Utility of Self-Reported Heat Stress Symptoms and NGAL Biomarker to Screen for Chronic Kidney Disease of Unknown Origin (CKDu) in Sri Lanka
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Measures
2.3. Laboratory Assessment of Urine Markers
2.4. Analyses
3. Results
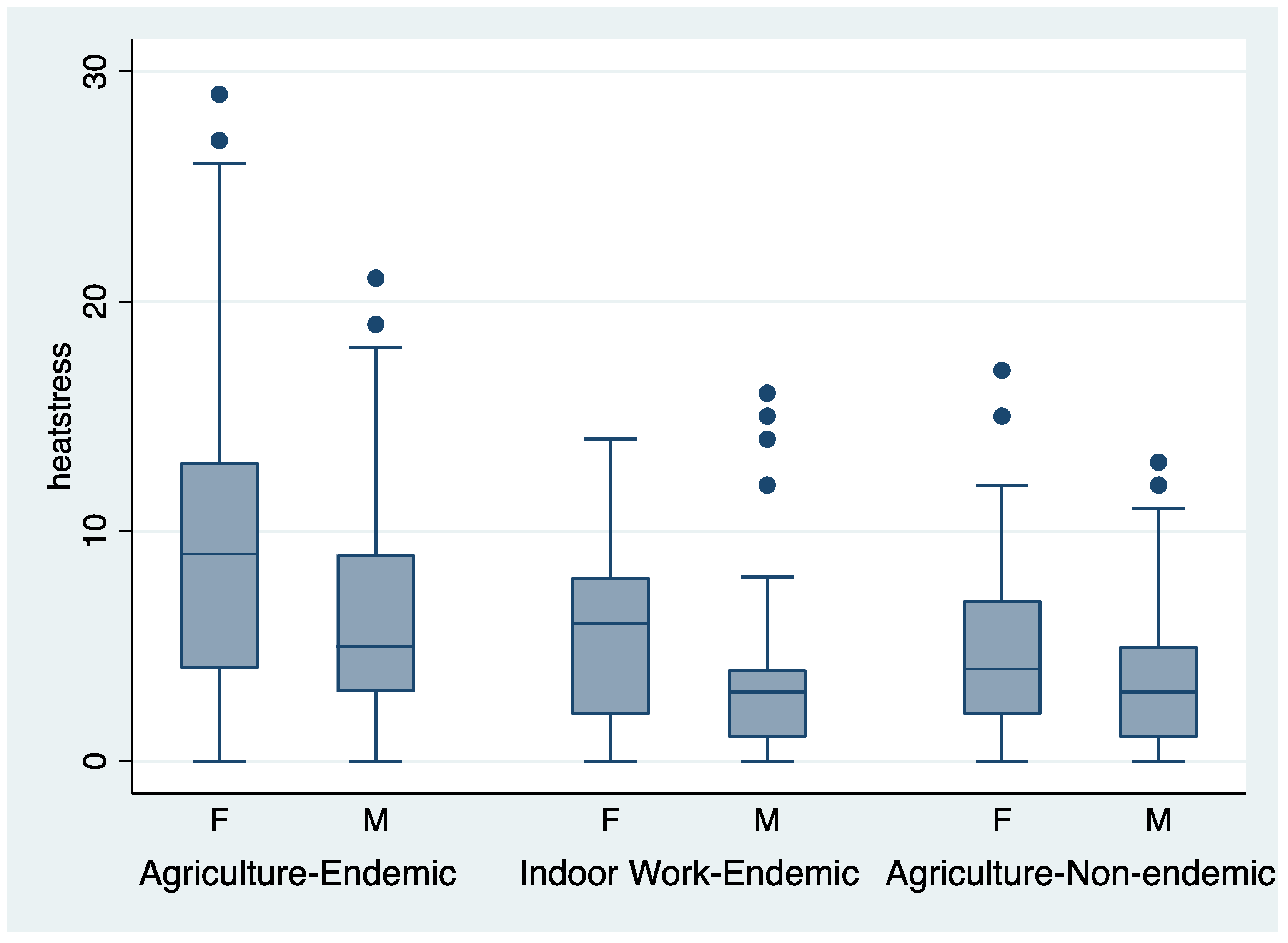
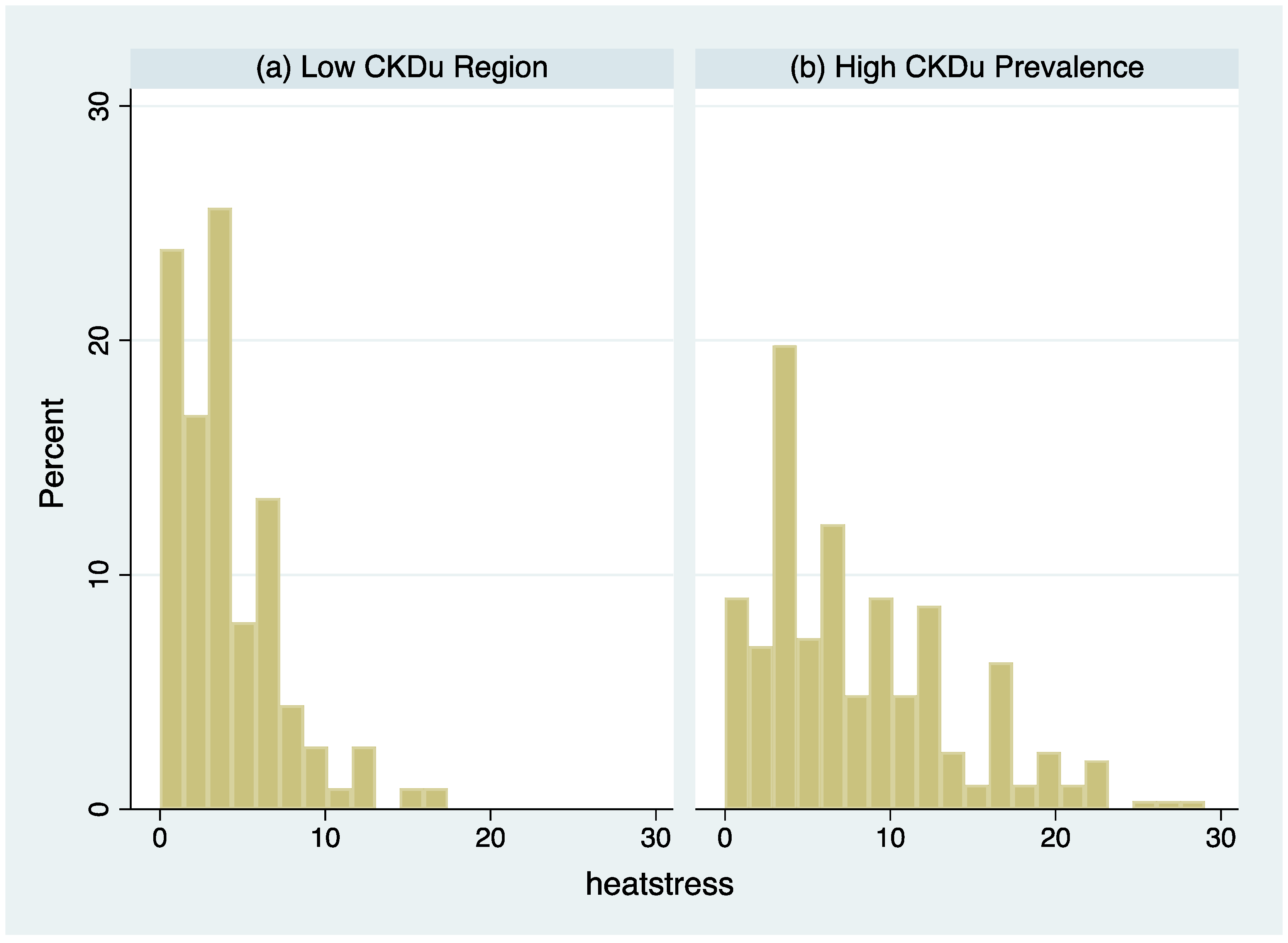
3.1. Heat Stress Symptoms Were More Frequent in CKDu Endemic Regions
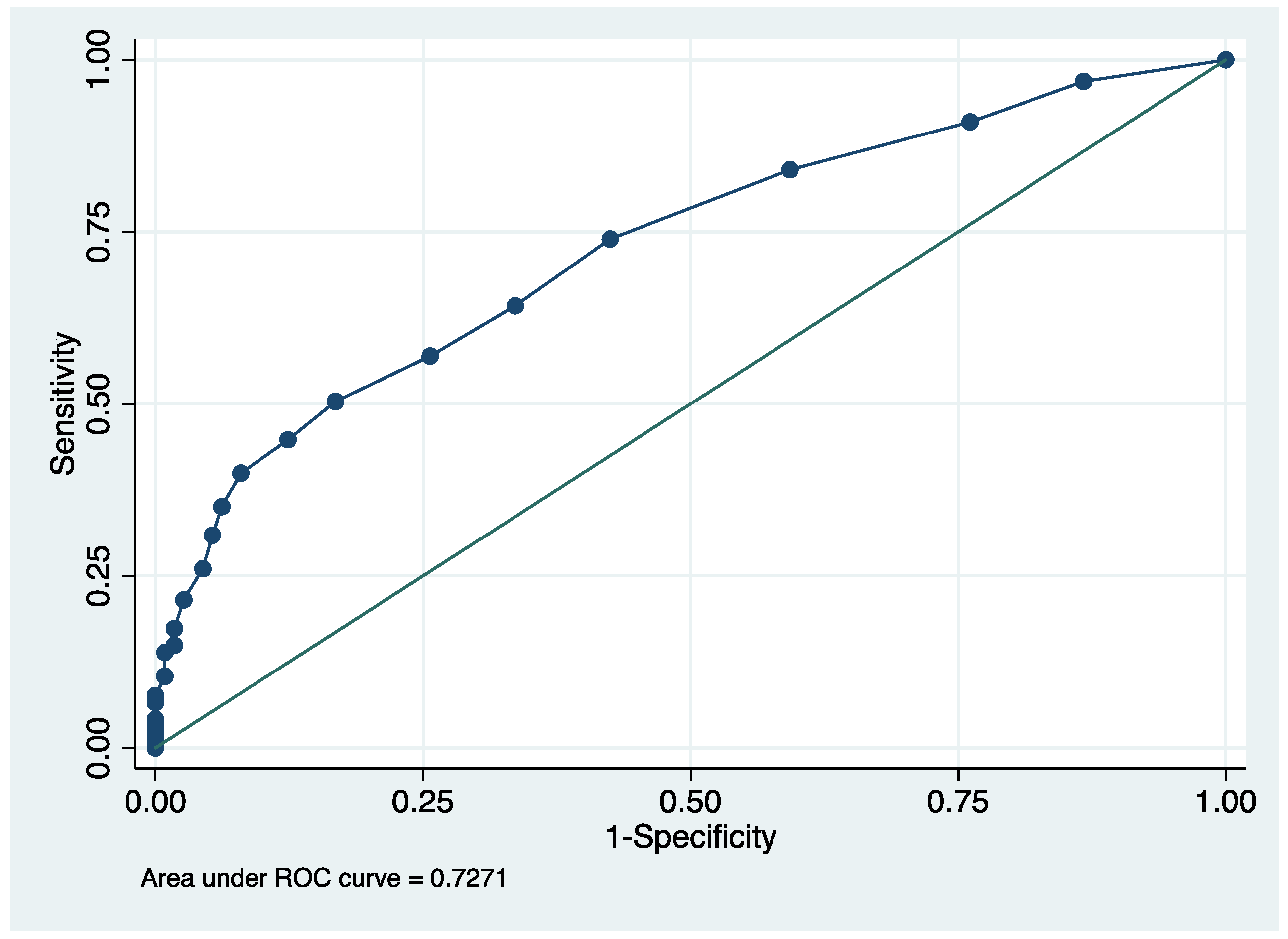
3.2. NGAL and CKDu Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, M.; Kjellstrom, T. Climate change impacts on working people: How to develop intervention policies. Glob. Heath Action 2010, 3, 5774. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia, G.G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Lunyera, J.; Mohottige, D.; Von