Diagnosis of Peritoneal Tuberculosis from Primary Peritoneal Cancer
Abstract
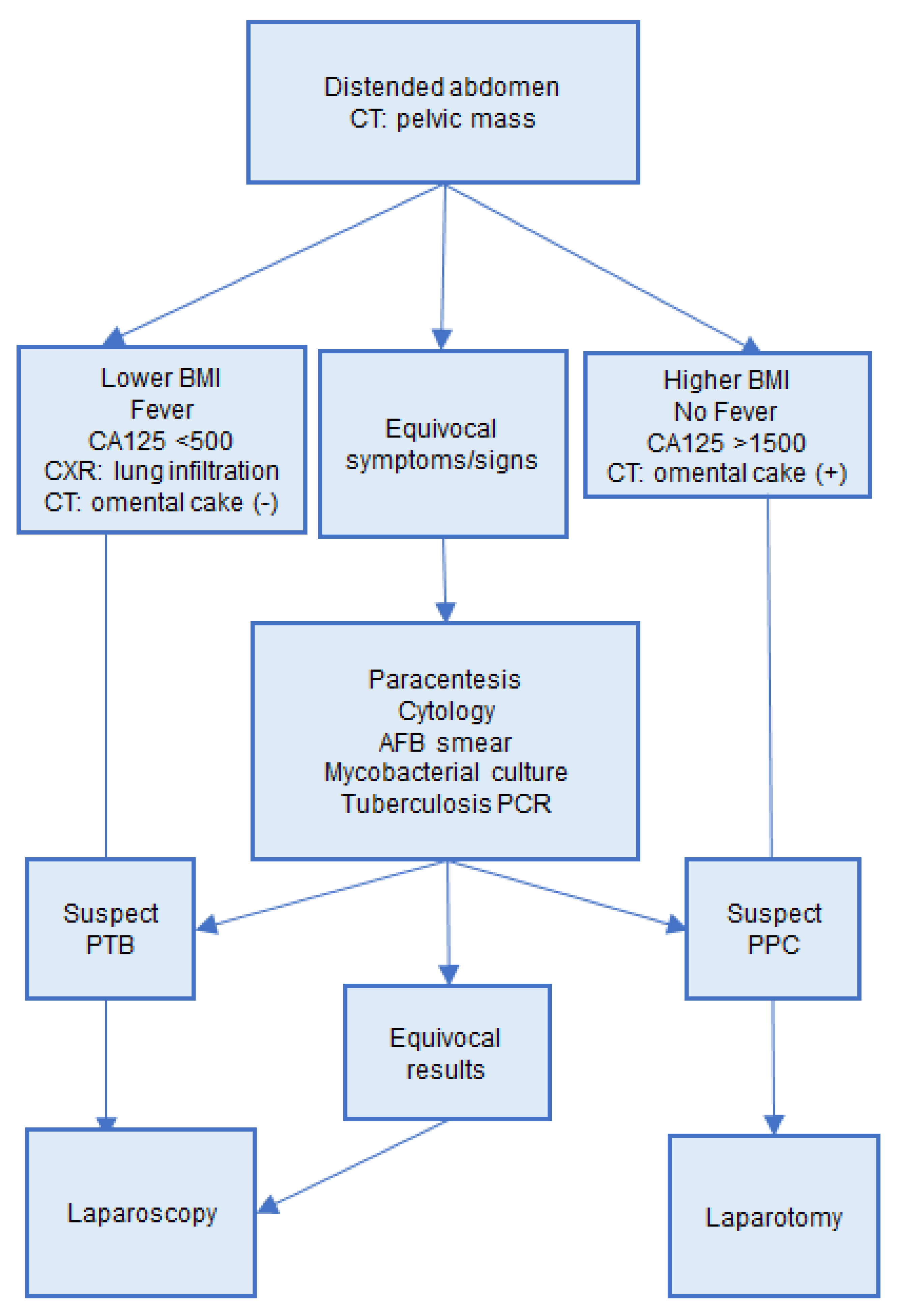
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Radiologic Characteristics
3.3. Intraoperative Findings
3.4. Duration before Anti-Tuberculosis Therapy in PTB Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Tuberculosis Report Fact Sheet 2019. Available online: https://www.who.int/tb/publications/factsheet_global.pdf (accessed on 2 October 2021).
