Increasing Physical Activity among Breast Cancer Survivors by Modulating Temporal Orientation with rTMS: Feasibility and Potential Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Equipment and Materials
2.3. Procedure
2.4. Measures
2.4.1. Feasibility
2.4.2. Limited Efficacy Testing
2.5. Data Analysis Plan
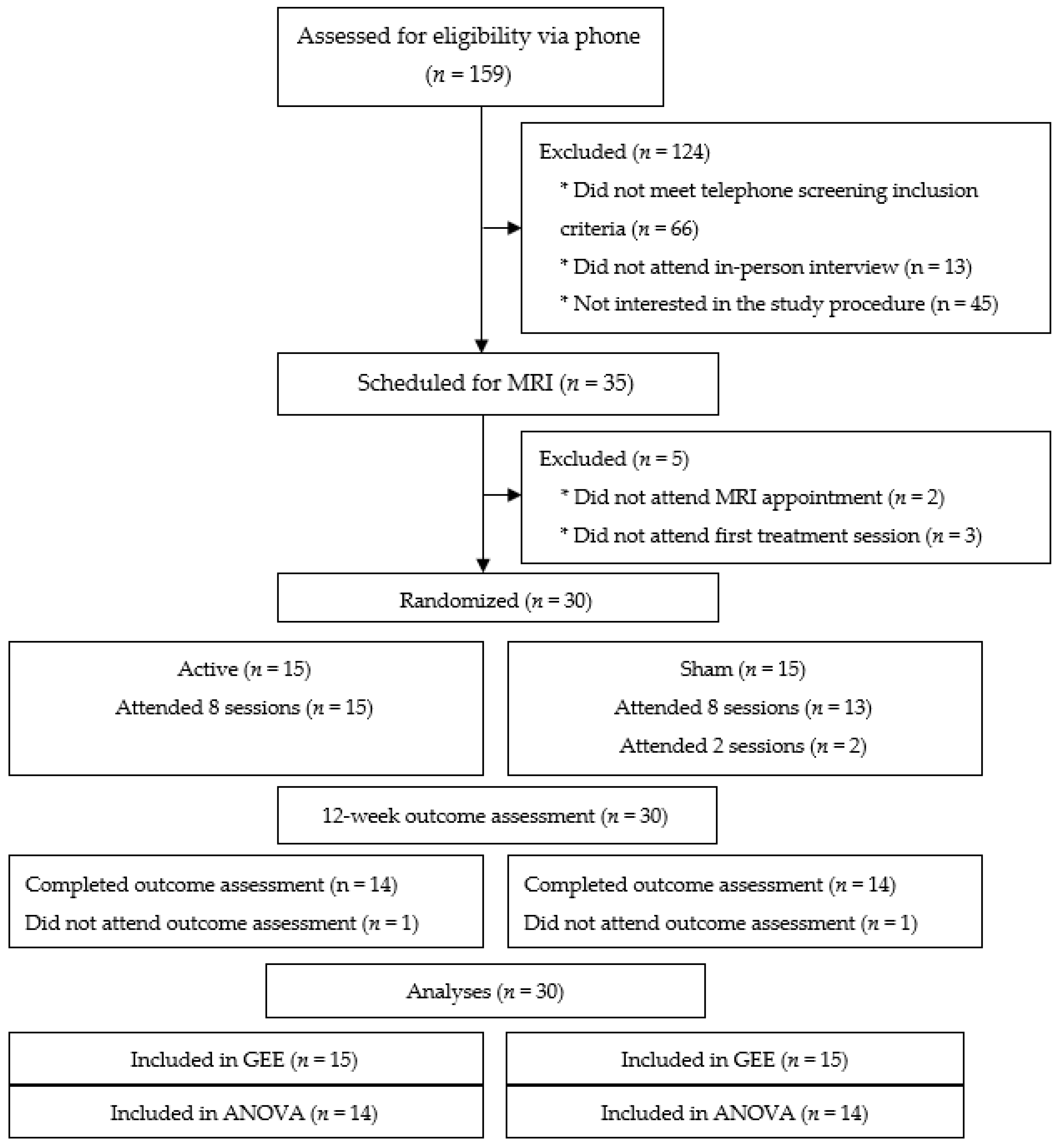
3. Results
3.1. Participants
3.2. Feasibility
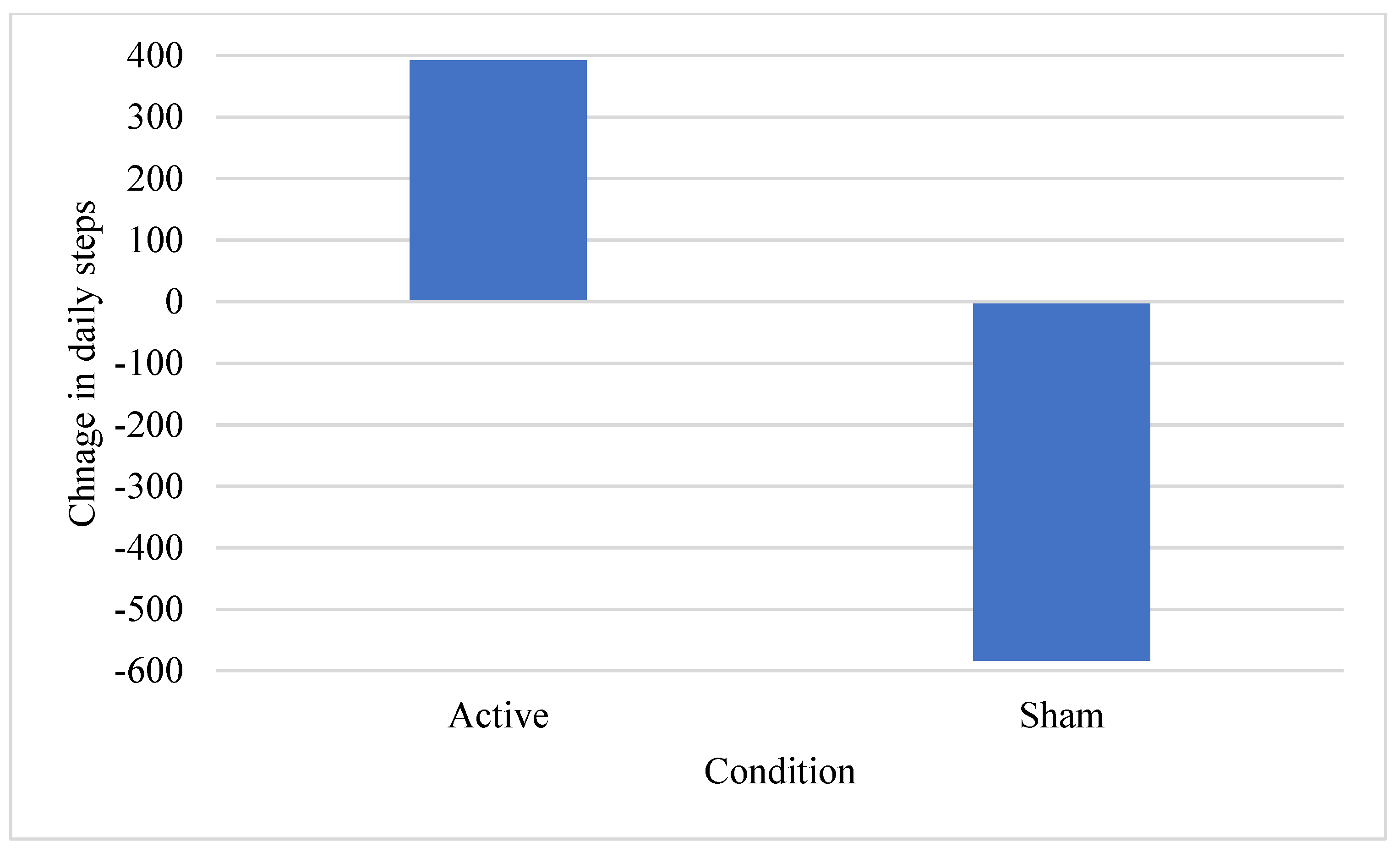
3.3. Limited Efficacy Testing
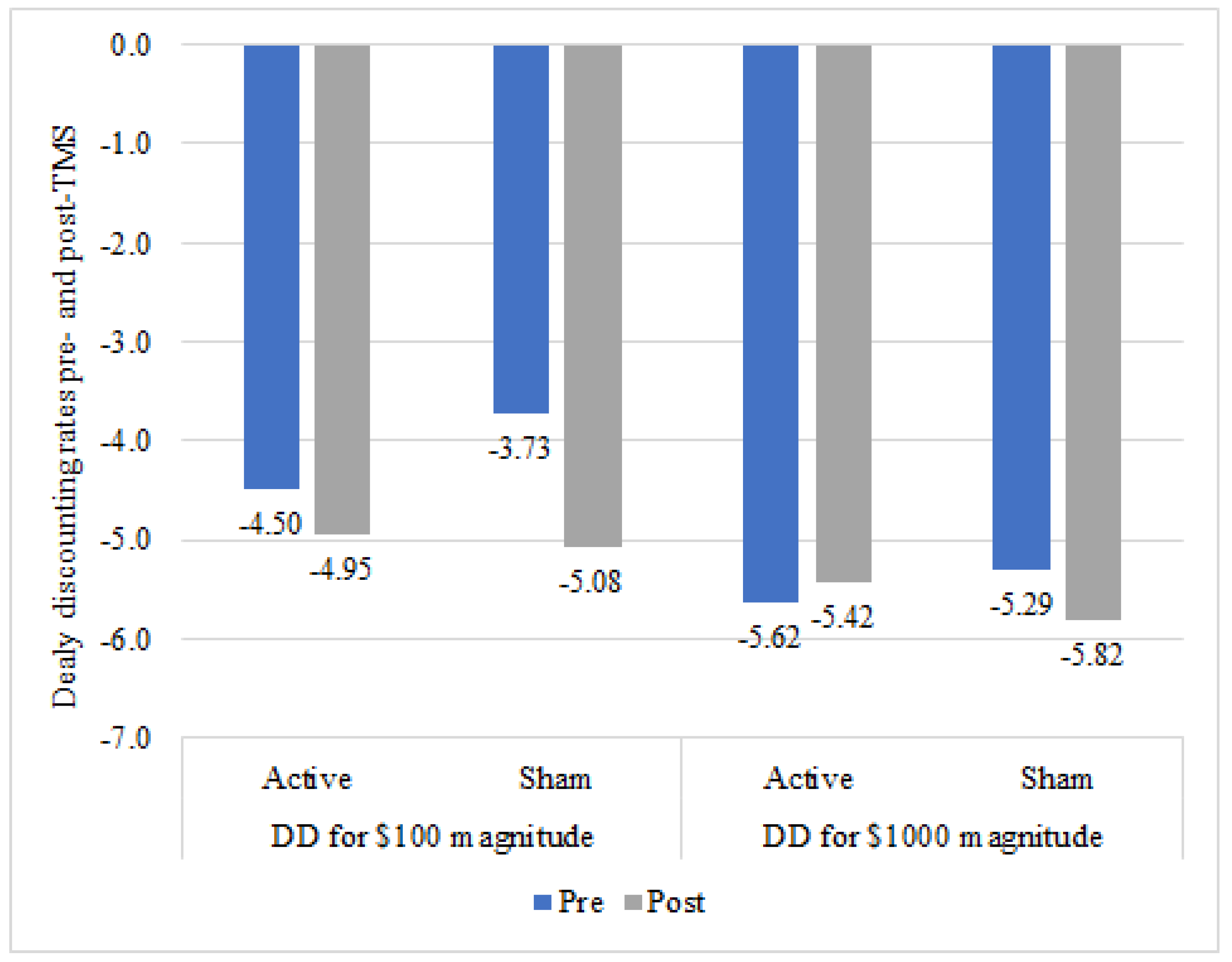
3.4. Delay Discounting
3.5. Other Self-Regulation Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cancer Facts & Figures 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 29 July 2021).
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef]
- Beasley, J.M.; Kwan, M.L.; Chen, W.Y.; Weltzien, E.K.; Kroenke, C.H.; Lu, W.; Nechuta, S.; Cadmus-Bertram, L.; Patterson, R.E.; Sternfeld, B.; et al. Meeting the physical activity guidelines and survival after breast cancer: Findings from the after breast cancer pooling project. Breast Cancer Res. Treat. 