Maternal Stressful Life Events during Pregnancy and Atopic Dermatitis in Children Aged Approximately 4–6 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Assessment of Exposure
2.3. Assessment of Outcomes
2.4. Covariates
2.5. Statistical Analysis
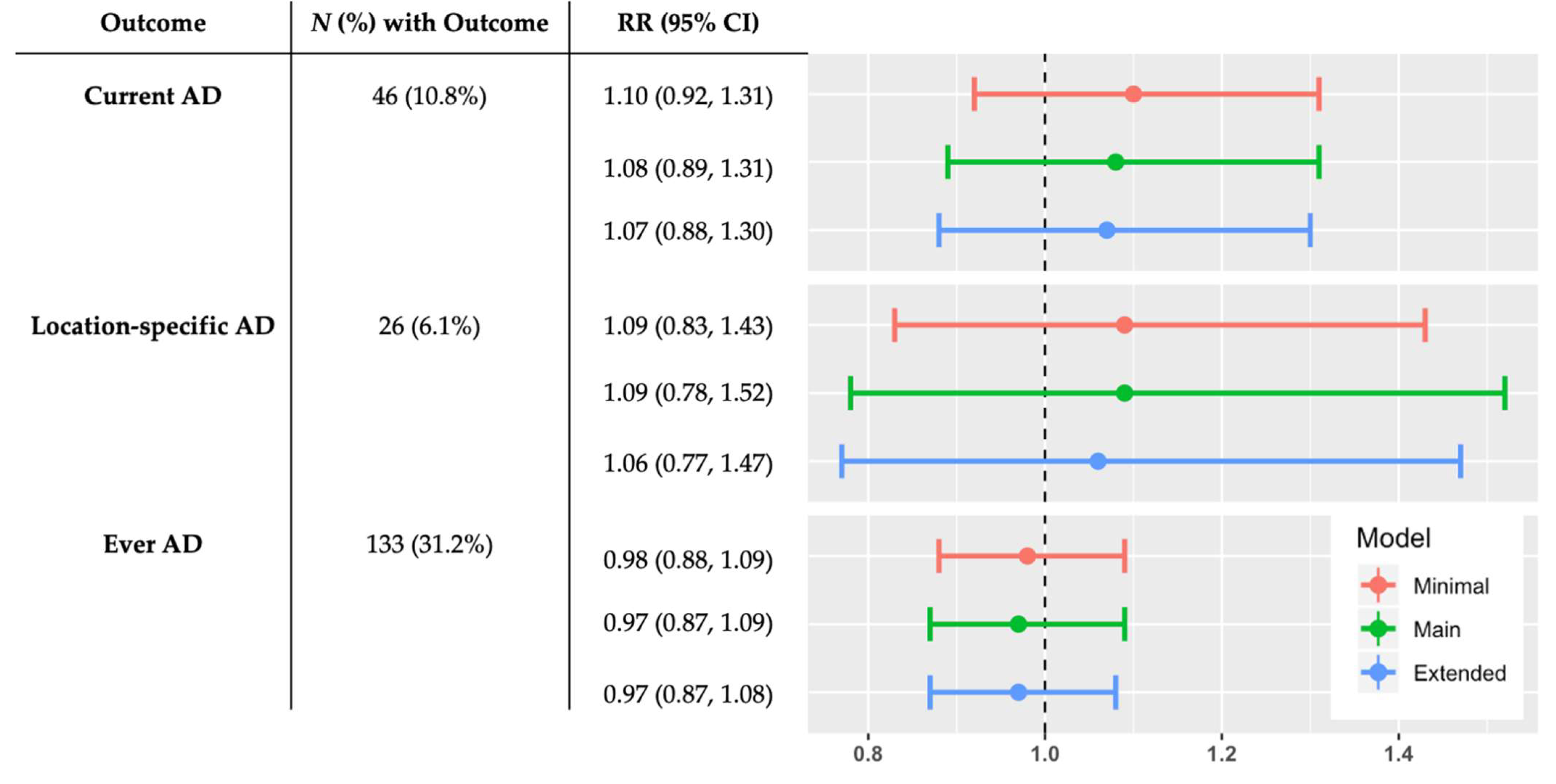
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Drucker, A.M.; Wang, A.R.; Li, W.-Q.; Sevetson, E.; Block, J.; Qureshi, A. The burden of atopic dermatitis: Summary of a report for the National Eczema Asso-ciation. J. Invest. Dermatol. 2017, 137, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-J.; Hsieh, W.-S.; Wu, K.-Y.; Guo, Y.L.; Hwang, Y.-H.; Jee, S.-H.; Chen, P.-C. Effect of gestational smoke exposure on atopic dermatitis in the offspring. Pediatr. Allergy Immunol. 2008, 19, 580–586. [Google Scholar] [CrossRef]
- Gardner, K.G.; Gebretsadik, T.; Hartman, T.J.; Rosa, M.J.; Tylavsky, F.A.; Adgent, M.A.; Moore, P.E.; Kocak, M.; Bush, N.R.; Davis, R.L.; et al. Prenatal omega-3 and omega-6 polyunsaturated fatty acids and childhood atopic dermatitis. J. Allergy Clin. Immunol. Pr. 2019, 8, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kim, E.M.; Lee, M.Y.; Jung, S.; Cho, H.-J.; Kim, Y.; Choi, Y.J.; Lee, E.; Yang, S.-I.; Lee, S.-Y.; et al. Perinatal maternal negative life events as risk factors of atopic dermatitis in female offspring. Ann. Allergy Asthma Immunol. 2018, 121, 641–642.e1. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Pesce, G.; Girardi, P.; Marchetti, P.; Rava, M.; Ricci, P.; Marcon, A. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Eur. Respir. J. 2012, 40 (Suppl. 56), 724–729. [Google Scholar] [CrossRef]
- Hartwig, I.R.; Sly, P.; Schmidt, L.A.; van Lieshout, R.J.; Bienenstock, J.; Holt, P.G.; Arck, P.C. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J. Allergy Clin. Immunol. 2014, 134, 160–169.e7. [Google Scholar] [CrossRef] [PubMed]
- Gitau, R.; Cameron, A.; Fisk, N.; Glover, V. Fetal exposure to maternal cortisol. Lancet 1998, 352, 707–708. [Google Scholar] [CrossRef]
- Chang, H.Y.; Suh, D.I.; Yang, S.-I.; Kang, M.-J.; Lee, S.-Y.; Lee, E.; Choi, I.A.; Lee, K.-S.; Shin, Y.J.; Shin, Y.H.; et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J. Allergy Clin. Immunol. 2016, 138, 468–475.e5. [Google Scholar] [CrossRef]
- Dahlerup, B.R.; Egsmose, E.L.; Siersma, V.; Mortensen, E.L.; Hedegaard, M.; Knudsen, L.E.; Mathiesen, L. Maternal stress and placental function, a study using questionnaires and bi-omarkers at birth. PLoS ONE 2018, 13, e0207184. [Google Scholar]