- Global Tuberculosis Report 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf (accessed on 2 October 2021).
- Chao, W.C.; Yen, C.L.; Wu, C.H.; Shieh, C.C. How mycobacteria take advantage of the weakness in human immune system in the modern world. J. Microbiol. Immunol. Infect. 2020, 53, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Guirat, A.; Koubaa, M.; Mzali, R.; Abid, B.; Ellouz, S.; Affes, N.; Ben Jemaa, M.; Frikha, F.; Ben Amar, M.; Beyrouti, M.I. Peritoneal tuberculosis. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Koc, S.; Beydilli, G.; Tulunay, G.; Ocalan, R.; Boran, N.; Ozgul, N.; Kose, M.F.; Erdogan, Z. Peritoneal tuberculosis mimicking advanced ovarian cancer: A retrospective review of 22 cases. Gynecol. Oncol. 2006, 103, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.; Fader, A.; Wilbur, M.; Salimian, K.; Azadi, J.R.; Johnson, P.T.; Stone, R. Peritoneal tuberculosis: The great mimicker. Int. J. Gynecol. Cancer 2020. [CrossRef]
- Tan, O.; Luchansky, E.; Rosenman, S.; Pua, T.; Azodi, M. Peritoneal tuberculosis with elevated serum Ca-125 level mimicking advanced stage ovarian cancer: A case report. Arch. Gynecol. Obs. 2009, 280, 333–335. [Google Scholar] [CrossRef]
- Oge, T.; Ozalp, S.S.; Yalcin, O.T.; Kabukcuoglu, S.; Kebapci, M.; Arik, D.; Isikci, T. Peritoneal tuberculosis mimicking ovarian cancer. Eur. J. Obs. Gynecol. Reprod. Biol. 2012, 162, 105–108. [Google Scholar] [CrossRef]
- Lataifeh, I.; Matalka, I.; Hayajneh, W.; Obeidat, B.; Al Zou’bi, H.; Abdeen, G. Disseminated peritoneal tuberculosis mimicking advanced ovarian cancer. J. Obs. Gynaecol. 2014, 34, 268–271. [Google Scholar] [CrossRef]
- Epstein, B.M.; Mann, J.H. CT of abdominal tuberculosis. AJR Am. J. Roentgenol. 1982, 139, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, E.; Pombo, F. Peritoneal tuberculosis versus peritoneal carcinomatosis: Distinction based on CT findings. J. Comput. Assist. Tomogr. 1996, 20, 269–272. [Google Scholar] [CrossRef]
- Sorensen, R.D.; Schnack, T.H.; Karlsen, M.A.; Hogdall, C.K. Serous ovarian, fallopian tube and primary peritoneal cancers: A common disease or separate entities—a systematic review. Gynecol. Oncol. 2015, 136, 571–581. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, C.J.; Lee, Y.Y.; Kim, J.S.; Song, T.; Park, H.S.; Kim, M.K.; Kim, T.J.; Lee, J.W.; Lee, J.H.; et al. Peritoneal tuberculosis: A retrospective review of 20 cases and comparison with primary peritoneal carcinoma. Int. J. Gynecol. Cancer 2010, 20, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Sanai, F.M.; Bzeizi, K.I. Systematic review: Tuberculous peritonitis—presenting features, diagnostic strategies and treatment. Aliment. Pharm. Ther. 2005, 22, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.D.; Lee, S.I.; Moon, H.Y. Comparison between laparoscopy and noninvasive tests for the diagnosis of tuberculous peritonitis. World J. Surg. 2011, 35, 2369–2375. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.J.; Huang, H.F.; Shen, K.; Cui, Q.C.; Xiang, Y. Comparison between peritoneal tuberculosis and primary peritoneal carcinoma: A 16-year, single-center experience. Chin. Med. J. 2012, 125, 3256–3260. [Google Scholar]
- Kaya, M.; Kaplan, M.A.; Isikdogan, A.; Celik, Y. Differentiation of tuberculous peritonitis from peritonitis carcinomatosa without surgical intervention. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2011, 17, 312–317. [Google Scholar] [CrossRef]
- Portillo-Gomez, L.; Morris, S.L.; Panduro, A. Rapid and efficient detection of extra-pulmonary Mycobacterium tuberculosis by PCR analysis. Int. J. Tuberc. lung Dis. Off. J. Int. Union against Tuberc. Lung Dis. 2000, 4, 361–370. [Google Scholar]
- Saleh, M.A.; Hammad, E.; Ramadan, M.M.; Abd El-Rahman, A.; Enein, A.F. Use of adenosine deaminase measurements and QuantiFERON in the rapid diagnosis of tuberculous peritonitis. J. Med Microbiol. 2012, 61 Pt 4, 514–519. [Google Scholar] [CrossRef]
- Liao, Y.J.; Wu, C.Y.; Lee, S.W.; Lee, C.L.; Yang, S.S.; Chang, C.S.; Lee, T.Y. Adenosine deaminase activity in tuberculous peritonitis among patients with underlying liver cirrhosis. World J. Gastroenterol. WJG 2012, 18, 5260–5265. [Google Scholar]
- Kang, S.J.; Kim, J.W.; Baek, J.H.; Kim, S.H.; Kim, B.G.; Lee, K.L.; Jeong, J.B.; Jung, Y.J.; Kim, J.S.; Jung, H.C.; et al. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J. Gastroenterol. WJG 2012, 18, 2837–2843. [Google Scholar] [CrossRef]
- Kurman, R.J.; Ellenson, L.H.; Ronnett, B.M. (Eds.) Blaustein’s Pathology of the Female Genital Tract, 7nd ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Yeh, H.F.; Chiu, T.F.; Chen, J.C.; Ng, C.J. Tuberculous peritonitis: Analysis of 211 cases in Taiwan. Dig. Liver Dis. 2012, 44, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Piura, B.; Rabinovich, A.; Leron, E.; Yanai-Inbar, I.; Mazor, M. Peritoneal tuberculosis mimicking ovarian carcinoma with ascites and elevated serum CA-125: Case report and review of literature. Eur. J. Gynaecol. Oncol. 2002, 23, 120–122. [Google Scholar] [PubMed]
- Ha, H.K.; Jung, J.I.; Lee, M.S.; Choi, B.G.; Lee, M.G.; Kim, Y.H.; Kim, P.N.; Auh, Y.H. CT differentiation of tuberculous peritonitis and peritoneal carcinomatosis. AJR Am. J. Roentgenol. 1996, 167, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Zissin, R.; Gayer, G.; Chowers, M.; Shapiro-Feinberg, M.; Kots, E.; Hertz, M. Computerized tomography findings of abdominal tuberculosis: Report of 19 cases. Isr. Med. Assoc. J. 2001, 3, 414–418. [Google Scholar]