2011, 131, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.L.; Smith, A.W.; McTiernan, A.; Ballard-Barbash, R.; Cronin, K.; Gilliland, F.D.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L. Influence of Pre- and Postdiagnosis Physical Activity on Mortality in Breast Cancer Survivors: The Health, Eating, Activity, and Lifestyle Study. J. Clin. Oncol. 2008, 26, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; De Backer, I.C.; Schep, G.; Vreugdenhil, A.; Brug, J.; Chinapaw, M.J. Fatigue mediates the relationship between physical fitness and quality of life in cancer survivors. J. Sci. Med. Sport 2013, 16, 99–104. [Google Scholar] [CrossRef]
- Demeyer, H.; Burtin, C.; Hornikx, M.; Camillo, C.A.; Van Remoortel, H.; Langer, D.; Janssens, W.; Troosters, T. The Minimal Important Difference in Physical Activity in Patients with COPD. PLoS ONE 2016, 11, e0154587. [Google Scholar] [CrossRef]
- Kaleth, A.S.; Slaven, J.E.; Ang, D.C. Does Increasing Steps Per Day Predict Improvement in Physical Function and Pain Interference in Adults With Fibromyalgia? Arthritis Rheum. 2014, 66, 1887–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, P.J. A primer on self-regulation and health behavior change. Arch. Exerc. Health Dis. 2015, 5, 326–337. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Carraça, E.V.; Marques, M.M.; Rutter, H.; Oppert, J.-M.; De Bourdeaudhuij, I.; Lakerveld, J.; Brug, J. Successful behavior change in obesity interventions in adults: A systematic review of self-regulation mediators. BMC Med. 2015, 13, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothman, A.J.; Baldwin, A.S.; Hertel, A.W.; Fuglestad, P.T. Self-regulation and behavior change: Disentangling behavioral initiation and behavioral maintenance. In Handbook of Self-Regulation: Research, Theory, and Applications, 2nd ed.; Guilford Press: New York, NY, USA; Volume 2, pp. 106–122.