- Larsen, A.D.; Schlunssen, V.; Christensen, B.H.; Bonde, J.; Obel, C.; Thulstrup, A.M.; Hannerz, H.; Hougaard, K.S. Exposure to psychosocial job strain during pregnancy and odds of asthma and atopic dermatitis among 7-year old children—A prospective cohort study. Scand. J. Work. Environ. Heal. 2014, 40, 639–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elbert, N.J.; Duijts, L.; Dekker, H.T.D.; De Jong, N.W.; Nijsten, T.E.; Jaddoe, V.W.; De Jongste, J.C.; Van Wijk, R.G.; Tiemeier, H.; Pasmans, S.G. Maternal psychiatric symptoms during pregnancy and risk of childhood atopic diseases. Clin. Exp. Allergy 2017, 47, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, N.L.; Kozyrskyj, A.L.; Cosic, N.; Ntanda, H.N.; Anis, L.; Hart, M.J.; Campbell, T.S.; Giesbrecht, G.F. Maternal sensitivity and social support protect against childhood atopic der-matitis. Allergy Asthma Clin. Immunol. 2017, 13, 26. [Google Scholar] [CrossRef]
- Braig, S.; Weiss, J.; Stalder, T.; Kirschbaum, C.; Rothenbacher, D.; Genuneit, J. Maternal prenatal stress and child atopic dermatitis up to age 2 years: The Ulm SPATZ health study. Pediatr. Allergy Immunol. 2016, 28, 144–151. [Google Scholar] [CrossRef]
- Kawaguchi, C.; Murakami, K.; Obara, T.; Ishikuro, M.; Ueno, F.; Noda, A.; Kuriyama, S. Maternal psychological distress during and after pregnancy and atopic dermatitis in children. Eur. J. Public Heal. 2020, 30, ckaa166.972. [Google Scholar] [CrossRef]
- Sausenthaler, S.; Rzehak, P.; Chen, C.M.; Arck, P.; Bockelbrink, A.; Schäfer, T.; Schaaf, B.; Borte, M.; Herbarth, O.; Krämer, U.; et al. Stress-related maternal factors during pregnancy in relation to childhood eczema: Results from the LISA study. J. Investig. Allergol. Clin. Immunol. 2009, 19, 481–487. [Google Scholar]
- Illi, S.; von Mutius, E.; Lau, S.; Nickel, R.; Grüber, C.; Niggemann, B.; Wahn, U. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J. Allergy Clin. Immunol. 2004, 113, 925–931. [Google Scholar] [CrossRef]
- Carpenter, T.; Grecian, S.M.; Reynolds, R.M. Sex differences in early-life programming of the hypothalamic–pituitary–adrenal axis in humans suggest increased vulnerability in females: A systematic review. J. Dev. Orig. Heal. Dis. 2017, 8, 244–255. [Google Scholar] [CrossRef]
- Sutherland, S.; Brunwasser, S.M. Sex differences in vulnerability to prenatal stress: A review of the recent literature. Curr. Psychiatry Rep. 2018, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Wen, H.; Chiang, T.L.; Lin, S.J.; Guo, Y.L. Maternal psychologic problems increased the risk of childhood atopic dermatitis. Pediatr. Allergy Immunol. 2016, 27, 169–176. [Google Scholar] [CrossRef]