- Coupland, G.A.; Townend, D.M.; Martin, C.J. Peritoneoscopy—use in assessment of intra-abdominal malignancy. Surgery 1981, 89, 645–649. [Google Scholar] [PubMed]

| PTB (n = 23) | PPC (n = 47) | p Value | |
|---|---|---|---|
| Age, years, mean ± SD range | 57.5 ± 16.9 | 61.5 ± 8.6 | 0.29 |
| (23–83) | (41–78) | ||
| Underlying disease * n (%) | 15 | 26 | 0.23 |
| (65.2) | (55.3) | ||
| BMI, kg/m2, mean ± SD range | 21.9 ± 3.7 | 25.2 ± 4.1 | 0.003 |
| (17.1–32.4) | (18.9–38.8) | ||
| Presenting symptoms, n (%) | |||
| Abdominal distension | 15 (65.2) | 33 (70.2) | 0.44 |
| Abdominal pain, | 9 (39.1) | 12 (25.5) | 0.19 |
| Cough | 7 (30.4) | 12 (25.5) | 0.46 |
| Poor appetite | 8 (34.7) | 17 (36.2) | 0.56 |
| Body weight loss | 7 (30.4) | 11 (23.4) | 0.36 |
| Body temperature | 37.8 ± 1.3 | 36.7 ± 0.6 | 0.001 |
| Fever, n (%) | 8 (34.7) | 0 | <0.001 |
| Laboratory data | |||
| CA125, U/mL, mean ± SD | 508.0 ± 266.1 | 2130.1 ± 2367.2 | <0.001 |
| WBC count, K/uL, mean ± SD | 5179.6 ± 1502.2 | 7716.2 ± 2741.8 | <0.001 |
| PTB (n = 23) | PPC (n = 47) | p Value | |
|---|---|---|---|
| Chest X-ray | |||
| Pleural effusion, n (%) | 11 (47.8) | 12(25.5) | 0.11 |
| Pulmonary infiltration/ consolidation, n (%) | 12 (52.2) | 3 (6.4) | <0.001 |
| Computer Tomography | |||
| Pelvic mass | 5 (21.7) | 13(27.7) | 0.81 |
| Ascites | 0.51 | ||
| None | 2(8.7) | 7 (14.9) | |
| Small | 5 (21.7) | 5 (10.6) | |
| Moderate | 5 (21.7) | 6 (12.8) | |
| Massive | 11 (47.8) | 29 (61.7) | |
| Omental and mesentery changes | <0.001 | ||
| None | 9 (39.1) | 5 (10.6) | |
| Mild/nodular | 11 (47.8) | 4 (8.5) | |
| Severe/mass | 1 (4.3) | 35 (74.5) | |
| NA | 2 | 3 | |
| Peritoneum changes | 0.67 | ||
| None | 5 (21.7) | 7 (14.9) | |
| Thickening | 11 (47.8) | 28 (59.6) | |
| Nodular | 5 (21.7) | 12 (25.5) | |
| Lymphadenopathy | 0.16 | ||
| None/Small | 23 (100) | 42 (89.4) | |
| Large | 0 | 5 (10.6) |
| PTB (n = 16) | PPC (n = 47) | p Value | |
|---|---|---|---|
| Omentum | <0.001 | ||
| None, n (%) | 9 (52.9) | 6 (12.7) | |
| Nodule, n (%) | 3 (17.6) | 20 (42.6) | |
| Cake, n (%) | 0 | 16 (34.0) | |
| NA | 5 | 5 | |
| Peritoneum | 0.96 | ||
| None, n (%) | 2 (11.8) | 8 (17.0) | |
| Papule, n (%) | 2 (11.8) | 3 (6.4) | |
| Miliary seedings | 8 (47.1) | 26 (55.3) | |
| Nodule (>1 cm), n (%) | 5 (29.4) | 9 (19.1) | |
| NA | 0 | 1 | |
| Ascites, mL, mean ± SD | 1368 ± 1462.5 | 1844.2 ± 2008.8 | 0.33 |
| Laboratory | Symptoms/Signs | Radiographic Findings | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, Year | CA-125 Level | Abdominal Distension | Abdominal Pain | Fever | Pleural Effusion | Severe Omentum Involvement | Peritoneal Thickening | Ascites (Large Amount) | |
| Ha et al., 1996 (study period: 1991–1994) [25] | |||||||||
