Heart Rate Variability-Guided Training for Enhancing Cardiac-Vagal Modulation, Aerobic Fitness, and Endurance Performance: A Methodological Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Data Search and Sources
2.2. Study Selection
2.3. Data Extraction, Coding Study Characteristics, and Potential Moderator Variables
2.4. Risk of Bias
2.5. Computation of Effect Size and Statistical Analyses
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Outcomes
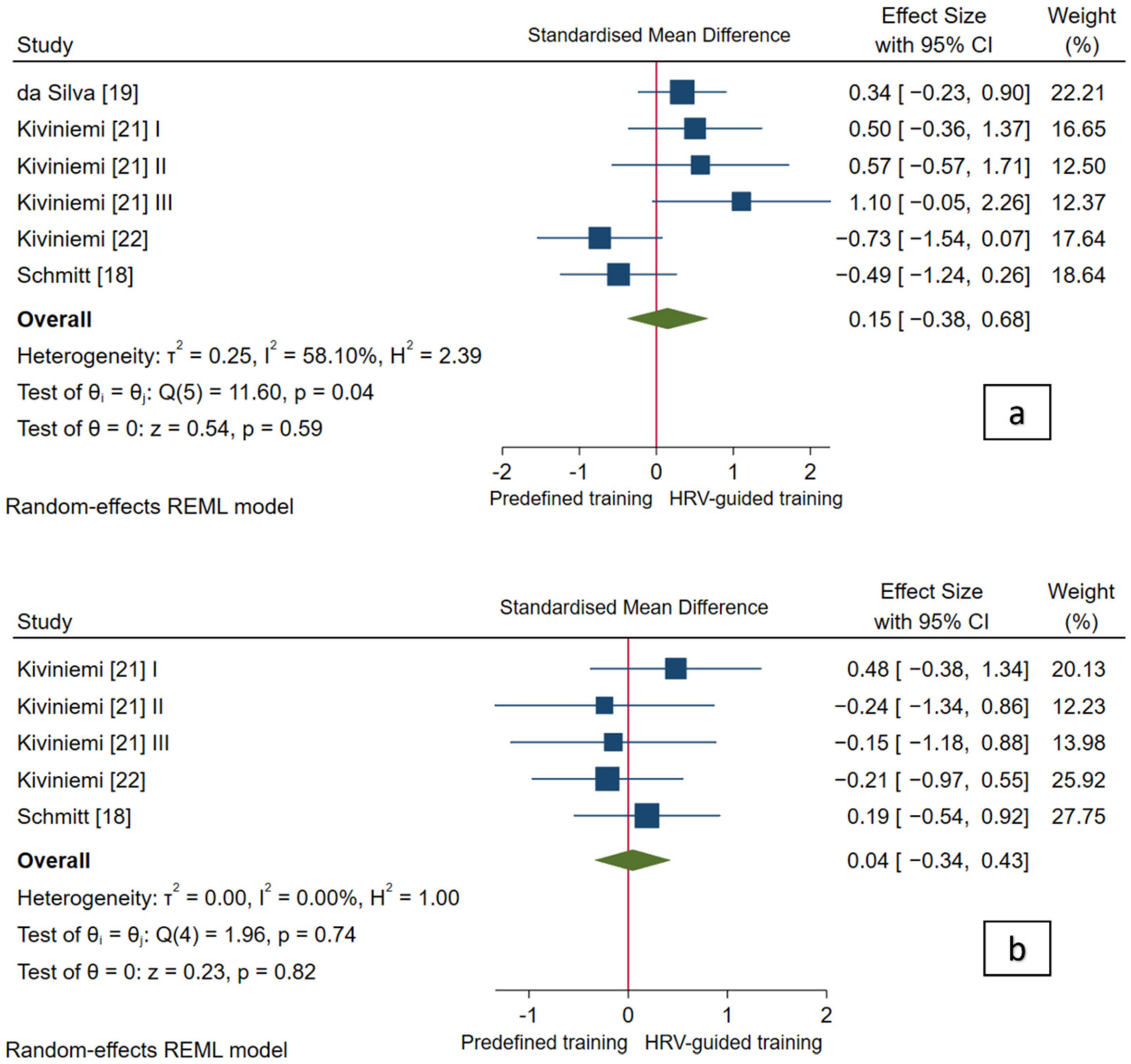
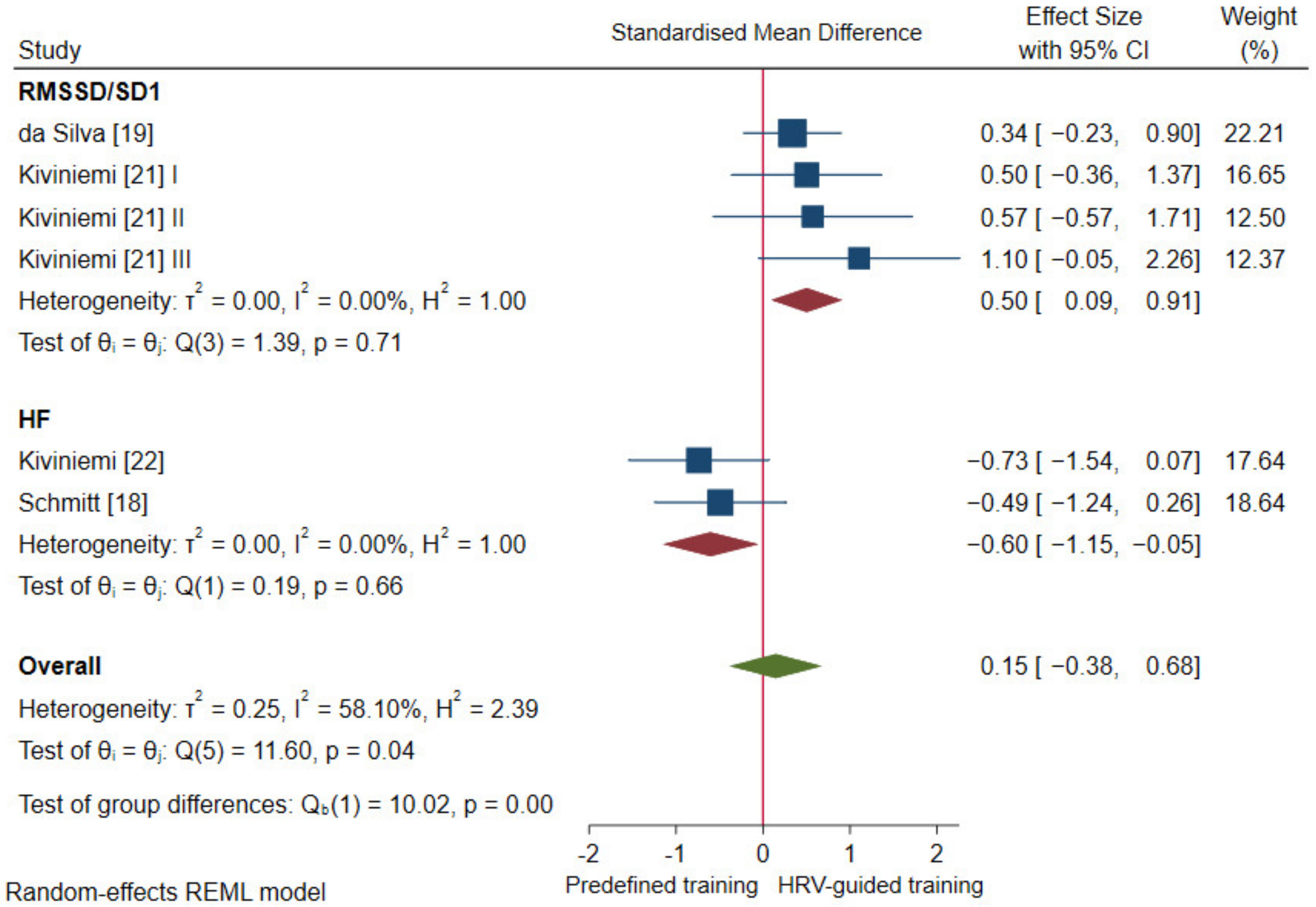
3.4.1. Cardiac-Vagal Modulation
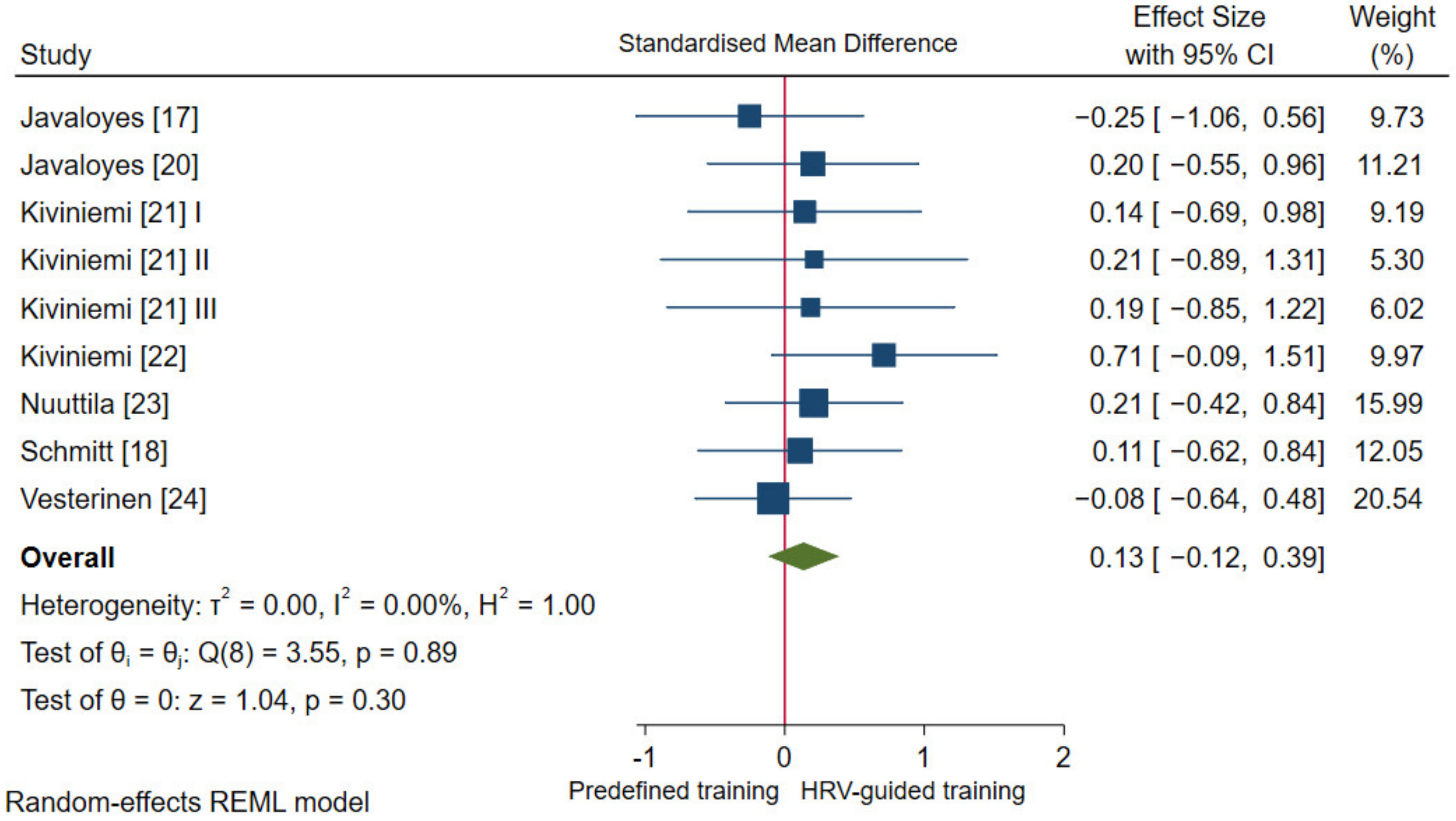
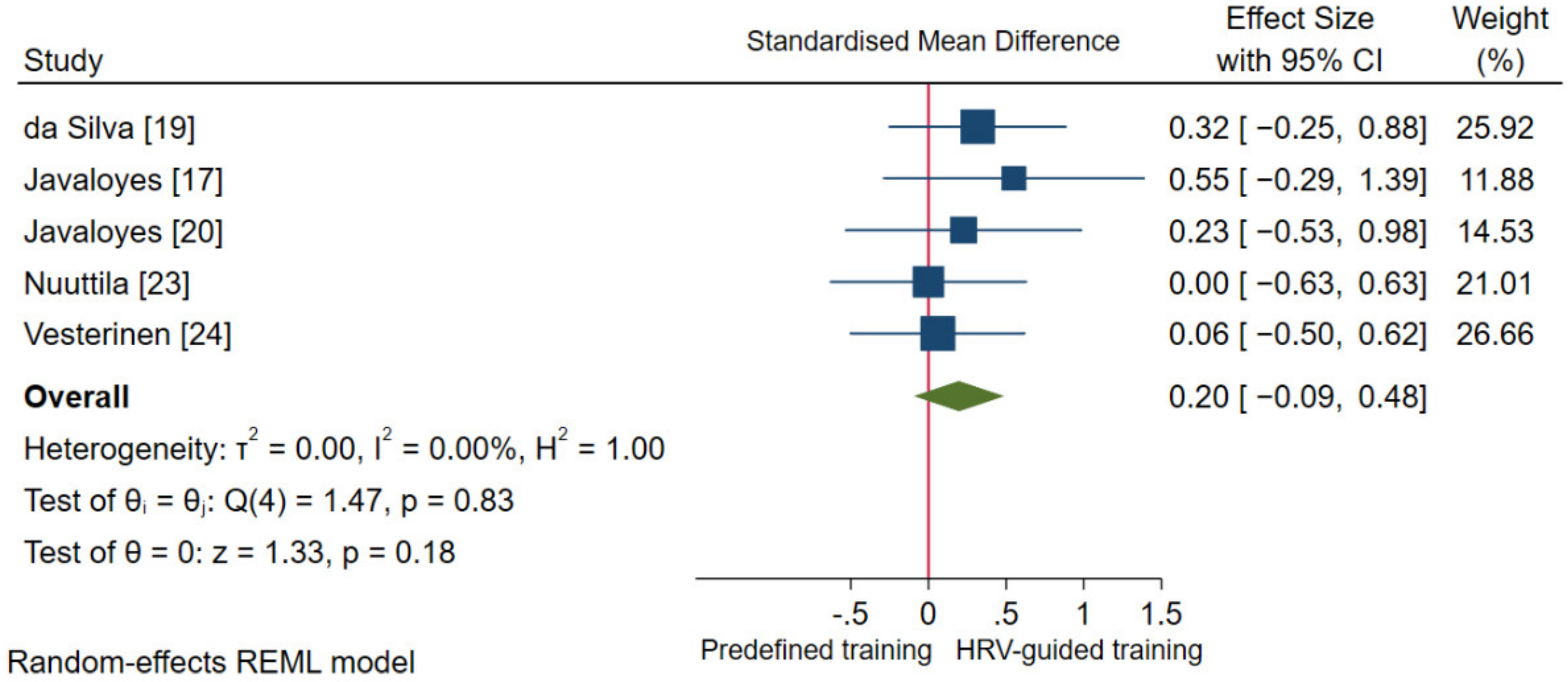
3.4.2. Aerobic Fitness Parameters and Endurance Performance
3.5. Publication Bias
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issurin, V. Block periodization versus traditional training theory: A review. J. Sports Med. Phys. Fit. 2008, 48, 65–75. [Google Scholar]
- Issurin, V.B. Benefits and Limitations of Block Periodized Training Approaches to Athletes’ Preparation: A Review. Sports Med. 2015, 46, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T. Individual differences in response to regular physical activity. Med. Sci. Sports Exerc. 2001, 33, S446–S451, Discussion S452–S453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, C.; An, P.; Rice, T.; Skinner, J.S.; Wilmore, J.H.; Gagnon, J.; Pérusse, L.; Leon, A.S.; Rao, D.C. Familial aggregation of vo(2max) response to exercise training: Results from the heritage family study. J. Appl. Physiol. 1999, 87, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Hautala, A.J.; Kiviniemi, A.; Mäkikallio, T.H.; Kinnunen, H.; Nissilä, S.; Huikuri, H.V.; Tulppo, M. Individual differences in the responses to endurance and resistance training. Graefe Arch. Clin. Exp. Ophthalmol. 2006, 96, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac Parasympathetic Reactivation Following Exercise: Implications for Training Prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]
- Carter, J.B.; Banister, E.W.; Blaber, A.P. The Effect of Age and Gender on Heart Rate Variability after Endurance Training. Med. Sci. Sports Exerc. 2003, 35, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Vesterinen, V.; Häkkinen, K.; Hynynen, E.; Mikkola, J.; Hokka, L.; Nummela, A. Heart rate variability in prediction of individual adap-tation to endurance training in recreational endurance runners. Scand. J. Med. Sci. Sports 2013, 23, 171–180. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Leicht, A.S.; Bishop, D.; Barbero-Álvarez, J.C.; Nakamura, F.Y. Seasonal Changes in Physical Performance and Heart Rate Variability in High Level Futsal Players. Endoscopy 2012, 34, 424–430. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Heart rate variability in elite triathletes, is variation in variability the key to effec-tive training? A case comparison. Eur. J. Appl. Physiol. 2012, 112, 3729–3741. [Google Scholar] [CrossRef]
- Stanley, J.; D’Auria, S.; Buchheit, M. Cardiac Parasympathetic Activity and Race Performance: An Elite Triathlete Case Study. Int. J. Sports Physiol. Perform. 2015, 10, 528–534. [Google Scholar] [CrossRef]
- Besnier, F.; Labrunee, M.; Pathak, A.; Pavy-Le Traon, A.; Galès, C.; Sénard, J.M.; Guiraud, T. Exercise training-induced modification in autonomic nervous system: An update for car-diac patients. Ann. Phys. Rehabil. Med. 2017, 60, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Phoemsapthawee, J.; Prasertsri, P.; Leelayuwat, N. Heart rate variability responses to a combined exercise training program: Correla-tion with adiposity and cardiorespiratory fitness changes in obese young men. J. Exerc. Rehabil. 2019, 15, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Grote, S.; Ricci, J.M.; Dehom, S.; Modeste, N.; Sealy, D.-A.; Tarleton, H.P. Heart Rate Variability and Cardiovascular Adaptations Among Cancer-Survivors Following a 26-Week Exercise Intervention. Integr. Cancer Ther. 2020, 19, 1534735420969816. [Google Scholar] [CrossRef]