Isenburg, M.; Jeuland, M.; Patel, U.D.; Stanifer, J.W. CKD of uncertain etiology: A systematic review. Clin. J. Am. Soc. Nephrol. 2016, 11, 7379–7385. [Google Scholar] [CrossRef] [PubMed]
- Wijkstrome, J.; Jayasumana, C.; Dassanayake, R.; Priyawardane, N.; Godakanda, N.; Siribaddana, S.; Ring, A.; Hultenby, K.; Soderberg, M.; Elinder, C.-G.; et al. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): Similarities and differences with Mesoamerican Nephropathy. PLoS ONE 2018, 13, e0193056. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, K.B.; Kulasooriya, P.N.; Wijayasiri, K.N.; Rajapakse, E.D.; Dulshika, D.S.; Bandara, P.; Fried, L.F.; De Silva, A.; Albert, S.M. Relevance of heat stress and dehydration to chronic kidney disease (CKDu) in Sri Lanka. Prev. Med. Rep. 2019, 15, 100928. [Google Scholar] [CrossRef] [PubMed]
- Tawatsupa, B.; Lim, L.L.-Y.; Kjellstrom, T.; Seubsman, S.-A.; Sleigh, A.; The Thai Cohort Study Team. Association between occupational heat stress and kidney disease among 37,816 workers in the Thai cohort study (TCS). Jpn. Epidemiol. Assoc. 2012, 22, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siriwardhana, E.A.; Perera, P.A.; Sivakanesan, R.; Abeysekara, T.; Nugegoda, D.B.; Jayaweera, J.A. Dehydration and malaria augment the risk of developing chronic kidney disease in Sri Lanka. Indian J. Nephrol. 2015, 25, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Department of Meteorology, Sri Lanka. Climate of Sri Lanka. 2016. Available online: http://www.meteo.gov.lk (accessed on 15 September 2021).
