A Health Technology Assessment in Maxillofacial Cancer Surgery by Using the Six Sigma Methodology
Abstract
:1. Introduction
Literature Review: Health Technology Assessment and Six Sigma
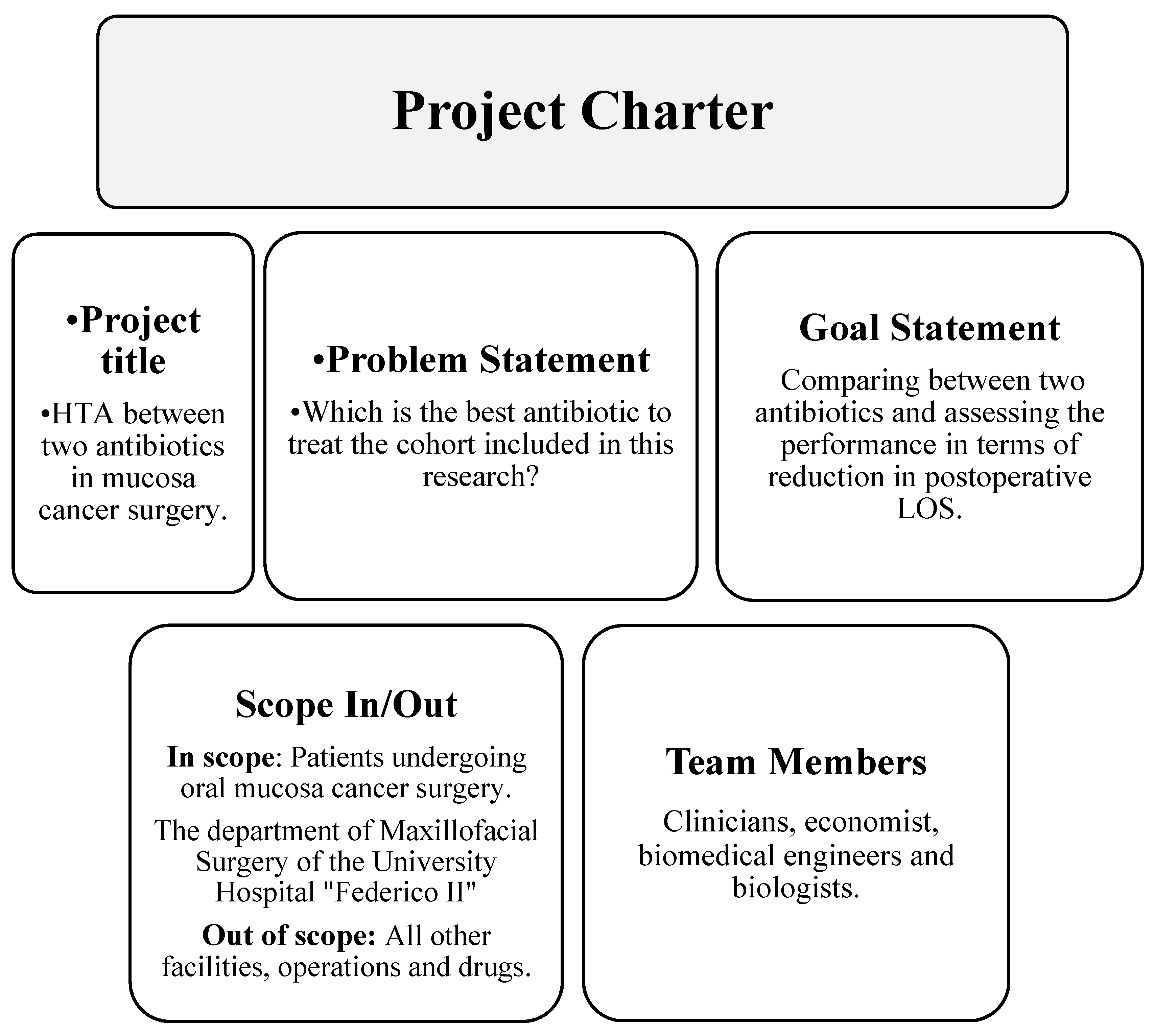
- The Define phase identifies the project, the problem and the objective.