- Javaloyes, A.; Sarabia, J.M.; Lamberts, R.P.; Plews, D.; Moya-Ramon, M. Training Prescription Guided by Heart Rate Variability Vs. Block Periodization in Well-Trained Cyclists. J. Strength Cond. Res. 2020, 34, 1511–1518. [Google Scholar] [CrossRef]
- Schmitt, L.; Willis, S.J.; Fardel, A.; Coulmy, N.; Millet, G.P. Live high–train low guided by daily heart rate variability in elite Nordic-skiers. Graefe Arch. Clin. Exp. Ophthalmol. 2018, 118, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.F.; Ferraro, Z.M.; Adamo, K.B.; Machado, F.A. Endurance running training individually guided by hrv in untrained women. J. Strength Cond. Res. 2019, 33, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Javaloyes, A.; Sarabia, J.M.; Lamberts, R.P.; Moya-Ramón, M. Training Prescription Guided by Heart-Rate Variability in Cycling. Int. J. Sports Physiol. Perform. 2019, 14, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiviniemi, A.M.; Hautala, A.; Kinnunen, H.; Nissilä, J.; Virtanen, P.; Karjalainen, J.; Tulppo, M. Daily Exercise Prescription on the Basis of HR Variability among Men and Women. Med. Sci. Sports Exerc. 2010, 42, 1355–1363. [Google Scholar] [CrossRef]
- Kiviniemi, A.M.; Hautala, A.J.; Kinnunen, H.; Tulppo, M.P. Endurance training guided individually by daily heart rate variability measurements. Eur. J. Appl. Physiol. 2007, 101, 743–751. [Google Scholar] [CrossRef]
- Nuuttila, O.-P.; Nikander, A.; Polomoshnov, D.; Laukkanen, J.; Häkkinen, K. Effects of HRV-Guided vs. Predetermined Block Training on Performance, HRV and Serum Hormones. Endoscopy 2017, 38, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Vesterinen, V.; Nummela, A.; Heikura, I.; Laine, T.; Hynynen, E.; Botella, J.; Häkkinen, K. Individual Endurance Training Prescription with Heart Rate Variability. Med. Sci. Sports Exerc. 2016, 48, 1347–1354. [Google Scholar] [CrossRef] [Green Version]
- Granero-Gallegos, A.; González-Quílez, A.; Plews, D.; Carrasco-Poyatos, M. HRV-Based Training for Improving VO2max in Endurance Athletes. A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7999. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Medellin Ruiz, J.P.; Rubio-Arias, J.A.; Clemente-Suarez, V.J.; Ramos-Campo, D.J. Effectiveness of training prescription guided by heart rate variability versus predefined training for physiological and aerobic performance improvements: A systematic review and meta-analysis. Appl. Sci. 2020, 10, 8532. [Google Scholar] [CrossRef]
- Düking, P.; Zinner, C.; Reed, J.L.; Holmberg, H.; Sperlich, B. Predefined vs data-guided training prescription based on autonomic nervous system variation: A systematic review. Scand. J. Med. Sci. Sports 2020, 30, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Hornikel, B.; Nakamura, F.Y.; Esco, M.R. Effect of competitive status and experience on heart rate variability profiles in col-legiate sprint-swimmers. J. Strength Cond. Res. 2021. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R. Worldwide survey of fitness trends for 2020. ACSM Health Fit. J. 2019, 23, 10–18. [Google Scholar] [CrossRef]
