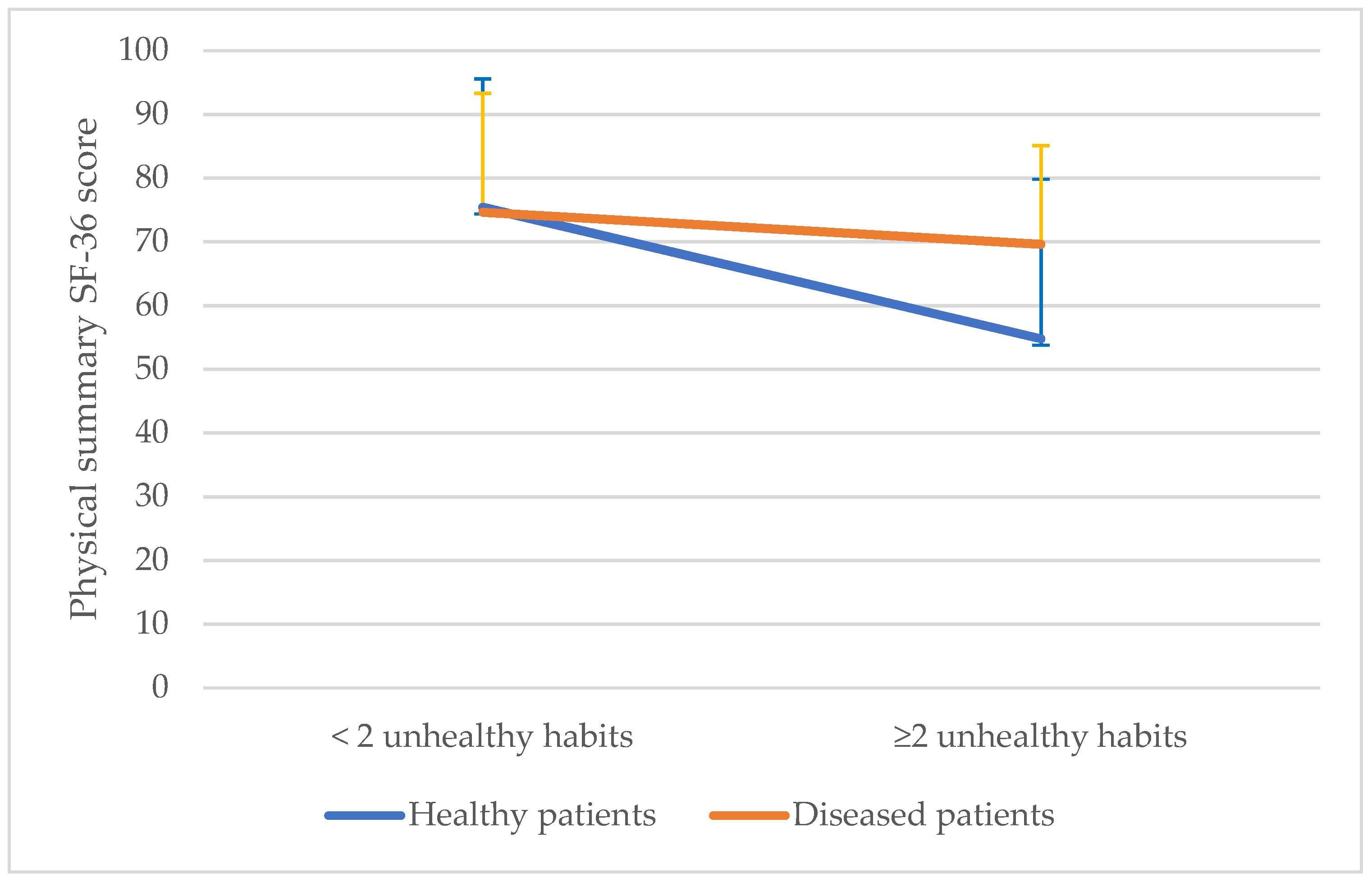
| Variables | Mean (SD) or n (%) |
|---|---|
| Age (years) | 55.3 (17.5) |
| Female sex (%) | 138 (52.70) |
| Comorbidity | |
| Myocardial infarction (%) | 13 (5.00) |
| Heart failure (%) | 6 (2.30) |
| Peripheral artery disease (%) | 18 (6.90) |
| Cerebrovascular disease (%) | 19 (7.30) |
| Diabetes mellitus (%) | 23 (8.80) |
| COPD (%) | 20 (7.60) |
| Mild dementia (%) | 6 (2.30) |
| Renal insufficiency (%) | 8 (3.10) |
| Liver disease (%) | 17 (6.50) |
| Tumoral disease (%) | 25 (9.50) |
| Depression (%) | 30 (11.50) |
| CCI (points) | 0.91 (1.39) |
| Unhealthy habits | |
| Smoker (%) | 32 (12.20) |
| Unhealthy dietary habits (%) | 109 (41.60) |
| No raw nuts consumption (%) | 146 (55.70) |
| Absence of physical activity (%) | 89 (34.00) |
| Sleep time less than 7 h (%) | 204 (77.90) |
| Unhealthy habits (aggregated) | |
| 4 or more habits (%) | 29 (11.10) |
| 3 habits (%) | 70 (26.70) |
| 2 habits (%) | 97 (37.00) |
| 0 or 1 habit (%) | 66 (25.20) |
| SF-36 subscales | |
| Physical QoL | 79.88 (25.73) |
| Physical role | 79.17 (26.01) |
| Emotional QoL | 86,26 (22.17) |
| Mental QoL | 70.74 (19.71) |
| Social QoL | 79.48 (26.76) |
| Vitality | 57.18 (22.00) |
| Pain | 65.88 (25.54) |
| General QoL | 56.01 (20.04) |
| SF-36 summaries | |
| Physical component of SF-36 | 65.31 (22.44) |
| Mental component of SF-36 | 71.91 (22.46) |
| SF-36 Subscales | Unhealthy Habits | Mean (SD) | p |
|---|---|---|---|
| Physical QoL | ≥4 habits | 59.31 (32.23) | <0.001 |
| 3 habits | 75.50 (23.68) | ||
| 2 habits | 82.40 (22.66) | ||
| ≤1 habits | 91.00 (22.36) | ||
| Physical role | ≥4 habits | 57.97 (35.27) | <0.001 |
| 3 habits | 74.55 (26.14) | ||
| 2 habits | 82.88 (23.91) | ||
| ≤1 habits | 88.65 (16.68) | ||
| Emotional QoL | ≥4 habits | 76.15 (28.32) | <0.001 |