- In the Measure phase, the current process that needs improvement is quantitatively described.
- In the Analyse phase, the statistical analysis is used to understand causes and effects in relation to the current process.
- The Improve phase allows users to develop a plan that can be validated by statistical data to improve the process. In this research, this phase will be used to compare the two analysed approaches.
2. Methods
2.1. Context
2.2. Collection of Data
- Patients with postoperative LOS ≤ 2 because the target of the study was ordinary hospitalization.
- Patients who underwent an antibiotic shift during their hospital stay (8 treated with Ceftriaxone and 15 with Cefazolin plus Clindamycin).
- Patients with missing data because they could have compromised the result of the analyses.
- Gender;
- Age;
- American Society of Anaesthesiologists (ASA) score;
- Oral hygiene;
- Diabetes;
- Cardiovascular diseases;
- Surgical intervention;
- Flap;
- Lymphadenectomy;
- Tracheotomy;
- Surgical site infections;
- Dehiscence;
- Fistulae.
2.3. Define
2.4. Measure
2.5. Analyse
2.6. Improve
2.7. Control
- Following the guidelines to improve administration, drawn up according to the influence of clinical characteristics and complications, as from the analyses carried out in this study;
- Periodic review meetings to evaluate the maxillofacial surgery process;
- Internal audit and production of reports that highlight the trend of patients’ LOS measured in days.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
Abbreviations
References
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral. Maxillofac. Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Tsantoulis, P.K.; Kastrinakis, N.G.; Tourvas, A.D.; Laskaris, G.; Gorgoulis, V.G. Advances in the biology of oral cancer. Oral Oncol. 2007, 43, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Singh, A.; Chien, C.Y.; Warnakulasuriya, S. Tobacco related Oral Cancer. BMJ 2019, 365, l2142. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [Green Version]
- Yete, S.; D’Souza, W.; Saranath, D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology 2018, 94, 133–141. [Google Scholar] [CrossRef]
- Yang, E.C.; Tan, M.T.; Schwarz, R.A.; Richards-Kortum, R.R.; Gillenwater, A.M.; Vigneswaran, N. Noninvasive diagnostic adjuncts for the evaluation of potentially premalignant oral epithelial lesions: Current limitations and future directions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 670–681. [Google Scholar] [CrossRef]
- Porter, S.; Gueiros, L.A.; Leão, J.C.; Fedele, S. Risk factors and etiopathogenesis of potentially premalignant oral epithelial lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63 (Suppl. 1), S91–S99. [Google Scholar] [CrossRef]
- Durand, M.L.; Yarlagadda, B.B.; Rich, D.L.; Lin, D.T.; Emerick, K.S.; Rocco, J.W.; Deschler, D.G. The time course and microbiology of surgical site infections after head and neck free flap surgery. Laryngoscope 2015, 125, 1084–1089. [Google Scholar] [CrossRef]
- Avery, C.M.; Ameerally, P.; Castling, B.; Swann, R.A. Infection of surgical wounds in the maxillofacial region and free flap donor sites with methicillin-resistant Staphylococcus aureus. Br. J. Oral Maxillofac. Surg. 2006, 44, 217–221. [Google Scholar] [CrossRef]