- Bickel, W.K.; Miller, M.L.; Yi, R.; Kowal, B.P.; Lindquist, D.M.; Pitcock, J.A. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007, 90, S85–S91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickel, W.K.; Yi, R.; Kowal, B.P.; Gatchalian, K.M. Cigarette smokers discount past and future rewards symmetrically and more than controls: Is discounting a measure of impulsivity? Drug Alcohol Depend. 2008, 96, 256–262. [Google Scholar] [CrossRef] [Green Version]
- McClure, S.M.; Bickel, W.K. A dual-systems perspective on addiction: Contributions from neuroimaging and cognitive training. Ann. N. Y. Acad. Sci. 2014, 1327, 62–78. [Google Scholar] [CrossRef] [Green Version]
- Bickel, W.K.; Mellis, A.M.; Snider, S.E.; Athamneh, L.N.; Stein, J.S.; Pope, D.A. 21st century neurobehavioral theories of decision making in addiction: Review and evaluation. Pharmacol. Biochem. Behav. 2018, 164, 4–21. [Google Scholar] [CrossRef] [PubMed]
- MacKillop, J. Integrating behavioral economics and behavioral genetics: Delayed reward discounting as an endophenotype for addictive disorders. J. Exp. Anal. Behav. 2013, 99, 14–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, C.A.; Dowdle, L.; Austelle, C.W.; DeVries, W.; Mithoefer, O.; Badran, B.; George, M.S. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015, 1628, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, G.E.; De Long, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.; Moody, L.; Quisenberry, A.; Ramey, C.; Sheffer, C. A Competing Neurobehavioral Decision Systems model of SES-related health and behavioral disparities. Prev. Med. 2014, 68, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Esch, T.; Stefano, G.B. Endogenous reward mechanisms and their importance in stress reduction, exercise and the brain. Arch. Med. Sci. 2010, 3, 447–455. [Google Scholar] [CrossRef]
- McClure, S.M.; Laibson, D.I.; Loewenstein, G.; Cohen, J.D. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science 2004, 306, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Logue, A.W.; Peña-Correal, T.E.; Rodriguez, M.L.; Kabela, E. Self-control in adult humans: Variation in positive reinforcer amount and delay. J. Exp. Anal. Behav. 1986, 46, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Logue, A.W. Research on self-control: An integrating framework. Behav. Brain Sci. 1988, 11, 665–679. [Google Scholar] [CrossRef]
- Koffarnus, M.N.; Jarmolowicz, D.P.; Mueller, E.T.; Bickel, W.K. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: A review. J. Exp. Anal. Behav. 2013, 99, 32–57. [Google Scholar] [CrossRef] [Green Version]
- LeComte, R.S.; Sofis, M.J.; Jarmolowicz, D.P. Independent Effects of Ideal Body Image Valuation and Delay Discounting on Proximal and Typical Levels of Physical Activity. Psychol. Rec. 2020, 70, 75–82. [Google Scholar] [CrossRef]
- Sofis, M.J.; Carrillo, A.; Jarmolowicz, D.P. Maintained Physical Activity Induced Changes in Delay Discounting. Behav. Modif. 2017, 41, 499–528. [Google Scholar] [CrossRef]
- Tate, L.M.; Tsai, P.-F.; Landes, R.D.; Rettiganti, M.; Lefler, L.L. Temporal discounting rates and their relation to exercise behavior in older adults. Physiol. Behav. 2015, 152, 295–299. [Google Scholar] [CrossRef]
- Sheffer, C.E.; Miller, A.; Bickel, W.K.; Devonish, J.A.; O’Connor, R.J.; Wang, C.; Rivard, C.; Gage-Bouchard, E.A. The treasure of now and an uncertain future: Delay discounting and health behaviors among cancer survivors. Cancer 2018, 124, 4711–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabroff, K.R.; Lawrence, W.F.; Clauser, S.; Davis, W.W.; Brown, M.L. Burden of Illness in Cancer Survivors: Findings From a Population-Based National Sample. J. Natl. Cancer Inst. 2004, 96, 1322–1330. [Google Scholar] [CrossRef]
- Cancer Survivors and Physical Activity. Available online: https://www.progressreport.cancer.gov/after/physical_activity (accessed on 30 July 2021).