| 3 habits | 89.64 (20.63) | ||
| 2 habits | 82.47 (24.29) | ||
| ≤1 habits | 93.08 (12.97) | ||
| Mental QoL | ≥4 habits | 60.17 (20.15) | <0.001 |
| 3 habits | 72.14 (21.65) | ||
| 2 habits | 69.58 (20.10) | ||
| ≤1 habits | 75.77 (14.56) | ||
| Social QoL | ≥4 habits | 62.93 (31.07) | 0.004 |
| 3 habits | 78.39 (29.86) | ||
| 2 habits | 79.82 (24.89) | ||
| ≤1 habits | 87.88 (19.64) | ||
| Vitality | ≥4 habits | 45.26 (23.48) | <0.001 |
| 3 habits | 51.61 (23.30) | ||
| 2 habits | 59.83 (20.57) | ||
| ≤1 habits | 64.81 (18.41) | ||
| Pain | ≥4 habits | 56.09 (22.33) | 0.043 |
| 3 habits | 62.83 (29.08) | ||
| 2 habits | 67.45 (23.89) | ||
| ≤1 habits | 70.86 (24.12) | ||
| General QoL | ≥4 habits | 36.55 (18.71) | <0.001 |
| 3 habits | 51.86 (20.22) | ||
| 2 habits | 58.65 (18.59) | ||
| ≤1 habits | 65.00 (15.69) | ||
| Physical SF-36 | ≥4 habits | 45.31 (24.81) | <0.001 |
| 3 habits | 58.73 (23.01) | ||
| 2 habits | 69.88 (19.53) | ||
| ≤1 habits | 74.89 (16.99) | ||
| Mental SF-36 | ≥4 habits | 63.51 (26.85) | 0.019 |
| 3 habits | 74.34 (23.98) | ||
| 2 habits | 69.24 (23.02) | ||
| ≤1 habits | 77.13 (14.89) |
| SF-36 Subscales | Beta for Unhealthy Habits -Per Habit- (SE) | R2 for the Model | p |
|---|---|---|---|
| Physical QoL | −0.243 (1.407) | 0.366 | <0.001 |
| Physical role | −0.295 (1.648) | 0.158 | <0.001 |
| Emotional QoL | −0.149 (1.435) | 0.123 | 0.016 |
| Mental QoL | −0.142 (1.278) | 0.115 | 0.022 |
| Social QoL | −0.179 (1.720) | 0.134 | 0.004 |
| Vitality | −0.254 (1.431) | 0.121 | <0.001 |
| Pain | −0.152 (1.654) | 0.101 | <0.001 |
| General QoL | −0.317 (1.199) | 0.253 | <0.001 |
| Physical SF-36 | −0.313 (1.280) | 0.310 | <0.001 |