- De Bree, R.; Takes, R.P.; Shah, J.P.; Hamoir, M.; Kowalski, L.P.; Robbins, K.T.; Rodrigo, J.P.; Sanabria, A.; Medina, J.E.; Rinaldo, A.; et al. Elective neck dissection in oral squamous cell carcinoma: Past, present and future. Oral Oncol. 2019, 90, 87–93. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.F.; Chang, J.T.; Liao, C.T.; Kang, C.J.; Lin, C.Y.; Fan, K.H.; Wang, H.M.; Chen, I.H. The role of elective neck dissection in early stage buccal cancer. Laryngoscope 2015, 125, 128–133. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.; de Mast, J. A Rational Reconstruction of Six Sigma’s Breakthrough Cookbook. Int. J. Qual. Reliab. Manag. 2006, 23, 766–787. Available online: https://dare.uva.nl/search?identifier=0c29924f-fbb9-4877-9014-13b0680e083b (accessed on 19 November 2020). [CrossRef] [Green Version]
- Yousef, N.; Yousef, F. Using total quality management approach to improve patient safety by preventing medication error incidences. BMC Health Serv. Res. 2017, 17, 621. [Google Scholar] [CrossRef]
- Ricciardi, C.; Fiorillo, A.; Valente, A.S.; Borrelli, A.; Verdoliva, C.; Triassi, M.; Improta, G. Lean Six Sigma approach to reduce LOS through a diagnostic-therapeutic-assistance path at A.O.R.N.A. Cardarelli. TQM J. 2019, 31, 657–672. [Google Scholar] [CrossRef]
- Geerlinks, A.V.; Digout, C.; Bernstein, M.; Chan, A.; MacPhee, S.; Pambrun, C.; Gallant, G.; Wyatt, L.; Fernandez, C.V.; Price, V.E. Improving Time to Antibiotics for Pediatric Oncology Patients With Fever and Suspected Neutropenia by Applying Lean Principles. Pediatr. Emerg. Care 2020, 36, 509–514. [Google Scholar] [CrossRef]
- Maniscalco, G.T.; Cerillo, I.; Servillo, G.; Napolitano, M.; Guarcello, G.; Abate, V.; Improta, G.; Florio, C. Early neutropenia with thrombocytopenia following alemtuzumab treatment for multiple sclerosis: Case report and review of literature. Clin. Neurol. Neurosurg. 2018, 175, 134–136. [Google Scholar] [CrossRef]
- Downen, J.; Jaeger, C. Quality improvement of intravenous to oral medication conversion using Lean Six Sigma methodologies. BMJ Open Qual. 2020, 9, e000804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayai, K.; Kumar, P. Implementing Six Sigma to improve hospital discharge process. Int. J. Pharm. Sci. Res. 2012, 3, 4528–4532. [Google Scholar]
- Akifuddin, S.; Khatoon, F. Reduction of Complications of Local Anaesthesia in Dental Healthcare Setups by Application of the Six Sigma Methodology: A Statistical Quality Improvement Technique. J. Clin. Diagn. Res. 2015, 9, 5989. [Google Scholar] [CrossRef]
- Improta, G.; Mazzella, V.; Vecchione, D.; Santini, S.; Triassi, M. Fuzzy logic–based clinical decision support system for the evaluation of renal function in post-Transplant Patients. J. Eval. Clin. Pract. 2019, 26, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Cortesi, P.A.; Castaman, G.; Trifiro, G.; Creazzola, S.S.; Improta, G.; Mazzaglia, G.; Molinari, A.C.; Mantovani, L.G. Cost-Effectiveness and Budget Impact of Emicizumab Prophylaxis in Haemophilia A Patients with Inhibitors. Thromb. Haemost. 