- French-Rosas, L.N.; Moye, J.; Naik, A.D. Improving the Recognition and Treatment of Cancer-Related Posttraumatic Stress Disorder. J. Psychiatr. Pract. 2011, 17, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boat, R.; Cooper, S.B. Self-Control and Exercise: A Review of the Bi-Directional Relationship. Brain Plast. 2019, 5, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A.; et al. Ageing, fitness and neurocognitive function. Nat. Cell Biol. 1999, 400, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.K.; Yi, R.; Landes, R.D.; Hill, P.F.; Baxter, C. Remember the Future: Working Memory Training Decreases Delay Discounting Among Stimulant Addicts. Biol. Psychiatry 2011, 69, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Oxley, T.; Laird, A.; Kulkarni, J.; Egan, G.; Daskalakis, Z.J. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. Neuroimaging 2006, 148, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Sheffer, C.E.; Mennemeier, M.; Landes, R.D.; Bickel, W.K.; Brackman, S.; Dornhoffer, J.; Kimbrell, T.; Brown, G. Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J. Subst. Abus. Treat. 2013, 45, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheffer, C.E.; Bickel, W.K.; Brandon, T.H.; Franck, C.T.; Deen, D.; Panissidi, L.; Abdali, S.A.; Pittman, J.C.; Lunden, S.E.; Prashad, N.; et al. Preventing relapse to smoking with transcranial magnetic stimulation: Feasibility and potential efficacy. Drug Alcohol Depend. 2018, 182, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, B.; Byeon, K.; Park, H.; Kim, Y.; Eun, Y.; Chung, J. The effects of high-frequency repetitive transcranial magnetic stimulation on resting-state functional connectivity in obese adults. Diabetes Obes. Metab. 2019, 21, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Chung, J.-H.; Kim, T.-H.; Lim, S.H.; Kim, Y.; Lee, Y.-A.; Song, S.-W. The effects of repetitive transcranial magnetic stimulation on eating behaviors and body weight in obesity: A randomized controlled study. Brain Stimul. 2018, 11, 528–535. [Google Scholar] [CrossRef]
- Amiaz, R.; Levy, D.; Vainiger, D.; Grunhaus, L.; Zangen, A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009, 104, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Eichhammer, P.; Johann, M.; Kharraz, A.; Binder, H.; Pittrow, D.; Wodarz, N.; Hajak, G. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J. Clin. Psychiatry 2003, 64, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kikuchi, S.; Nisijima, K.; Suda, S. Actigraphy in Patients with Major Depressive Disorder Undergoing Repetitive Transcranial Magnetic Stimulation: An Open Label Pilot Study. J. ECT 2017, 33, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Speer, A.M.; Kimbrell, T.A.; Wassermann, E.M.; Repella, J.D.; Willis, M.W.; Herscovitch, P. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry 2000, 48, 1133–1141. [Google Scholar] [CrossRef]
- Guse, B.; Falkai, P.; Wobrock, T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: A systematic review. J. Neural Transm. 2010, 117, 105–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, G.; Anderson, E.; Silva, C.; Goldberg, T.; Wassermann, E.M. Effects of 10 Hz rTMS on the Neural Efficiency of Working Memory. J. Cogn. Neurosci. 2010, 22, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Esslinger, C.; Schüler, N.; Sauer, C.; Gass, D.; Mier, D.; Braun, U.; Ochs, E.; Schulze, T.G.; Rietschel, M.; Kirsch, P.; et al. Induction and quantification of prefrontal cortical network plasticity using 5 Hz rTMS and fMRI. Hum. Brain Mapp. 2014, 35, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.C.; Oathes, D.; Chang, C.; Bradley, T.; Zhou, Z.-W.; Williams, L.M.; Glover, G.H.; Deisseroth, K.; Etkin, A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 19944–19949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeken, C.; De Raedt, R. Neurobiological mechanisms of repetitive transcranial magnetic stimulation on the underlying neurocircuitry in unipolar depression. Dialogues Clin. Neurosci. 2011, 13, 139–145. [Google Scholar]