- Jandackova, V.K.; Scholes, S.; Britton, A.; Steptoe, A. Healthy Lifestyle and Cardiac Vagal Modulation Over 10 Years: Whitehall II Cohort Study. J. Am. Heart Assoc. 2019, 8, e012420. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, S.; Bossard, M.; Repilado, F.J.R.; Good, N.; Schön, T.; Zimny, M.; Probst-Hensch, N.M.; Schmidt-Trucksäss, A.; Risch, M.; Risch, L.; et al. Healthy lifestyle and heart rate variability in young adults. Eur. J. Prev. Cardiol. 2016, 23, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Jarczok, M.N.; Koenig, J.; Wittling, A.; Fischer, J.E.; Thayer, J.F. First Evaluation of an Index of Low Vagally-Mediated Heart Rate Variability as a Marker of Health Risks in Human Adults: Proof of Concept. J. Clin. Med. 2019, 8, 1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfiqar, U.; Jurivich, D.A.; Gao, W.; Singer, D.H. Relation of High Heart Rate Variability to Healthy Longevity. Am. J. Cardiol. 2010, 105, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Nummela, A.; Hynynen, E.; Kaikkonen, P.; Rusko, H. Endurance Performance and Nocturnal HRV Indices. Int. J. Sports Med. 2010, 31, 154–159. [Google Scholar] [CrossRef]
- Buchheit, M.; Chivot, A.; Parouty, J.; Mercier, D.; Al Haddad, H.; Laursen, P.B.; Ahmaidi, S. Monitoring endurance running performance using cardiac parasympathetic function. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 108, 1153–1167. [Google Scholar] [CrossRef]
- Pereira, L.A.; Abad CC, C.; Leiva, D.F.; Oliveira, G.; Carmo, E.C.; Kobal, R.; Loturco, I. Relationship between resting heart rate variability and intermittent endurance perfor-mance in novice soccer players. Res. Q. Exerc. Sport 2019, 90, 355–361. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R. Endurance performance relates to resting heart rate and its variability: A case study of a collegiate male cross-country athlete. Aust. Strength Cond. 2014, 22, 39–45. [Google Scholar]
- Lamberts, R.P.; Swart, J.; Capostagno, B.; Noakes, T.D.; Lambert, M.I. Heart rate recovery as a guide to monitor fatigue and predict changes in performance parameters. Scand. J. Med. Sci. Sports 2009, 20, 449–457. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Mertgen, A. A unifying conceptual framework of factors associated to cardiac vagal control. Heliyon 2018, 4, e01002. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ Clin. Res. Ed. 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; Sage: New York, NY, USA, 1991. [Google Scholar]
- Hopkins, W.; Marshall, S.; Batterham, A.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, A.B.; Siedlik, J.A.; Wecht, J.M.; Deckert, J.A.; Nguyen, N.D.; Weir, J.P. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 2017, 56, 674–678. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in me-ta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, H.R.; Sutton, A.J.; Borenstein, M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments; John Wiley & Sons: New York, NY, USA, 2006; pp. 1–7. [Google Scholar]
- Da Silva, D.F.; Peixoto, E.M.; Ferraro, Z.M.; Adamo, K.B.; Machado, F.A. Changes in mood state and recovery-stress perception after an HRV-guided running program in untrained women. Rev. De Psicol. Del Deporte 2020, 29, 0083–0094. [Google Scholar]
- Hottenrott, K.; Ludyga, S.; Gronwald, T.; Schulze, S. Effects of an individualized and time based training program on physical fitness and mood states in recreational endurance runners. Am. J. Sports Sci. 2014, 2, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.E.; Beightol, L.A.; Koh, J.; Eckberg, D.L. Important influence of respiration on human r-r interval power spectra is largely ig-nored. J. Appl. Physiol. 1993, 75, 2310–2317. [Google Scholar] [CrossRef] [Green Version]