- Seely, H.D.; Mickelson, K. Maternal resilience as a protective factor between financial stress and child outcomes. J. Fam. Issues 2019, 40, 1604–1626. [Google Scholar] [CrossRef]
- Verner, G.; Epel, E.; Lahti-Pulkkinen, M.; Kajantie, E.; Buss, C.; Lin, J.; Blackburn, E.; Räikkönen, K.; Wadhwa, P.D.; Entringer, S. Maternal psychological resilience during pregnancy and newborn telomere length: A prospective study. Am. J. Psychiatry 2021, 178, 183–192. [Google Scholar] [CrossRef]
- Whitehead, N.S.; Brogan, D.J.; Blackmore-Prince, C.; Hill, H.A. Correlates of experiencing life events just before or during pregnancy. J. Psychosom. Obstet. Gynecol. 2003, 24, 77–86. [Google Scholar] [CrossRef]
- Ahluwalia, I.B.; Helms, K.; Morrow, B. Assessing the validity and reliability of three indicators self-reported on the pregnancy risk assessment monitoring system survey. Public Heal. Rep. 2013, 128, 527–536. [Google Scholar] [CrossRef]
- Bush, N.R.; Savitz, J.; Coccia, M.; Jones-Mason, K.; Adler, N.; Boyce, W.T.; Laraia, B.; Epel, E. Maternal stress during pregnancy predicts infant infectious and noninfectious illness. J. Pediatr. 2020, 228, 117–125.e2. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Sinclair, V.G.; Wallston, K.A. The development and psychometric evaluation of the brief resilient coping scale. Assessment 2004, 11, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Kockum, I.; Söderhäll, C.; van Hage-Hamsten, M.; Luthman, H.; Nordenskjöld, M.; Wahlgren, C.F. Characterization by phenotype of families with atopic dermatitis. Acta Derm. Venereol. 2000, 80, 106–110. [Google Scholar] [PubMed]
- Wen, H.-J.; Wang, Y.-J.; Lin, Y.-C.; Chang, C.-C.; Shieh, C.-C.; Lung, F.-W.; Guo, Y.L. Prediction of atopic dermatitis in 2-yr-old children by cord blood IgE, genetic polymorphisms in cytokine genes, and maternal mentality during pregnancy. Pediatr. Allergy Immunol. 2011, 22, 695–703. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Karvonen, A.M.; Renz, H.; Braun-Fahrländer, C.; Schmausser-Hechfellner, E.; Pekkanen, J.; Riedler, J.; Dalphin, J.-C.; et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr. 2017, 171, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Bockelbrink, A.; Heinrich, J.; Schäfer, I.; Zutavern, A.; Borte, M.; Herbarth, O.; Schaaf, B.; Von Berg, A.; LISA Study Group. Atopic eczema in children: Another harmful sequel of divorce. Allergy 2006, 61, 1397–1402. [Google Scholar] [CrossRef]