- Kravitz, A.V.; Tomasi, D.; LeBlanc, K.; Baler, R.; Volkow, N.D.; Bonci, A.; Ferre, S. Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Res. 2015, 1628, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Siebner, H.R.; Rothwell, J. Transcranial magnetic stimulation: New insights into representational cortical plasticity. Exp. Brain Res. 2003, 148, 1–16. [Google Scholar] [CrossRef]
- Thickbroom, G.W. Transcranial magnetic stimulation and synaptic plasticity: Experimental framework and human models. Exp. Brain Res. 2007, 180, 583–593. [Google Scholar] [CrossRef]
- Cooke, S.F.; Bliss, T.V.P. Plasticity in the human central nervous system. Brain 2006, 129, 1659–1673. [Google Scholar] [CrossRef] [Green Version]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Serafini, G.; Pompili, M.; Murri, M.B.; Respino, M.; Ghio, L.; Girardi, P.; Fitzgerald, P.B.; Amore, M. The Effects of Repetitive Transcranial Magnetic Stimulation on Cognitive Performance in Treatment-Resistant Depression. A Systematic Review. Neuropsychobiology 2015, 71, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.J.; Kreuter, M.; Spring, B.; Cofta-Woerpel, L.; Linnan, L.; Weiner, D.; Bakken, S.; Kaplan, C.P.; Squiers, L.; Fabrizio, C.; et al. How We Design Feasibility Studies. Am. J. Prev. Med. 2009, 36, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Screening questionnaire before TMS: An update. Clin. Neurophysiol. 2011, 122, 1686. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, P.M.; Barr, M.S.; Farzan, F.; Arenovich, T.; Maller, J.J.; Fitzgerald, P.B.; Daskalakis, Z.J. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum. Brain Mapp. 2010, 31, 1643–1652. [Google Scholar] [CrossRef]
- Cincotta, M.; Giovannelli, F.; Borgheresi, A.; Balestrieri, F.; Toscani, L.; Zaccara, G.; Carducci, F.; Viggiano, M.P.; Rossi, S. Optically tracked neuronavigation increases the stability of hand-held focal coil positioning: Evidence from “transcranial” magnetic stimulation-induced electrical field measurements. Brain Stimul. 2010, 3, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory STAI (Form Y) (“Self-Evaluation Questionnaire”); Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal, and Coping; Springer Publishing Company: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Koffarnus, M.N.; Bickel, W.K. A 5-trial adjusting delay discounting task: Accurate discount rates in less than one minute. Exp. Clin. Psychopharmacol. 2014, 22, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, J.E. An adjusting procedure for studying delayed reinforcement. In The Effect of Delay and of Intervening Events on Reinforcement Value; Routledge: Oxfordshire, UK, 1987; Volume 344, pp. 55–73. [Google Scholar]
- Rajchert, J. BIS/BAS Systems. In Encyclopedia of Personality and Individual Differences; Springer: Berlin/Heidelberg, Germany, 2020; pp. 501–510. [Google Scholar] [CrossRef]
- Reise, S.P.; Moore, T.M.; Sabb, F.W.; Brown, A.K.; London, E.D. The Barratt Impulsiveness Scale–11: Reassessment of its structure in a community sample. Psychol. Assess. 2013, 25, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Manapat, P.D.; Edwards, M.C.; MacKinnon, D.P.; Poldrack, R.; Marsch, L. A Psychometric Analysis of the Brief Self-Control Scale. Assessment 2021, 28, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.L.; Christopher, M.; Neuhaus, E.C. Development and Validation of the Cognitive-Behavioral Therapy Skills Questionnaire. Behav. Modif. 2011, 35, 595–618. [Google Scholar] [CrossRef]