- Saboul, D.; Pialoux, V.; Hautier, C. The impact of breathing on HRV measurements: Implications for the longitudinal follow-up of athletes. Eur. J. Sport Sci. 2013, 13, 534–542. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Evaluating training adaptation with heart-rate measures: A methodological com-parison. Int. J. Sports Physiol. Perform. 2013, 8, 688–691. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med. Sci. Sports Exerc. 2013, 45, 2061–2071. [Google Scholar]
- Manresa-Rocamora, A.; Flatt, A.A.; Casanova-Lizón, A.; Ballester-Ferrer, J.A.; Sarabia, J.M.; Vera-Garcia, F.J.; Moya-Ramón, M. Heart rate-based indices to detect parasympathetic hyperactivity in functionally overreached athletes. A meta-analysis. Scand. J. Med. Sci. Sports 2021, 31, 1164–1182. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Le Meur, Y.; Hausswirth, C.; Kilding, A.E.; Buchheit, M. Monitoring Training with Heart-Rate Variability: How Much Compliance Is Needed for Valid Assessment? Int. J. Sports Physiol. Perform. 2014, 9, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Esco, M.R. Smartphone-Derived Heart-Rate Variability and Training Load in a Women’s Soccer Team. Int. J. Sports Physiol. Perform. 2015, 10, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Magagnin, V.; Bassani, T.; Bari, V.; Turiel, M.; Maestri, R.; Pinna, G.D.; Porta, A. Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indices. Physiol. Meas. 2011, 32, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Simon, C.; Piquard, F.; Ehrhart, J.; Brandenberger, G. Effects of increased training load on vagal-related indexes of heart rate variability: A novel sleep approach. Am. J. Physiol. Circ. Physiol. 2004, 287, H2813–H2818. [Google Scholar] [CrossRef] [Green Version]
- Goldberger, J.J.; Challapalli, S.; Tung, R.; Parker, M.A.; Kadish, A.H. Relationship of Heart Rate Variability to Parasympathetic Effect. Circulation 2001, 103, 1977–1983. [Google Scholar] [CrossRef] [Green Version]
- Kiviniemi, A.M.; Hautala, A.; Seppänen, T.; Mäkikallio, T.H.; Huikuri, H.V.; Tulppo, M.P. Saturation of high-frequency oscillations of R-R intervals in healthy subjects and patients after acute myocardial infarction during ambulatory conditions. Am. J. Physiol. Circ. Physiol. 2004, 287, H1921–H1927. [Google Scholar] [CrossRef] [Green Version]
- Mourot, L.; Bouhaddi, M.; Tordi, N.; Rouillon, J.-D.; Regnard, J. Short- and long-term effects of a single bout of exercise on heart rate variability: Comparison between constant and interval training exercises. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 92, 508–517. [Google Scholar] [CrossRef]
- Vescovi, J.D. Intra-Individual Variation of HRV during Orthostatic Challenge in Elite Male Field Hockey Players. J. Med. Syst. 2019, 43, 328. [Google Scholar] [CrossRef]
- Quinto, S.S.; Lopez-Grueso, R.; Brizuela, G.; Flatt, A.A.; Moya-Ramón, M. Influence of Training Models at 3900-m Altitude on the Physiological Response and Performance of a Professional Wheelchair Athlete: A Case Study. J. Strength Cond. Res. 2019, 33, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Esco, M.R. Evaluating Individual Training Adaptation with Smartphone-Derived Heart Rate Variability in a Collegiate Female Soccer Team. J. Strength Cond. Res. 2016, 30, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Howells, D. Effects of varying training load on heart rate variability and running performance among an Olympic rugby sevens team. J. Sci. Med. Sport 2019, 22, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.H.; Moreira, A.; Gonçalves, H.R.; Stanganelli, L.C. Effect of Overload and Tapering on Individual Heart Rate Variability, Stress Tolerance, and Intermittent Running Performance in Soccer Players during a Preseason. J. Strength Cond. Res. 2019, 33, 1222–1231. [Google Scholar] [CrossRef]