| PTB (n = 42) | 37 (8–74) | NA | NA | NA | NA | NA | 5% | NA | 31% |
| PPC (n = 93) | 51 (19–89) | NA | NA | NA | NA | NA | 17% | NA | 30% |
| Choi et al., 2010 (study period: 1996–2006) [13] | |||||||||
| PTB (n = 20) | 39 (23–69) | 448 (32–1725) | 70% | 50% | 20% | 20% | 45% | 35% | 55% |
| PPC (n = 17) | 63 (50–73) | 1484 (42–14,380) | 53% | 24% | 0 | 18% | 76% | 41% | 41% |
| Wang et al., 2012 (study period: 1995–2010) [16] | |||||||||
| PTB (n = 30) | 39 ± 17 | 345 (0.6–850) | 53% | 23% | NA | 13% | NA | 3% | 67% |
| PPC (n = 38) | 60 ± 11 | 2626 (35–>500) | 79% | 18% | NA | 24% | NA | 34% | 76% |
| Koc et al., 2006 (study period: 1992–2004) [5] | |||||||||
| PTB (n = 22) | 37 (21–68) | 565 (3–2021) | 82% | 55% | 9% | 23% | 9% | 5% | 100% |
| Lataifeh et al., 2014 (study period: 2002–2012) [9] | |||||||||
| PTB (n = 16) | 30 (13–65) | 319 (45–1072) | 81% | 81% | 38% | 25% | 50% | 6% | 100% |
| Oge et al., 2012 (study period 2000–2011) [8] | |||||||||
| PTB (n = 20) | 38 (16–70) | 289 (4–793) | 65% | 70% | 15% | NA | 30% | 65% | 85% |
| Hong et al.; 2011 (study period 2002–2010) [15] | |||||||||
| PTB (n = 60) | 49 (21–80) | 474 (61–1251) | 71% | 37% | 37% | 29% | NA | NA | 93% |
| Zissin et al., 2001, (study period 1988–1999) [26] | |||||||||
| PTB (n = 19) | 48 (20–85) | NA | 16% | 16% | NA | 5% | 53% | 42% | 53% |
| Current Study (study period: 2006–2018) | |||||||||
| PTB (n = 23) | 58 (23–83) | 508 (82.3–1214) | 65% | 39% | 48% | 48% | 4% | 70% | 48% |
| PPC (n = 47) | 62 (41–78) | 2130 (56.9–11751) | 70% | 26% | 4.2% | 26% | 74% | 85% | 62% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.-H.; Torng, P.-L.; Lee, C.-Y.; Lee, K.-H.; Hsu, H.-C.; Cheng, W.-F. Diagnosis of Peritoneal Tuberculosis from Primary Peritoneal Cancer. Int. J. Environ. Res. Public Health 2021, 18, 10407. https://doi.org/10.3390/ijerph181910407
Chen I-H, Torng P-L, Lee C-Y, Lee K-H, Hsu H-C, Cheng W-F. Diagnosis of Peritoneal Tuberculosis from Primary Peritoneal Cancer. International Journal of Environmental Research and Public Health. 2021; 18(19):10407. https://doi.org/10.3390/ijerph181910407
Chicago/Turabian StyleChen, I-Hui, Pao-Ling Torng, Chia-Yi Lee, Kuang-Han Lee, Heng-Cheng Hsu, and Wen-Fang Cheng. 2021. "Diagnosis of Peritoneal Tuberculosis from Primary Peritoneal Cancer" International Journal of Environmental Research and Public Health 18, no. 19: 10407. https://doi.org/10.3390/ijerph181910407
APA StyleChen, I. -H., Torng, P. -L., Lee, C. -Y., Lee, K. -H., Hsu, H. -C., & Cheng, W. -F. (2021). Diagnosis of Peritoneal Tuberculosis from Primary Peritoneal Cancer. International Journal of Environmental Research and Public Health, 18(19), 10407. https://doi.org/10.3390/ijerph181910407