2020, 120, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Improta, G.; Perrone, A.; Russo, M.A.; Triassi, M. Health technology assessment (HTA) of optoelectronic biosensors for oncology by analytic hierarchy process (AHP) and Likert scale. BMC Med. Res. Methodol. 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Improta, G.; Converso, G.; Murino, T.; Gallo, M.; Perrone, A.; Romano, M. Analytic Hierarchy Process (AHP) in Dynamic Configuration as a Tool for Health Technology Assessment (HTA): The Case of Biosensing Optoelectronics in Oncology. Int. J. Inf. Technol. Decis. Mak. 2019, 18, 1533–1550. [Google Scholar] [CrossRef] [Green Version]
- Improta, G.; Russo, M.A.; Triassi, M.; Converso, G.; Murino, T.; Santillo, L.C. Use of the AHP methodology in system dynamics: Modelling and simulation for health technology assessments to determine the correct prosthesis choice for hernia diseases. Math. Biosci. 2018, 299, 19–27. [Google Scholar] [CrossRef]
- Improta, G.; Simone, T.; Bracale, M. HTA (Health Technology Assessment): A means to reach governance goals and to guide health politics on the topic of clinical risk management. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009. [Google Scholar] [CrossRef]
- Cesarelli, M.; Romano, M.; Bifulco, P.; Improta, G.; D’Addio, G. Prognostic decision support using symbolic dynamics in CTG monitoring. In Proceedings of the 13th EFMI Special Topic Conference Data and Knowledge for Medical Decision Support, Prague, Czech Republic, 17–19 April 2013; pp. 140–144. [Google Scholar] [CrossRef]
- Romano, M.; D’Addio, G.; Clemente, F.; Ponsiglione, M.A.; Improta, G.; Cesarelli, M. Symbolic dynamic and frequency analysis in foetal monitoring. IEEE Comput. Soc. 2014, 1–5. [Google Scholar] [CrossRef]
- Romano, M.; Bifulco, P.; Ponsiglione, A.M.; Gargiulo, G.D.; Amato, F.; Cesarelli, M. Evaluation of floatingline and foetal heart rate variability. Biomed. Signal Process. Control 2018, 39, 185–196. [Google Scholar] [CrossRef]
- Ricciardi, C.; Cuocolo, R.; Cesarelli, G.; Lorenzo, U.; Giovanni, I.; Domenico, S.; Valeria, R.; Elia, G.; Maria, C.L.; Mario, C. Distinguishing Functional from Non-functional Pituitary Macroadenomas with a Machine Learning Analysis. In Mediterranean Conference on Medical and Biological Engineering and Computing; Henriques, J., Neves, N., de Carvalho, P., Eds.; Springer: Cham, Switzerland, 2019; Volume 76. [Google Scholar] [CrossRef]
- Stanzione, A.; Ricciardi, C.; Cuocolo, R.; Romeo, V.; Petrone, J.; Sarnataro, M.; Mainenti, P.P.; Improta, G.; De Rosa, F.; Insabato, L.; et al. MRI Radiomics for the Prediction of Fuhrman Grade in Clear Cell Renal Cell Carcinoma: A Machine Learning Exploratory Study. J. Digit. Imaging 2020, 33, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Cantoni, V.; Improta, G.; Iuppariello, L.; Latessa, I.; Cesarelli, M.; Triassi, M.; Cuocolo, A. Application of data mining in a cohort of Italian subjects undergoing myocardial perfusion imaging at an academic medical center. Comput. Methods Programs Biomed. 2020, 189, 105343. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of tumor grade and nodal status in oropharyngeal and oral cavity squamous-cell carcinoma using a radiomic approach. Anticancer Res. 2020, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Amboni, M.; De Santis, C.; Ricciardelli, G.; Improta, G.; D’Addio, G.; Cuoco, S.; Picillo, M.; Barone, P.; Cesarelli, M. Machine learning can detect the presence of Mild cognitive impairment in patients affected by Parkinson’s Disease. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Sunder, M.V.; Kunnath, N.R. Six Sigma to reduce claims processing errors in a healthcare payer firm. Prod. Plan. Control 2020, 31, 496–511. [Google Scholar] [CrossRef]
- Arafeh, M.; Barghash, M.A.; Haddad, N.; Musharbash, N.; Nashawati, D.; Al-Bashir, A.; Assaf, F. Using Six Sigma DMAIC Methodology and Discrete Event Simulation to Reduce Patient Discharge Time in King Hussein Cancer Center. J. Healthc. Eng. 2018, 2018, 3832151. [Google Scholar] [CrossRef] [Green Version]
- Improta, G.; Balato, G.; Ricciardi, C.; Russo, M.A.; Santalucia, I.; Triassi, M.; Cesarelli, M. Lean Six Sigma in healthcare: Fast track surgery for patients undergoing prosthetic hip replacement surgery. TQM J. 2019, 31, 526–540. [Google Scholar] [CrossRef]
- Ricciardi, C.; Balato, G.; Romano, M.; Santalucia, I.; Cesarelli, M.; Improta, G. Fast track surgery for knee replacement surgery: A lean six sigma approach. TQM J. 2020, 32, 461–474. [Google Scholar] [CrossRef]
- Improta, G.; Guizzi, G.; Ricciardi, C.; Giordano, V.; Ponsiglione, A.M.; Converso, G.; Triassi, M. Agile Six Sigma in Healthcare: Case Study at Santobono Pediatric Hospital. Int. J. Environ. Res. Public Health 2020, 17, 1052. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, C.; Sorrentino, A.; Improta, G.; Abbate, V.; Latessa, I.; Perrone, A.; Triassi, M.; Orabona, G.D. A health technology assessment between two pharmacological therapies through Six Sigma: The case study of bone cancer. TQM J. 2020, 32, 1507–1524. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Ricciardi, C.; Improta, G.; Orabona, G.D.A.; Sorrentino, A.; Amato, F.; Romano, M. A Six Sigma DMAIC methodology as a support tool for Health Technology Assessment of two antibiotics. Math. Biosci. Eng. 2021, 18, 3469–3490. [Google Scholar] [CrossRef]
- Latessa, I.; Ricciardi, C.; Jacob, D.; Jónsson, H.; Gambacorta, M., Jr.; Improta, G.; Gargiulo, P. Health technology assessment through Six Sigma Methodology to assess cemented and uncemented protheses in total hip arthroplasty. Eur. J. Transl. Myol. 2021, 31, 9651. [Google Scholar] [CrossRef]
- Ricciardi, C.; Gubitosi, A.; Lanzano, G.; Parisi, S.; Grella, E.; Ruggiero, R.; Izzo, S.; Docimo, L.; Ferraro, G.; Improta, G. Health technology assessment through the six sigma approach in abdominoplasty: Scalpel vs electrosurgery. Med. Eng. Phys. 2021, 93, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Gubitosi, A.; Lanzano, G.; Pieretti, G.; Improta, G.; Crisci, E.; Ferraro, G.A. The Use of Six Sigma to Assess Two Prostheses for Immediate Breast Reconstruction. In 8th European Medical and Biological Engineering Conference, Proceedings of the EMBEC 2020, Portorož, Slovenia, 29 November–3 December 2020; Jarm, T., Cvetkoska, A., Mahnič-Kalamiza, S., Miklavcic, D., Eds.; Springer: Cham, Switzerland, 2020; Volume 80. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Ricciardi, C.; Scala, A.; Fiorillo, A.; Sorrentino, A.; Triassi, M.; Dell’Aversana Orabona, G.; Improta, G. Application of DMAIC Cycle and Modeling as Tools for Health Technology Assessment in a University Hospital. J. Healthc. Eng. 2021, 2021, 8826048. [Google Scholar] [CrossRef]