| Mental SF-36 | −0.118 (1.472) | 0.096 | 0.060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Urbistondo, D.; Suarez del Villar, R.; Ramos-Lopez, O.; Fernández, M.A.; Segovia, R.C.; Domínguez, A.; de la Garza, R.G.; Gómez, M.L.-C.; Ramos, L.P.; San-Cristobal, R.; et al. Interactions of Comorbidity and Five Simple Environmental Unhealthy Habits Concerning Physical and Mental Quality of Life in the Clinical Setting. Int. J. Environ. Res. Public Health 2021, 18, 9590. https://doi.org/10.3390/ijerph18189590
Martínez-Urbistondo D, Suarez del Villar R, Ramos-Lopez O, Fernández MA, Segovia RC, Domínguez A, de la Garza RG, Gómez ML-C, Ramos LP, San-Cristobal R, et al. Interactions of Comorbidity and Five Simple Environmental Unhealthy Habits Concerning Physical and Mental Quality of Life in the Clinical Setting. International Journal of Environmental Research and Public Health. 2021; 18(18):9590. https://doi.org/10.3390/ijerph18189590
Chicago/Turabian StyleMartínez-Urbistondo, Diego, Rafael Suarez del Villar, Omar Ramos-Lopez, María Agud Fernández, Ramón Costa Segovia, Andrea Domínguez, Rocío García de la Garza, María López-Cano Gómez, Laura Prósper Ramos, Rodrigo San-Cristobal, and et al. 2021. "Interactions of Comorbidity and Five Simple Environmental Unhealthy Habits Concerning Physical and Mental Quality of Life in the Clinical Setting" International Journal of Environmental Research and Public Health 18, no. 18: 9590. https://doi.org/10.3390/ijerph18189590
APA StyleMartínez-Urbistondo, D., Suarez del Villar, R., Ramos-Lopez, O., Fernández, M. A., Segovia, R. C., Domínguez, A., de la Garza, R. G., Gómez, M. L.-C., Ramos, L. P., San-Cristobal, R., Daimiel, L., Fernández, P. V., & Martinez, J. A. (2021). Interactions of Comorbidity and Five Simple Environmental Unhealthy Habits Concerning Physical and Mental Quality of Life in the Clinical Setting. International Journal of Environmental Research and Public Health, 18(18), 9590. https://doi.org/10.3390/ijerph18189590