- González-Fimbres, R.A.; Hernández-Cruz, G.; Flatt, A.A. Ultrashort Versus Criterion Heart Rate Variability among International-Level Girls’ Field Hockey Players. Int. J. Sports Physiol. Perform. 2021, 16, 985–992. [Google Scholar] [CrossRef]
- Nakamura, F.Y.; Antunes, P.; Nunes, C.; Costa, J.A.; Esco, M.R.; Travassos, B. Heart rate variability changes from traditional vs. Ul-tra-short-term recordings in relation to preseason training load and performance in futsal players. J. Strength Cond. Res. 2020, 34, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Boullosa, D.A.; Abreu, L.; Nakamura, F.Y.; Muñoz, V.E.; Domínguez, E.; Leicht, A.S. Cardiac Autonomic Adaptations in Elite Spanish Soccer Players during Preseason. Int. J. Sports Physiol. Perform. 2013, 8, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Flatt, A.A.; Hornikel, B.; Esco, M.R. Heart rate variability and psychometric responses to overload and tapering in collegiate sprint-swimmers. J. Sci. Med. Sport 2017, 20, 606–610. [Google Scholar] [CrossRef]
- Schmitt, L.; Regnard, J.; Desmarets, M.; Mauny, F.; Mourot, L.; Fouillot, J.-P.; Coulmy, N.; Millet, G. Fatigue Shifts and Scatters Heart Rate Variability in Elite Endurance Athletes. PLoS ONE 2013, 8, e71588. [Google Scholar] [CrossRef]
- Hautala, A.J.; Kiviniemi, A.; Tulppo, M. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009, 33, 107–115. [Google Scholar] [CrossRef]
- Bellenger, C.R.; Fuller, J.; Thomson, R.; Davison, K.; Robertson, E.Y.; Buckley, J. Monitoring Athletic Training Status through Autonomic Heart Rate Regulation: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1461–1486. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zuegel, M.; Steinacker, J.M.; Schumann, U. Heart rate recovery and risk of cardiovascular events and all-cause mortality: A meta-analysis of pro-spective cohort studies. J. Am. Heart Assoc. 2017, 6, e005505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjønna, A.E.; Leinan, I.M.; Bartnes, A.T.; Jenssen, B.M.; Gibala, M.J.; Winett, R.A.; Wisløff, U. Low- and High-Volume of Intensive Endurance Training Significantly Improves Maximal Oxygen Uptake after 10-Weeks of Training in Healthy Men. PLoS ONE 2013, 8, e65382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carte, H.; Jones, A.M.; Doust, J.H. Effect of 6 weeks of endurance training on the lactate minimum speed. J. Sports Sci. 1999, 17, 957–967. [Google Scholar] [CrossRef]
- Jones, A.M.; Carter, H. The Effect of Endurance Training on Parameters of Aerobic Fitness. Sports Med. 2000, 29, 373–386. [Google Scholar] [CrossRef]
- Gisselman, A.S.; Baxter, G.D.; Wright, A.; Hegedus, E.; Tumilty, S.; Information, P.E.K.F.C. Musculoskeletal overuse injuries and heart rate variability: Is there a link? Med. Hypotheses 2016, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.S.; Jaskólski, A.; Jaskólska, A.; Krasnoff, J.; Gagnon, J.; Leon, A.S.; Rao, D.C.; Wilmore, J.H.; Bouchard, C. Age, sex, race, initial fitness, and response to training: The HERITAGE Family Study. J. Appl. Physiol. 2001, 90, 1770–1776. [Google Scholar] [CrossRef] [Green Version]
- Behrens, K.; Hottenrott, K.; Weippert, M.; Montanus, H.; Kreuzfeld, S.; Rieger, A.; Lübke, J.; Werdan, K.; Stoll, R. Individualization of exercise load control for inpatient cardiac rehabilitation. Development and evaluation of a hrv-based intervention program for patients with ischemic heart failure. Herz 2015, 40 (Suppl. 1), 61–69. [Google Scholar] [CrossRef]