- Vander Poorten, V.; Uyttebroek, S.; Robbins, K.T.; Rodrigo, J.P.; de Bree, R.; Laenen, A.; Saba, N.F.; Suarez, C.; Mäkitie, A.; Rinaldo, A.; et al. Perioperative Antibiotics in Clean-Contaminated Head and Neck Surgery: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 1360–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Improta, G.; Ricciardi, C.; Borrelli, A.; D’alessandro, A.; Verdoliva, C.; Cesarelli, M. The application of six sigma to reduce the pre-operative length of hospital stay at the hospital Antonio Cardarelli. Int. J. Lean Six Sigma 2019, 11, 555–576. [Google Scholar] [CrossRef]
- Hackett, N.J.; De Oliveira, G.S.; Jain, U.K.; Kim, J.Y. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int. J. Surg. 2015, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Wolters, U.; Wolf, T.; Stutzer, H.; Schroder, T. ASA classification and perioperative variables as predictors of postoperative outcome. Br. J. Anaesth. 1996, 77, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.A.; Tung, K.C.; Shiao, J.Y.; Chiu, Y.T. Preliminary report of associated factors in wound infection after major head and neck neoplasm operations--does the duration of prophylactic antibiotic matter? J. Laryngol. Otol. 2008, 122, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, C.J.; Cavalcanti Rde, C.; Silva, A.M.; Latorre, M.D.; Ribeiro, K.D.; Carvalho, A.L.; Kowalski, L.P. Risk factors for surgical-site infections in head and neck cancer surgery. Otolaryngol. Head Neck Surg. 2008, 138, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Ji, T.; Ow, A.; Zhang, C.P.; Sun, J.; Zhou, X.H.; Wang, L.Z.; Sun, K.D.; Han, W. Surgical site infection in elderly oral cancer patients: Is the evaluation of comorbid conditions helpful in the identification of high-risk ones? J. Oral Maxillofac. Surg. 2012, 70, 2445–2452. [Google Scholar] [CrossRef]
- Becker, G.D.; Parell, J.; Busch, D.F.; Finegold, S.M.; Acquarelli, M.J. Anaerobic and Aerobic Bacteriology in Head and Neck Cancer Surgery. Arch. Otolaryngol. 1978, 104, 591–594. [Google Scholar] [CrossRef]




| Supplier | Inputs | Process | Outputs | Customers | |
|---|---|---|---|---|---|
| University Hospital of Naples “Federico II” | Needs of patients | Arrival at the hospital | Surgery | Shorter recovery | Patients |
| Clinical staff | Maxillofacial surgery | Recovery | Postoperative activities | Improved outcome of patients | University Hospital of Naples “Federico II” |
| Preoperative activities | Discharge | Ensuring fewer complications | |||
| Variable | Category | LOS (Mean ± dev std) | n | p-Value |
|---|---|---|---|---|
| Gender | Men | 13.48 ± 10.77 | 44 | 0.441 |
| Women | 16.02 ± 12.17 | 41 | ||
| Age | <50 | 13.27 ± 12.80 | 15 | 0.640 |
| 50 ≤ Age ≤ 70 | 16.00 ± 13.52 | 34 | ||
| >70 | 14.08 ± 8.65 | 36 | ||
| ASA score | Low | 12.60 ± 10.28 | 55 | 0.008 ** |
| High | 18.57 ± 12.67 | 30 | ||