- Shaw, T.E.; Currie, G.P.; Koudelka, C.W.; Simpson, E.L. Eczema prevalence in the United States: Data from the 2003 National Survey of Children’s Health. J. Investig. Dermatol. 2011, 131, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.R.; Farr, S.L.; Howards, P.P. Stressful life events experienced by women in the year before their infants’ births—United States, 2000–2010. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 247–251. [Google Scholar] [PubMed]
- Hogue, C.J.R.; Parker, C.B.; Willinger, M.; Temple, J.R.; Bann, C.; Silver, R.M.; Dudley, N.J.; Koch, M.A.; Coustan, D.R.; Stoll, B.J.; et al. A population-based case-control study of stillbirth: The relationship of significant life events to the racial disparity for African Americans. Am. J. Epidemiolog. 2013, 177, 755–767. [Google Scholar] [CrossRef]
- Lu, M.C.; Chen, B. Racial and ethnic disparities in preterm birth: The role of stressful life events. Am. J. Obstet. Gynecol. 2004, 191, 691–699. [Google Scholar] [CrossRef]
- Salm Ward, T.; Kanu, F.A.; Robb, S.W. Prevalence of stressful life events during pregnancy and its association with postpartum depressive symptoms. Arch. Womens Ment. Health 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Krinsley, K.E.; Gallagher, J.G.; Weathers, F.W.; Kutter, C.J.; Kaloupek, D.G. Consistency of retrospective reporting about exposure to traumatic events. J. Trauma. Stress 2003, 16, 399–409. [Google Scholar] [CrossRef]
- Garg, N.; Silverberg, J.I. Epidemiology of childhood atopic dermatitis. Clin. Dermatol. 2015, 33, 281–288. [Google Scholar] [CrossRef]
| Characteristic | Overall N = 426 | 0 Prenatal SLEs 1 N = 158 | ≥1 Prenatal SLEs N = 268 |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Child age (years) | 5.5 (5.1–6.1) | 5.5 (5.1–6.0) | 5.5 (5.2–6.1) |
| Maternal age (years) | 31.0 (27.0–34.0) | 32.0 (28.0–35.0) | 30.5 (27.0–34.0) |
| Missing, N (%) | 4 (0.9%) | 2 (1.3%) | 2 (0.7%) |
| Maternal current stress (score on PSS) 2 | 9.0 (7.0–11.0) | 8.0 (6.3–10.0) | 9.0 (8.0–11.0) |
| Child sex | N (%) | N (%) | N (%) |
| Male | 208 (48.8) | 74 (46.8) | 134 (50.0) |
| Female | 218 (51.2) | 84 (53.2) | 134 (50.0) |
| Maternal education | |||
| <High school degree | 15 (3.5) | 2 (1.3) | 13 (4.9) |
| High school graduate or GED | 39 (9.2) | 9 (5.7) | 30 (11.2) |
| Some college or technical/ vocational school graduate | 119 (27.9) | 39 (24.7) | 80 (29.9) |
| ≥College degree | 240 (56.3) | 104 (65.8) | 136 (50.7) |
| Missing | 13 (3.1) | 4 (2.5) | 9 (3.3) |
| Prenatal household income | |||
| <USD 50,000 | 129 (30.3) | 34 (21.5) | 95 (35.6) |
| USD 50,000–79,999 | 84 (19.7) | 40 (25.3) | 44 (16.4) |
| ≥USD 80,000 | 180 (42.3) | 76 (48.1) | 104 (38.8) |
| Missing | 33 (7.7) | 8 (5.1) | 25 (9.3) |
| Maternal history of atopy | |||
| No | 245 (57.5) | 95 (60.1) | 150 (56.0) |
| Yes | 181 (42.5) | 63 (39.9) | 118 (44.0) |