- Jayasekara, K.B.; Dissanayake, D.M.; Sivakanesan, R.; Ranasinghe, A.; Karunarathna, R.H.; Priyantha Kumara, G.W. Epidemiology of chronic kidney disease withspecial emphasis on chronic kidney disease of uncertain etiology in the north centralregion of Sri Lanka. J. Epidemiol. 2015, 25, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; García-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate change and the emergentepidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 1472–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Silva, M.W.A.; Albert, S.M. Kidney Disease, Health, and Commodification of Drinking Water: An Anthropological Inquiry into the Introduction of Reverse Osmosis Water in the North Central Province of Sri Lanka. Hum. Organ. 2021, 80, 140–151. [Google Scholar] [CrossRef]
- Soni, S.S.; Cruz, D.; Bobek, I.; Chionh, C.Y.; Nalesso, F.; Lentini, P.; de Cal, M.; Corradi, V.; Virzi, G.; Ronco, C. NGAL: A biomarker of acute kidney injury and other systemic conditions. Int. Urol. Nephrol. 2010, 42, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lumlertgul, N.; Amprai, M.; Tachaboon, S.; Dinhuzen, J.; Peerapornratana, S.; Kerr, S.J.; Srisawat, N. Urine Neutrophil Gelatinase-associated Lipocalin (NGAL) for Prediction of Persistent AKI and Major Adverse Kidney Events. Sci. Rep. 2020, 10, 8718. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institute of Occupational Safety and Health. NIOSH Health Hazard Evaluation Program. Available online: https://www.osha.gov/complianceassistance/hhe-program (accessed on 2 October 2021).
- Rodríguez, E.; Arias-Cabrales, C.; Bermejo, S.; Sierra, A.; Burballa, C.; Soler, M.J.; Barrios, C.; Pacual, J. Impact of Recurrent Acute Kidney Injury on Patient Outcomes. Kidney Blood Press. Res. 2018, 43, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Christianson, A.; Himmelfarb, J.; Leonard, A.C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2011, 6, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Rimes-Stigare, C.; Frumento, P.; Bottai, M.; Mårtensson, J.; Martling, C.R.; Walther, S.M.; Karlström, G.; Bell, M. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit. Care 2015, 19, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Kidney Foundation. Acute Kidney Injury. Available online: www.kidney.org/atoz/content/AcuteKidneyInjury (accessed on 15 August 2021).