- Rankinen, T.; Rice, T.; Boudreau, A.; Leon, A.S.; Skinner, J.S.; Wilmore, J.H.; Rao, D.C.; Bouchard, C. Titin is a candidate gene for stroke volume response to endurance training: The HERITAGE Family Study. Physiol. Genom. 2003, 15, 27–33. [Google Scholar] [CrossRef] [Green Version]


| Study (Author, Year) | Training Group | Study Characteristics | Participant Characteristics | ||
|---|---|---|---|---|---|
| Country; Study Design; Journal | Sample Size; Men Percentage | Age; Weight; O2 Max | Athletic Status; Sport (If Applicable) | ||
| da Silva et al. [19] 2019 | HRV-G | Brazil; randomized controlled trial; J Strength Cond Res | 15; 0% | 25.8 ± 3.1 years; 62.9 ± 10.3 kg; NR | Sedentary; NA |
| PRED-G | 15; 0% | 27.7 ± 3.6 years; 61.3 ± 10.5 kg; NR | |||
| Javaloyes et al. [17] 2020 | HRV-G | Spain; non-randomized controlled trial; J Strength Cond Res | 7; 100% | 28.1 ± 13.2 years; 73.8 ± 4.6 kg; 58.9 ± 5.6 mL·kg−1·min−1 | Well-trained; cyclists |
| PRED-G | 8; 100% | 30.8 ± 10.5 years; 72.6 ± 10.4 kg; 59.0 ± 6.2 mL·kg−1·min−1 | |||
| Javaloyes et al. [20] 2019 | HRV-G | Spain; randomized controlled trial; Int J Sport Physiol Perform | 9; 100% | 39.2 ± 5.3 years; 76.9 ± 12.5 kg; 55.0 ± 7.6 mL·kg−1·min−1 | Well-trained; cyclists |
| PRED-G | 8; 100% | 37.6 ± 7.1 years; 78.7 ± 11.7 kg; 52.2 ± 6.5 mL·kg−1·min−1 | |||
| Kiviniemi et al. [21] 2010 | HRV-G | Finland; randomized controlled trial; Med Sci Sports Exerc | 7; 100% | 35.0 ± 4.0 years; 82.0 ± 9.0 kg; 50.0 ± 6.0 mL·kg−1·min−1 | Physically active; NA |
| PRED-G | 7; 100% | 37.0 ± 3.0 years; 81.0 ± 14.0 kg; 50.0 ± 7.0 mL·kg−1·min−1 | |||
| HRV-G | 7; 0% | 33.0 ± 4.0 years; 64.0 ± 5.0 kg; 36.0 ± 4.0 mL·kg−1·min−1 | |||
| HRV-G | 10; 0% | 35.0 ± 4.0 years; 64.0 ± 9.0 kg; 37.0 ± 5.0 mL·kg−1·min−1 | |||
| PRED-G | 7; 0% | 34.0 ± 4.0 years; 67.0 ± 6.0 kg; 35.0 ± 5.0 mL·kg−1·min−1 | |||
| Kiviniemi et al. [22] 2007 | HRV-G | Finland; randomized controlled trial; Eur J Appl Physiol | 9; 100% | 31.0 ± 6.0 years; 80.0 ± 8.0 kg; 56.0 ± 4.0 mL·kg−1·min−1 | Recreationally trained; runners |
| PRED-G | 8; 100% | 32.0 ± 5.0 years; 78.0 ± 8.0 kg; 54.0 ± 4.0 mL·kg−1·min−1 | |||
| Nuuttila et al. [23] 2017 | HRV-G | Finland; randomized controlled trial; Int J Sports Med | 13; 100% | 29.0 ± 4.0 years; 76.4 ± 9.4 kg; 53.6 ± 4.2 mL·kg−1·min−1 | Recreationally trained; endurance athletes |
| PRED-G | 11; 100% | 31.5 ± 5.0 years; 74.0 ± 5.7 kg; 54.2 ± 4.1 mL·kg−1·min−1 | |||
| Schmitt et al. [18] 2018 | HRV-G | France, randomized controlled trial; Eur J Appl Physiol | 9; 78% | 22.4 ± 3.9 years; 65.5 ± 7.2 kg; 66.7 ± 5.9 mL·kg−1·min−1 | Highly trained; cross-country and nordic-skiers |
| PRED-G | 9; 67% | 22.6 ± 3.2 years; 66.7 ± 10.1 kg; 63.7 ± 4.4 mL·kg−1·min−1 | |||
| Vesterinen et al. [24] 2016 | HRV-G | Finland; randomized controlled trial; Me Sci Sports Exerc | 20; NR * | 34.5 ± 7.5 years #; NR; 54.4 ± 6.2 mL·kg−1·min−1 | Recreationally trained; runners |
| PRED-G | 20; NR * | 34.5 ± 7.5 years #; NR; 53.0 ± 5.8 mL·kg−1·min−1 | |||
| Study (Author) | Intervention Characteristics | Methodological Approach Characteristics | ||
|---|---|---|---|---|
| Type of Exercise; Length; Training Frequency | Device; Time of Day; Stabilization Period (Min); Recording Posture (Length) *; Breathing Control | HRV Index; Single Day vs. Averaged; Number of Averaged Values | Fixed vs. Moving; Number of Averaged Values; Range Used | |