| Paternal history of atopy | |||
| No | 291 (68.3) | 114 (72.2) | 177 (66.0) |
| Yes | 135 (31.7) | 44 (27.8) | 91 (34.0) |
| Maternal report of race | |||
| White | 341 (80.0) | 136 (86.1) | 205 (76.4) |
| Black or African American | 8 (1.9) | 0 (0) | 8 (3.0) |
| Asian | 12 (2.8) | 4 (2.5) | 8 (3.0) |
| American Indian/Alaskan Native | 4 (0.9) | 0 (0) | 4 (1.5) |
| Multiple Race | 33 (7.8) | 9 (5.7) | 24 (9.0) |
| Other | 12 (2.8) | 4 (2.5) | 8 (3.0) |
| Missing | 16 (3.8) | 5 (3.2) | 11 (4.1) |
| Maternal report of ethnicity | |||
| Not Hispanic/Latino | 363 (85.2) | 142 (89.9) | 221 (82.5) |
| Hispanic/Latino | 60 (14.1) | 16 (10.1) | 44 (16.4) |
| Missing | 3 (0.7) | 0 (0) | 3 (1.1) |
| Other children living in home | |||
| No | 52 (12.2) | 19 (12.0) | 33 (12.3) |
| Yes | 374 (87.8) | 139 (88.0) | 235 (87.7) |
| Recruitment site 3 | |||
| Seattle | 206 (48.4) | 78 (49.4) | 128 (47.8) |
| Yakima | 220 (51.6) | 80 (50.6) | 140 (52.2) |
| Prenatal farm animal exposure | |||
| No | 400 (93.9) | 149 (94.3) | 251 (93.7) |
| Yes | 25 (5.9) | 9 (5.7) | 16 (6.0) |
| Missing | 1 (0.2) | 0 (0) | 1 (0.3) |
| Prenatal cat or dog ownership | |||
| No | 183 (43.0) | 67 (42.4) | 116 (43.3) |
| Yes | 243 (57.0) | 91 (57.6) | 152 (56.7) |
| Prenatal smoking | |||
| No | 401 (94.1) | 150 (94.9) | 251 (93.7) |
| Yes | 12 (2.8) | 3 (1.9) | 9 (3.3) |
| Missing | 13 (3.1) | 5 (3.2) | 8 (3.0) |
| Delivery type | |||
| Vaginal | 272 (63.8) | 102 (64.6) | 170 (63.4) |
| Cesarean | 154 (36.2) | 56 (35.4) | 98 (36.6) |
| Breastfeeding duration | |||
| Did not breastfeed | 25 (5.9) | 7 (4.4) | 18 (6.7) |
| >0 to <6 months | 133 (31.2) | 46 (29.1) | 87 (32.5) |
| >6 months | 265 (62.2) | 104 (65.8) | 161 (60.1) |
| Missing | 3 (0.7) | 1 (0.7) | 2 (0.7) |
| Prenatal antibiotics | |||
| No | 419 (98.4) | 154 (97.5) | 265 (98.9) |
| Yes | 7 (1.6) | 4 (2.5) | 3 (1.1) |
| Maternal resilient coping 4 | |||
| Low resilient coping | 129 (30.3) | 44 (27.8) | 85 (31.7) |
| Medium resilient coping | 145 (34.0) | 57 (36.1) | 88 (32.8) |
| High resilient coping | 133 (31.2) | 52 (32.9) | 81 (30.3) |
| Missing | 19 (4.5) | 5 (3.2) | 14 (5.2) |
| Reported Total Sum of Prenatal SLEs, Mean (SD) | 1.4 (1.6) |
|---|---|
| Reported Total Sum of prenatal SLEs, range | 0–9 |
| Specific Prenatal SLE | N (%) |
| Moved addresses | 107 (25.1%) |
| Sick family member in hospital | 79 (18.5%) |
| More arguments than usual with partner | 74 (17.4%) |
| Problems paying bills | 59 (13.8%) |
| Someone close had drinking or drug problem | 57 (13.4%) |
| Someone close died | 48 (11.3%) |
| Cut in work hours or pay | 43 (10.1%) |