| Region | Date | CKDu Region and Occupation (n) | Villages (n) | Biomarkers |
|---|---|---|---|---|
| Anuradhapura District, Thanthirimale | May 2017 | Endemic Agricultural (190) Non-agricultural (28) | 3 | Urine SG Urine albumin Urine creatinine ACR |
| Non-endemic Agricultural (39) | 1 | |||
| Anuradhapura, Thanthirimale | May–August 2019 | Endemic Agricultural (75) Non-agricultural (67) | 3 | Urine SG Urine albumin Urine creatinine ACR Urine NGAL |
| Hambanthota, Sooriyawewa | March 2020 | Non-endemic Agricultural (76) | 3 | Urine SG Urine albumin Urine creatinine ACR Urine NGAL |
| Total Village Sample | ||||
| Region | Endemic Region | Non-Endemic | ||
| Occupation | Agricultural Workers (n = 288) | Non-Agricultural Workers (n = 67) | Agricultural Workers (n = 115) | p |
| Demography | ||||
| Female, % | 57.1 | 43.3 | 25.2 | <0.001 |
| Age | 46.6 | 43.4 | 42.9 | 0.001 |
| Health Conditions | ||||
| CKD, % | 11.1 | 0.0 | 0.0 | <0.001 |
| Diabetes, % | 5.2 | 10.5 | 3.5 | NS |
| BMI | 23.4 | 23.4 | 22.2 | 0.014 |
| Heat stress | 8.0 | 4.6 | 3.8 | <0.001 |
| Water Intake | ||||
| l/day | 2.7 | 2.1 | 2.1 | <0.001 |
| NGAL Sub-Sample | ||||
| Region | Endemic Region | Non-Endemic | ||
| Occupation | Agricultural Workers (n = 71) | Non-Agricultural Workers (n = 67) | Agricultural Workers (n = 76) | p |
| Demography | ||||
| Female, % | 21.1 | 43.3 | 29.0 | 0.017 |
| Age | 42.8 | 43.4 | 40.5 | NS |
| Health Conditions | ||||
| CKD, % | 7.1 | 0.0 | 0.0 | 0.005 |
| Diabetes, % | 4.3 | 10.5 | 1.3 | 0.044 |
| BMI | 21.9 | 23.4 | 22.0 | NS |
| Heat stress | 5.4 | 4.6 | 2.8 | <0.001 |
| Water Intake | ||||
| l/day | 2.0 | 2.1 | 1.7 | <0.001 |
| Urine Parameters | ||||
| Specific gravity | 1.015 | 1.012 | 1.016 | 0.004 |
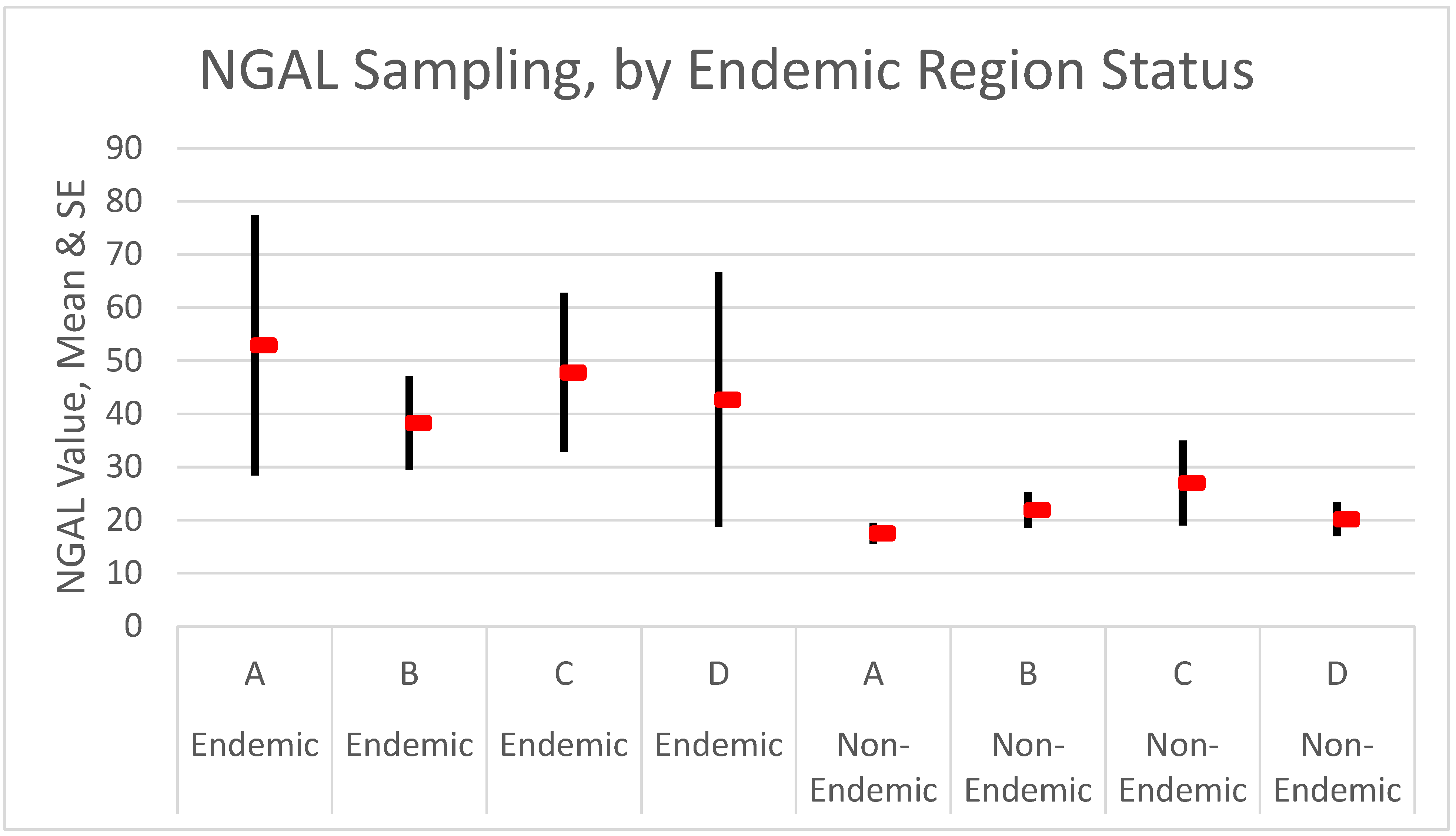
| NGAL (se) | 52.9 (24.5) | 48.9 (13.3) | 17.5 (2.0) | NS |
| Microalbumin (se) | 17.9 (5.4) | 13.5 (2.8) | 25.8 (6.1) | NS |
| Creatinine (se) | 158.4 (14.3) | 107.0 (8.7) | 130.7 (8.2) | 0.007 |
| ACR (se) | 26.9 (11.6) | 18.8 (3.9) | 26.6 (8.0) | NS |
| Correlate | b | se | t | p | 95% CI |
|---|---|---|---|---|---|
| Constant | 6.89 | 2.86 | 2.41 | 0.02 | 1.26, 12.5 |
| NGAL (morning sample) | 0.005 | 0.002 | 2.54 | 0.012 | 0.001, 0.008 |