| Oral hygiene | Low | 12.93 ± 9.42 | 43 | 0.305 |
| High | 16.52 ± 13.11 | 42 | ||
| Diabetes | No | 14.41 ± 11.63 | 74 | 0.355 |
| Yes | 16.73 ± 10.63 | 11 | ||
| Cardiovascular disease | No | 14.42 ± 9.89 | 50 | 0.601 |
| Yes | 15.11 ± 13.56 | 35 | ||
| Surgical Procedure | Removal | 12.82 ± 10.27 | 66 | 0.003 ** |
| Removal and reconstruction | 21.26 ± 13.22 | 19 | ||
| Flap | No | 11.78 ± 8.30 | 64 | <0.001 *** |
| Yes | 23.62 ± 14.99 | 21 | ||
| Lymphadenectomy | No | 12.54 ± 8.96 | 76 | <0.001 *** |
| Yes | 33.00 ± 14.44 | 9 | ||
| Tracheotomy | No | 12.71 ± 8.98 | 77 | 0.001 *** |
| Yes | 33.88 ± 15.51 | 8 | ||
| Infections | No | 13.20 ± 10.38 | 75 | 0.005 ** |
| Yes | 26.00 ± 13.47 | 10 | ||
| Dehiscence | No | 13.70 ± 10.29 | 76 | 0.061 |
| Yes | 23.22 ±17.23 | 9 | ||
| Fistulae | No | 14.24 ±11.20 | 83 | 0.017 * |
| Yes | 34.00 ± 1.41 | 2 |
| Variable | Category | LOS (Mean ± dev std) | n | p-Value |
|---|---|---|---|---|
| Gender | Men | 17.00 ± 11.40 | 23 | 0.515 |
| Women | 15.18 ± 8.82 | 28 | ||
| Age | <50 | 8.29 ± 4.96 | 7 | 0.585 |
| 50 ≤ Age ≤ 70 | 17.57 ± 11.18 | 27 | ||
| >70 | 16.71 ± 7.66 | 17 | ||
| ASA score | Low | 10.53 ± 9.59 | 15 | 0.009 ** |
| High | 18.28 ± 9.11 | 36 | ||
| Oral hygiene | Low | 16.63 ± 10.31 | 30 | 0.587 |
| High | 15.10 ± 9.25 | 21 | ||
| Diabetes | No | 15.50 ± 9.37 | 48 | 0.148 |
| Yes | 24.00 ± 15.72 | 3 | ||
| Cardiovascular disease | No | 13.77 ± 8.22 | 26 | 0.099 |
| Yes | 18.32 ± 10.94 | 25 | ||
| Surgical Procedure | Removal | 13.20 ± 8.21 | 15 | 0.192 |
| Removal and reconstruction | 17.17 ± 10.30 | 36 | ||
| Flap | No | 13.25 ± 7.83 | 16 | 0.179 |
| Yes | 17.26 ± 10.47 | 35 | ||
| Lymphadenectomy | No | 11.12 ± 6.75 | 25 | <0.001 *** |
| Yes | 20.69 ± 10.12 | 26 | ||
| Tracheotomy | No | 13.65 ± 9.10 | 37 | 0.004 ** |
| Yes | 22.21 ± 9.19 | 14 | ||
| Infections | No | 14.81 ± 8.72 | 48 | <0.001 *** |
| Yes | 35.00 ± 7.00 | 3 | ||
| Dehiscence | No | 14.43 ± 8.71 | 46 | <0.001 *** |
| Yes | 30.40 ± 8.08 | 5 | ||
| Fistulae | No | 16.00 ± 9.82 | 51 | N.A. |
| Yes | N.A. | 0 |
| Variables | Category | Ceftriaxone (Mean ± dev std) | Cefazolin Plus Clindamycin (Mean ± dev std) | Difference of the Mean (%) | p-Value |
|---|---|---|---|---|---|
| All patients | 14.71 ± 11.47 | 16.00 ± 9.82 | −8.1% | 0.197 | |
| Gender | Men | 13.48 ± 10.77 | 17.00 ± 11.40 | −20.7% | 0.236 |
| Women | 16.02 ± 12.17 | 15.18 ± 8.82 | 5.5% | 0.732 | |
| Age | <50 | 13.27 ± 12.80 | 8.29 ± 4.96 | 60.1% | 0.490 |
| 50 ≤ Age ≤ 70 | 16.00 ± 13.52 | 17.57 ± 11.18 | −8.9% | 0.299 | |
| >70 | 14.08 ± 8.65 | 16.71 ± 7.66 | −15.7% | 0.185 | |
| ASA score | Low | 12.60 ± 10.28 | 10.53 ± 9.59 | 19.7% | 0.615 |
| High | 18.57 ± 12.67 | 18.28 ± 9.11 | 1.6% | 0.671 | |
| Oral hygiene | Low | 12.93 ± 9.42 | 16.63 ± 10.31 | −22.2% | 0.078 |
| High | 16.52 ± 13.11 | 15.10 ± 9.25 | 9.4% | 0.907 | |
| Diabetes | No | 14.41 ± 11.63 | 15.50 ± 9.37 | −7.0% | 0.175 |
| Yes | 16.73 ±10.63 | 24.00 ± 15.72 | −30.3% | 0.456 | |
| Cardiovascular disease | No | 14.42 ± 9.89 | 13.77 ± 8.22 | 4.7% | 0.969 |
| Yes | 15.11 ± 13.56 | 18.32 ± 10.94 | −17.5% | 0.086 | |
| Surgical Procedure | Removal | 12.82 ± 10.27 | 13.20 ± 8.21 | −2.9% | 0.630 |