| da Silva et al. [19] | Endurance training; 8 weeks; 3 days a week | Polar RS800cx; afternoon/evening; yes (2 min); standing (3 min); no (spontaneous) | RMSSD; single day; NA | Moving; 5 up to 10 values; mean − (1·SD) |
| Javaloyes et al. [17] | Endurance training; 8 weeks; NA (habitual training volume) | HRV4training app; morning; yes (30 s); supine (1 min); NR | RMSSD; averaged; 7 values | Fixed $; 28 values; mean ± (0.5·SD) |
| Javaloyes et al. [20] | Endurance training; 8 weeks; NA (habitual training volume) | Polar H7 strap; morning; yes (30 s); supine (1 min); NR | RMSSD; averaged; 7 values | Fixed $; 28 values; mean ± (0.5·SD) |
| Kiviniemi et al. [21] | Endurance training; 8 weeks; at least 5 days a week # | Polar RS800; morning; yes (2 min); standing (3 min); no (spontaneous) | SD1; single day; NA | Moving; 7 up to 10 values; mean − (1·SD) |
| Kiviniemi et al. [22] | Endurance training; 4 weeks; 6 days a week # | Polar S180i; morning; yes (5 min); standing (5 min); NR | HF (auto-regressive method); single day; NA | Moving; 10 values; mean − (1·SD) |
| Nuuttila et al. [23] | Endurance and strength training; 8 weeks; 6 days a week # | Garmin 920XT; morning; supine (3 min); yes (until heart rate became steady); NR | RMSSD; averaged; 3 values | Fixed; 21 values; Mean |
| Schmitt et al. [18] | NR; 2 weeks; NR | Suunto; morning; yes (3 in supine and 1 in standing); supine and standing (5 + 5 min); NR | HF (Fast-Fourier Transform); single day; NA | Moving; 1 value; 70% of the previous day |
| Vesterinen et al. [24] | Endurance and strength training; 8 weeks; 2–4 days a week # | Omegawave Pro Mobile System; morning; no stabilization; supine (4 min); no (spontaneous) | RMSSD; averaged; 7 values | Fixed $; 28 values; mean ± (0.5·SD) |
| Study (Author); N (HRV-G/PRED-G) | Aerobic Fitness Parameters and Endurance Performance | Cardiac-Vagal Modulation | ||
|---|---|---|---|---|
| Assessment Characteristics | Parameter Assessed: SMD (95% CI) | Assessment Characteristics | Parameter Assessed: SMD (95% CI) | |
| da Silva et al. [19] N (15/15) | Incremental running test until volitional exhaustion | Maximal velocity (MAC): 0.07 (−0.49, 0.63) | Incremental maximal running test; recovery characteristics no reported | $ HRR 1 min: 0.20 (−0.36, 0.77) |
| 5 km running performance | Time (EP): 0.31 (−0.26, 0.87) | 3-day averaged values measured in standing position in the afternoon/evening | Standing RMSSD: 0.34 (−0.23, 0.90) | |
| Javaloyes et al. [17] N (7/8) | Incremental cardiopulmonary cycling test until volitional exhaustion | O2 max: −0.25 (−1.06, 0.56) | ||
| Maximal PO (MAC): 0.21 (−0.60, 1.02) | ||||
| PO at VT2 (AC_VT2): 0.42 (−0.41, 1.24) | ||||
| PO at VT1 (AC_VT1): 1.77 (0.67, 2.87) | ||||
| 40 min all-out time trial | Mean PO (EP): 0.55 (−0.29, 1.39) | |||
| Javaloyes et al. [20] N (9/8) | Incremental cardiopulmonary cycling test until volitional exhaustion | O2 max: 0.20 (−0.55, 0.96) | ||
| Maximal PO (MAC): 0.39 (−0.38, 1.15) | ||||
| PO at VT2 (AC_VT2): 0.32 (−0.44, 1.09) | ||||
| PO at VT1 (AC_VT1): 0.19 (−0.56, 0.95) | ||||
| 40 min all-out time trial | Mean PO (EP): 0.23 (−0.53, 0.98) | |||
| Kiviniemi et al. [21] I N (7/7) | Incremental cardiopulmonary cycling test until volitional exhaustion | O2 max: 0.14 (−0.69, 0.98) | 7-day averaged values measured in standing position in the morning | Standing SD1: 0.50 (−0.36, 1.37) |
| Maximal PO (MAC): 0.39 (−0.46, 1.24) | Standing HR: 0.48 (−0.38, 1.34) | |||
| Kiviniemi et al. [21] II N (7/3) | O2 max: 0.21 (−0.89, 1.31) | Standing SD1: 0.57 (−0.57, 1.71) | ||
| Maximal PO (MAC): −0.12 (−1.22, 0.97) | Standing HR: −0.24 (−1.34, 0.86) | |||