| Age | 0.004 | 0.023 | 0.18 | 0.86 | −0.04, 0.05 |
| Gender (male) | −1.61 | 0.57 | 2.84 | 0.005 | −2.73, 0.49 |
| Kidney disease | −1.67 | 1.71 | 0.97 | 0.33 | −5.05, 1.71 |
| Diabetes | 2.83 | 1.22 | 2.33 | 0.02 | 0.43, 5.24 |
| BMI | −0.01 | 0.06 | 0.16 | 0.87 | −0.13, 0.11 |
| Sample | |||||
| Agriculture–Endemic | Ref | ||||
| Indoor–Endemic | −1.40 | 0.66 | 2.12 | 0.04 | −2.70, −0.10 |
| Agriculture–Non-endemic | −2.62 | 0.63 | 4.15 | <0.001 | −3.87, −1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulasooriya, P.N.; Jayasekara, K.B.; Nisansala, T.; Kannangara, S.; Karunarathna, R.; Karunarathne, C.; Wikramarathne, M.; Albert, S.M. Utility of Self-Reported Heat Stress Symptoms and NGAL Biomarker to Screen for Chronic Kidney Disease of Unknown Origin (CKDu) in Sri Lanka. Int. J. Environ. Res. Public Health 2021, 18, 10498. https://doi.org/10.3390/ijerph181910498
Kulasooriya PN, Jayasekara KB, Nisansala T, Kannangara S, Karunarathna R, Karunarathne C, Wikramarathne M, Albert SM. Utility of Self-Reported Heat Stress Symptoms and NGAL Biomarker to Screen for Chronic Kidney Disease of Unknown Origin (CKDu) in Sri Lanka. International Journal of Environmental Research and Public Health. 2021; 18(19):10498. https://doi.org/10.3390/ijerph181910498
Chicago/Turabian StyleKulasooriya, Pavithra N., Kithsiri B. Jayasekara, Thilini Nisansala, Sajani Kannangara, Ranawaka Karunarathna, Chaminda Karunarathne, Mahinda Wikramarathne, and Steven M. Albert. 2021. "Utility of Self-Reported Heat Stress Symptoms and NGAL Biomarker to Screen for Chronic Kidney Disease of Unknown Origin (CKDu) in Sri Lanka" International Journal of Environmental Research and Public Health 18, no. 19: 10498. https://doi.org/10.3390/ijerph181910498
APA StyleKulasooriya, P. N., Jayasekara, K. B., Nisansala, T., Kannangara, S., Karunarathna, R., Karunarathne, C., Wikramarathne, M., & Albert, S. M. (2021). Utility of Self-Reported Heat Stress Symptoms and NGAL Biomarker to Screen for Chronic Kidney Disease of Unknown Origin (CKDu) in Sri Lanka. International Journal of Environmental Research and Public Health, 18(19), 10498. https://doi.org/10.3390/ijerph181910498