- Lingler, J.H.; Schmidt, K.L.; Gentry, A.L.; Hu, L.; Terhorst, L.A. A New Measure of Research Participant Burden: Brief Report. J. Empir. Res. Hum. Res. Ethics 2014, 9, 46–49. [Google Scholar] [CrossRef]
- Berlim, M.T.; Eynde, F.V.D.; Daskalakis, Z.J. Clinically Meaningful Efficacy and Acceptability of Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) for Treating Primary Major Depression: A Meta-Analysis of Randomized, Double-Blind and Sham-Controlled Trials. Neuropsychopharmacology 2013, 38, 543–551. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp: Armonk, NY, USA, 2015. [Google Scholar]
- Hubbard, A.E.; Ahern, J.; Fleischer, N.L.; Van der Laan, M.; Lippman, S.A.; Jewell, N.; Bruckner, T.; Satariano, W.A. To GEE or Not to GEE. Epidemiology 2010, 21, 467–474. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.-Y.; Albert, P.S. Models for Longitudinal Data: A Generalized Estimating Equation Approach. Biometrics 1988, 44, 1049. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.G.; Seiden, A.; Koffarnus, M.N.; Bickel, W.K.; Wing, R.R. Delayed reward discounting and grit in men and women with and without obesity. Obes. Sci. Pract. 2015, 1, 131–135. [Google Scholar] [CrossRef]
- Shin, G.; Feng, Y.; Jarrahi, M.H.; Gafinowitz, N. Beyond novelty effect: A mixed-methods exploration into the motivation for long-term activity tracker use. JAMIA Open 2019, 2, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Figner, B.; Knoch, D.; Johnson, E.; Krosch, A.R.; Lisanby, S.H.; Fehr, E.; Weber, E.U. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010, 13, 538–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.S.; Ko, J.H.; Pellecchia, G.; Van Eimeren, T.; Cilia, R.; Strafella, A.P. Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stimul. 2010, 3, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Suuberg, E.M. Very low concentration adsorption isotherms of trichloroethylene on common building materials. Build. Environ. 2020, 179, 106954. [Google Scholar] [CrossRef] [PubMed]

| Variable | Range or Categories | Percent (n) or Mean (SD) | ||
|---|---|---|---|---|
| Total (30) | Active (15) | Sham (15) | ||
| Age | 53.7 (7.9) | 55.2 (8.2) | 52.1 (7.7) | |
| Race | Caucasian or White | 93.3 (28) | 93.3 (14) | 93.3 (14) |
| African American or Black | 6.7 (2) | 6.7 (1) | 6.7 (1) | |
| Partnered status | Partnered | 66.7 (20) | 66.7 (10) | 66.7 (10) |
| Annual household income | ≤USD 34,999 | 13.3 (4) | 13.3 (2) | 13.3 (2) |
| USD 35,000–USD 74,999 | 23.3 (7) | 20.0 (3) | 26.7 (4) | |
| ≥USD 75,000 | 63.3 (19) | 66.7 (10) | 60.0 (9) | |
| Education | High school | 13.3 (4) | 13.3 (2) | 13.3 (2) |
| College | 66.7 (20) | 66.7 (10) | 66.7 (10) | |
| Graduate school | 20.0 (6) | 20.0 (3) | 20.0 (3) | |
| Employment status * | Full Time | 76.3 (22) | 53.3 (8) | 93.3 (14) |
| Part Time | 10.0 (3) | 13.3 (2) | 6.7 (1) | |
| Retired | 13.3 (4) | 26.7 (4) | - | |
| Unemployed | 3.3 (1) | 6.7 (1) | - | |
| Health insurance | Medicaid | 3.3 (1) | 6.7 (1) | - |
| None | 6.7 (2) | 6.7 (1) | 6.7 (1) | |
| Private | 90.0 (27) | 86.7 (13) | 93.3 (14) | |
| Self-reported minutes of moderate to vigorous physical activity per week | 0–300 | 65.6 (91.9) | 101.0 (105.9) | 30.1 (59.9) |
| PANAS | Positive | 34.1 (7.4) | 34.3 (7.2) | 34.0 (7.8) |
| Negative | 13.1 (4.3) | 12.6 (4.9) | 13.7 (3.6) | |
| STAI | State | 28.2 (7.7) | 26.5 (6.1) | 29.9 (8.9) |
| Trait | 33.3 (8.6) | 32.2 (8.9) | 34.5 (8.4) | |