| Kiviniemi et al. [21] III N (10/3) | O2 max: 0.19 (−0.85, 1.22) | Standing SD1: 1.10 (−0.05, 2.26) | ||
| Maximal PO (MAC): −0.07 (−1.10, 0.96) | Standing HR: −0.15 (−1.18, 0.88) | |||
| Kiviniemi et al. [22] N (9/8) | Incremental cardiopulmonary running test until volitional exhaustion | O2 max: 0.71 (−0.09, 1.51) | 3-day averaged values measured in sitting and standing position in the morning | $ Sitting HF: 0.66 (−0.14, 1.45) |
| Maximal velocity (MAC): 0.25 (−0.51, 1.01) | $ Sitting HR: 0.00 (−0.75, 0.75) | |||
| Velocity at VT2 (AC_VT2): 0.38 (−0.39, 1.14) | Standing HF: −0.73 (−1.54, 0.07) | |||
| Standing HR: −0.21 (−0.97, 0.55) | ||||
| Nuuttila et al. [23] N (13/11) | Incremental cardiopulmonary running test until volitional exhaustion | O2 max: 0.21 (−0.42, 0.84) | Averaged 3-control weeks (11 days, measured every other night) and averaged last training week (4 days, measured every other night) | $ Night RMSSD: −0.05 (−0.68, 0.58) |
| Maximal velocity (MAC): 0.32 (−0.32, 0.96) | $ Night HF: 0.10 (−0.53, 0.73) | |||
| Velocity at VT2 (AC_VT2): 0.30 (−0.33, 0.94) | $ Night HR: 0.14 (−0.49, 0.77) | |||
| Velocity at VT1 (AC_VT1): 0.14 (−0.49, 0.77) | 21-day averaged (pre-intervention) and 7-day averaged (post-intervention) measured in supine position the morning | No reported | ||
| 3 km running performance | Time (EP): 0.00 (−0.63, 0.63) | |||
| Schmitt et al. [18] N (9/9) | Incremental cardiopulmonary running test until volitional exhaustion | O2 max: 0.11 (−0.62, 0.84) | Single day (pre-intervention) and 21-day averaged (post-intervention) values measured in supine and standing position in the morning | $ Supine HF: −0.17 (−0.90, 0.57) |
| $O2 at VT2: 0.24 (−0.49, 0.98) | $ Supine HR: 0.44 (−0.31, 1.18) | |||
| Standing HF: −0.49 (−1.24, 0.26) | ||||
| Standing HR: 0.19 (−0.55, 0.92) | ||||
| Vesterinen et al. [24] N (13/18) | Incremental cardiopulmonary running test until volitional exhaustion | O2 max: −0.08 (−0.64, 0.48) | ||
| Velocity at VT2 (AC_VT2): 0.06 (−0.49, 0.62) | ||||
| Velocity at VT1 (AC_VT1): 0.15 (−0.41, 0.71) | ||||
| 3 km running performance | Mean velocity (EP): 0.06 (−0.50, 0.62) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manresa-Rocamora, A.; Sarabia, J.M.; Javaloyes, A.; Flatt, A.A.; Moya-Ramón, M. Heart Rate Variability-Guided Training for Enhancing Cardiac-Vagal Modulation, Aerobic Fitness, and Endurance Performance: A Methodological Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10299. https://doi.org/10.3390/ijerph181910299
Manresa-Rocamora A, Sarabia JM, Javaloyes A, Flatt AA, Moya-Ramón M. Heart Rate Variability-Guided Training for Enhancing Cardiac-Vagal Modulation, Aerobic Fitness, and Endurance Performance: A Methodological Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(19):10299. https://doi.org/10.3390/ijerph181910299
Chicago/Turabian StyleManresa-Rocamora, Agustín, José Manuel Sarabia, Alejandro Javaloyes, Andrew A. Flatt, and Manuel Moya-Ramón. 2021. "Heart Rate Variability-Guided Training for Enhancing Cardiac-Vagal Modulation, Aerobic Fitness, and Endurance Performance: A Methodological Systematic Review with Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 19: 10299. https://doi.org/10.3390/ijerph181910299
APA StyleManresa-Rocamora, A., Sarabia, J. M., Javaloyes, A., Flatt, A. A., & Moya-Ramón, M. (2021). Heart Rate Variability-Guided Training for Enhancing Cardiac-Vagal Modulation, Aerobic Fitness, and Endurance Performance: A Methodological Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health, 18(19), 10299. https://doi.org/10.3390/ijerph181910299