| Partner lost job | 30 (7.0%) |
| Partner did not want pregnancy | 25 (5.9%) |
| Lost job | 22 (5.2%) |
| Partner deployed | 18 (4.2%) |
| Separation or divorce | 15 (3.5%) |
| Partner or self-jailed | 9 (2.1%) |
| Homelessness | 5 (1.2%) |
| Characteristic | Current AD 1,2 RR (95% CI) | Location-Specific AD 1,2 RR (95% CI) |
|---|---|---|
| Child sex | ||
| Male | 1.07 (0.82, 1.41) | 1.14 (0.77, 1.72) |
| Female | 1.08 (0.80, 1.47) | 0.95 (0.54, 1.69) |
| p for interaction | 0.96 | 0.59 |
| Maternal history of atopy | ||
| Yes | 1.08 (0.84, 1.38) | 1.09 (0.77, 1.54) |
| No | 1.07 (0.84, 1.37) | 1.08 (0.71, 1.64) |
| p for interaction | 0.98 | 0.97 |
| Prenatal maternal resilient coping 3 | ||
| Low resilient coping | 0.89 (0.69, 1.14) | 1.00 (0.68, 1.46) |
| Medium resilient coping | 1.25 (0.79, 1.97) | 0.87 (0.41, 1.87) |
| High resilient coping | 1.28 (0.94, 1.74) | 1.29 (0.87, 1.92) |
| p for interaction | 0.20 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senter, C.C.; Bush, N.R.; Loftus, C.T.; Szpiro, A.A.; Fitzpatrick, A.L.; Carroll, K.N.; LeWinn, K.Z.; Mason, W.A.; Sathyanarayana, S.; Akingbade, O.A.; et al. Maternal Stressful Life Events during Pregnancy and Atopic Dermatitis in Children Aged Approximately 4–6 Years. Int. J. Environ. Res. Public Health 2021, 18, 9696. https://doi.org/10.3390/ijerph18189696
Senter CC, Bush NR, Loftus CT, Szpiro AA, Fitzpatrick AL, Carroll KN, LeWinn KZ, Mason WA, Sathyanarayana S, Akingbade OA, et al. Maternal Stressful Life Events during Pregnancy and Atopic Dermatitis in Children Aged Approximately 4–6 Years. International Journal of Environmental Research and Public Health. 2021; 18(18):9696. https://doi.org/10.3390/ijerph18189696
Chicago/Turabian StyleSenter, Camilla C., Nicole R. Bush, Christine T. Loftus, Adam A. Szpiro, Annette L. Fitzpatrick, Kecia N. Carroll, Kaja Z. LeWinn, W. Alex Mason, Sheela Sathyanarayana, Oluwatobiloba A. Akingbade, and et al. 2021. "Maternal Stressful Life Events during Pregnancy and Atopic Dermatitis in Children Aged Approximately 4–6 Years" International Journal of Environmental Research and Public Health 18, no. 18: 9696. https://doi.org/10.3390/ijerph18189696
APA StyleSenter, C. C., Bush, N. R., Loftus, C. T., Szpiro, A. A., Fitzpatrick, A. L., Carroll, K. N., LeWinn, K. Z., Mason, W. A., Sathyanarayana, S., Akingbade, O. A., & Karr, C. J. (2021). Maternal Stressful Life Events during Pregnancy and Atopic Dermatitis in Children Aged Approximately 4–6 Years. International Journal of Environmental Research and Public Health, 18(18), 9696. https://doi.org/10.3390/ijerph18189696