| CES-D | 0–60 | 10.2 (8.5) | 9.3 (8.6) | 11.1 (8.6) |
| PSS-4 | 0–16 | 4.8 (2.6) | 4.6 (2.9) | 5.0 (2.3) |
| Motivation to increase physical activity | 0–10 | 9.0 (1.4) | 8.9 (1.5) | 8.4 (1.2) |
| Efficacy to achieve 10,000 steps per day | 0–10 | 8.1 (1.9) | 8.1 (2.3) | 8.1 (1.8) |
| Efficacy to achieve 150 min of moderate to vigorous activity per week | 0–10 | 8.2 (1.8) | 8.5 (1.6) | 7.9 (1.9) |
| Delay discounting (USD 100) logk | −3.9 (1.6) | −4.4 (0.4) | −3.6 (1.6) | |
| Delay discounting (USD 1000) logk | −5.3 (1.1) | −5.5 (1.4) | −5.2 (0.9) | |
| BIS/BAS | BAS: Drive | 10.8 (2.4) | 10.9 (2.2) | 10.8 (2.7) |
| BAS: Fun Seeking | 11.5 (1.5) | 11.4 (1.3) | 11.5 (1.6) | |
| BAS: Reward Response | 17.4 (1.9) | 17.1 (2.3) | 17.7 (1.4) | |
| BIS | 20.1 (3.4) | 19.9 (3.8) | 20.3 (3.1) | |
| BIS | Attentional | 16.1 (3.1) | 16.0 (3.8) | 16.3 (2.4) |
| Motor | 23.0 (2.4) | 23.3 (2.1) | 22.8 (2.7) | |
| Non-planning | 23.5 (2.9) | 22.8 (2.4) | 24.1 (3.4) | |
| Total | 62.6 (6.6) | 62.1 (6.2) | 63.2 (7.1) | |
| BSCS | 44.3 (7.3) | 46.0 (7.4) | 42.7 (7.0) | |
| SSRQ * | 95.8 (7.8) | 92.9 (4.6) | 98.6 (9.4) | |
| CBTSQ | Behavioral | 24.9 (3.3) | 25.1 (3.4) | 24.8 (3.3) |
| Cognitive | 27.9 (5.6) | 28.7 (4.1) | 27.2 (6.9) | |
| Total | 52.9 (7.3) | 64.7 (2.1) | 52.0 (8.2) | |
| Height (inches) | 64.7 (2.7) | 64.7 (2.1) | 64.7 (3.3) | |
| Weight (pounds) | 185.1 (34.8) | 185.9 (35.8) | 184.2 (34.9) | |
| Body fat percentage | 0–100 | 41.7 (6.7) | 41.6 (7.1) | 41.7 (6.5) |
| Muscle Mass (pounds) | 99.4 (11.0) | 101.1 (11.9) | 100.2 (10.4) | |
| Bone Mass (pounds) | 5.4 (0.6) | 5.4 (0.6) | 5.4 (0.6) | |
| Visceral Fat | 10.2 (3.1) | 10.5 (2.9) | 10.0 (3.5) | |
| Body Mass Index | 31.0 (5.1) | 31.1 (5.2) | 30.9 (5.1) | |
| Waist Circumference | 42.8 (16.1) | 45.6 (22.3) | 39.9 (4.8) | |
| Hip Circumference | 47.9 (13.9) | 50.3 (19.2) | 45.6 (4.0) | |
| Systolic Blood Pressure | 123.2 (17.2) | 125.5 (20.3) | 120.8 (13.7) | |
| Diastolic Blood Pressure | 78.8 (7.9) | 78.9 (9.3) | 78.7 (6.6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carl, E.; Shevorykin, A.; Liskiewicz, A.; Alberico, R.; Belal, A.; Mahoney, M.; Bouchard, E.; Ray, A.; Sheffer, C.E. Increasing Physical Activity among Breast Cancer Survivors by Modulating Temporal Orientation with rTMS: Feasibility and Potential Efficacy. Int. J. Environ. Res. Public Health 2021, 18, 10052. https://doi.org/10.3390/ijerph181910052
Carl E, Shevorykin A, Liskiewicz A, Alberico R, Belal A, Mahoney M, Bouchard E, Ray A, Sheffer CE. Increasing Physical Activity among Breast Cancer Survivors by Modulating Temporal Orientation with rTMS: Feasibility and Potential Efficacy. International Journal of Environmental Research and Public Health. 2021; 18(19):10052. https://doi.org/10.3390/ijerph181910052
Chicago/Turabian StyleCarl, Ellen, Alina Shevorykin, Amylynn Liskiewicz, Ronald Alberico, Ahmed Belal, Martin Mahoney, Elizabeth Bouchard, Andrew Ray, and Christine E. Sheffer. 2021. "Increasing Physical Activity among Breast Cancer Survivors by Modulating Temporal Orientation with rTMS: Feasibility and Potential Efficacy" International Journal of Environmental Research and Public Health 18, no. 19: 10052. https://doi.org/10.3390/ijerph181910052
APA StyleCarl, E., Shevorykin, A., Liskiewicz, A., Alberico, R., Belal, A., Mahoney, M., Bouchard, E., Ray, A., & Sheffer, C. E. (2021). Increasing Physical Activity among Breast Cancer Survivors by Modulating Temporal Orientation with rTMS: Feasibility and Potential Efficacy. International Journal of Environmental Research and Public Health, 18(19), 10052. https://doi.org/10.3390/ijerph181910052