| Removal and reconstruction | 21.26 ±13.22 | 17.17 ± 10.30 | 23.8% | 0.357 | |
| Flap | No | 11.78 ± 8.30 | 13.25 ± 7.83 | −11.1% | 0.344 |
| Yes | 23.62 ± 14.99 | 17.26 ± 10.47 | 36.8% | 0.165 | |
| Lymphadenectomy | No | 12.54 ± 8.96 | 11.12 ± 6.75 | 12.8% | 0.714 |
| Yes | 33.00 ±14.44 | 20.69 ± 10.12 | 59.5% | 0.023 * | |
| Tracheotomy | No | 12.71 ± 8.98 | 13.65 ± 9.10 | −6.9% | 0.471 |
| Yes | 33.88 ±15.51 | 22.21 ± 9.19 | 52.5% | 0.050 * | |
| Infections | No | 13.20 ± 10.38 | 14.81 ± 8.72 | −10.9% | 0.114 |
| Yes | 26.00 ±13.47 | 35.00 ± 7.00 | −25.7% | 0.287 | |
| Dehiscence | No | 13.70 ± 10.29 | 14.43 ± 8.71 | −5.1% | 0.317 |
| Yes | 23.22 ±17.23 | 30.40 ± 8.08 | −23.6% | 0.298 | |
| Fistulae | No | 14.24 ±11.20 | 16.00 ± 9.82 | −11.0% | 0.130 |
| Yes | 34.00 ± 1.41 | 0 | N.A. | ||
| Variables | Category | Ceftriaxone (n) | Cefazolin Plus Clindamycin (n) | p-Value |
|---|---|---|---|---|
| Gender | Men | 44 | 23 | 0.452 |
| Women | 41 | 28 | ||
| Age | <50 | 15 | 7 | 0.340 |
| 50 ≤ Age ≤ 70 | 34 | 27 | ||
| >70 | 36 | 17 | ||
| ASA score | Low | 55 | 15 | <0.001 *** |
| High | 30 | 36 | ||
| Oral hygiene | Low | 43 | 30 | 0.351 |
| High | 42 | 21 | ||
| Diabetes | No | 74 | 48 | 0.190 |
| Yes | 11 | 3 | ||
| Cardiovascular disease | No | 50 | 26 | 0.372 |
| Yes | 35 | 25 | ||
| Surgical Procedure | Removal | 66 | 15 | <0.001 *** |
| Removal and reconstruction | 19 | 36 | ||
| Flap | No | 64 | 16 | <0.001 *** |
| Yes | 21 | 35 | ||
| Lymphadenectomy | No | 76 | 25 | <0,001 *** |
| Yes | 9 | 26 | ||
| Tracheotomy | No | 77 | 37 | 0.006 ** |
| Yes | 8 | 14 | ||
| Infections | No | 75 | 48 | 0.259 |
| Yes | 10 | 3 | ||
| Dehiscence | No | 76 | 46 | 0.884 |
| Yes | 9 | 5 | ||
| Fistulae | No | 83 | 51 | 0.270 |
| Yes | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, C.; Orabona, G.D.; Picone, I.; Latessa, I.; Fiorillo, A.; Sorrentino, A.; Triassi, M.; Improta, G. A Health Technology Assessment in Maxillofacial Cancer Surgery by Using the Six Sigma Methodology. Int. J. Environ. Res. Public Health 2021, 18, 9846. https://doi.org/10.3390/ijerph18189846
Ricciardi C, Orabona GD, Picone I, Latessa I, Fiorillo A, Sorrentino A, Triassi M, Improta G. A Health Technology Assessment in Maxillofacial Cancer Surgery by Using the Six Sigma Methodology. International Journal of Environmental Research and Public Health. 2021; 18(18):9846. https://doi.org/10.3390/ijerph18189846
Chicago/Turabian StyleRicciardi, Carlo, Giovanni Dell’Aversana Orabona, Ilaria Picone, Imma Latessa, Antonella Fiorillo, Alfonso Sorrentino, Maria Triassi, and Giovanni Improta. 2021. "A Health Technology Assessment in Maxillofacial Cancer Surgery by Using the Six Sigma Methodology" International Journal of Environmental Research and Public Health 18, no. 18: 9846. https://doi.org/10.3390/ijerph18189846
APA StyleRicciardi, C., Orabona, G. D., Picone, I., Latessa, I., Fiorillo, A., Sorrentino, A., Triassi, M., & Improta, G. (2021). A Health Technology Assessment in Maxillofacial Cancer Surgery by Using the Six Sigma Methodology. International Journal of Environmental Research and Public Health, 18(18), 9846. https://doi.org/10.3390/ijerph18189846








