Prevalence of Hyperuricemia and the Use of Allopurinol in Older Poles—Results from a Population-Based PolSenior Study
Abstract
1. Introduction
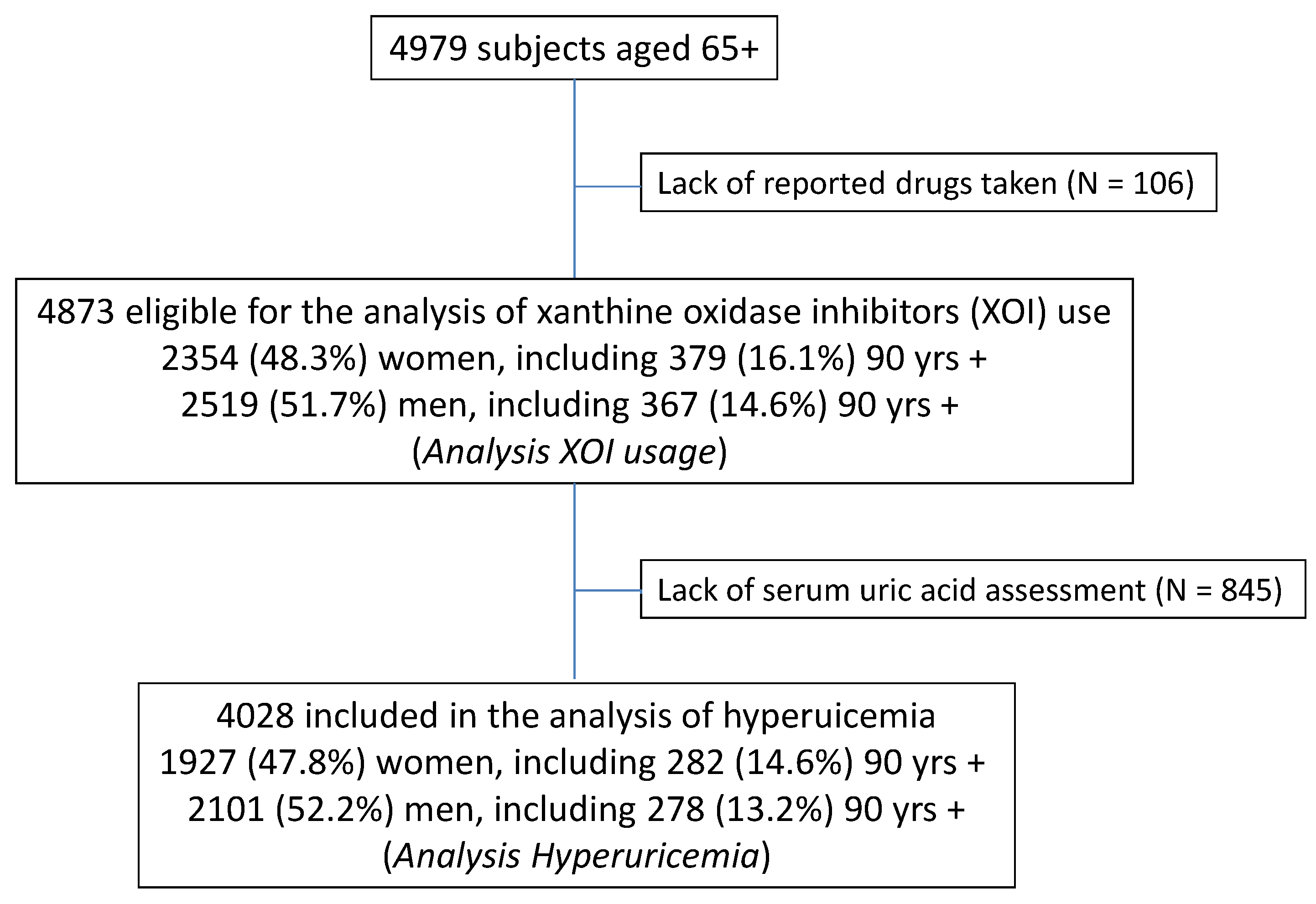
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Measurements
2.3. Data Analysis
2.4. Sociodemographic Variables
2.5. Statistical Analysis
3. Results
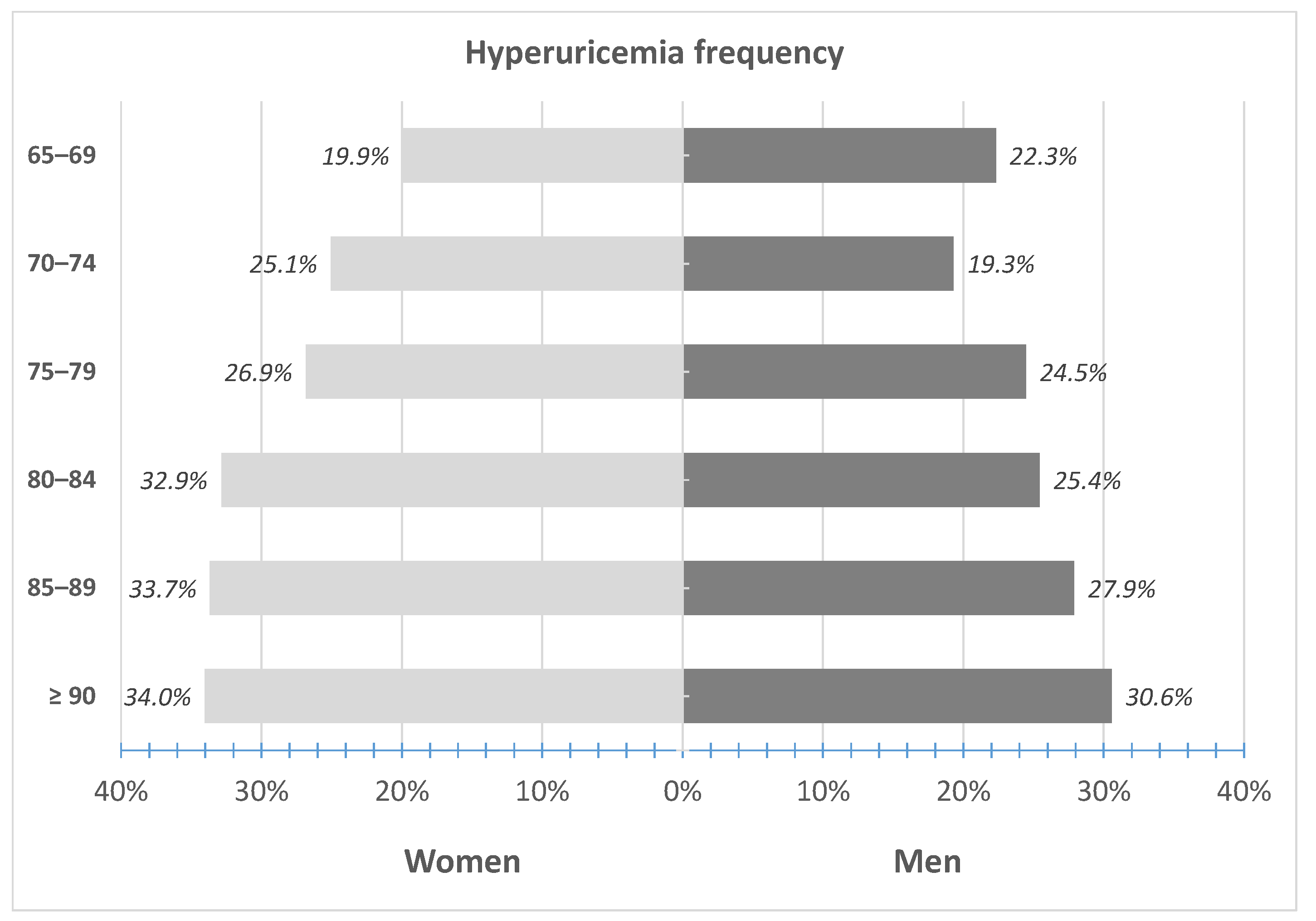
3.1. Prevalence of Hyperuricemia
3.2. Risk Factors of Hyperuricemia
3.3. The Use of Xanthine Oxidase Inhibitors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabbrini, E.; Serafini, M.; Baric, I.C.; Hazen, S.L.; Klein, S. Effect of Plasma Uric Acid on Antioxidant Capacity, Oxidative Stress, and Insulin Sensitivity in Obese Subjects. Diabetes 2013, 63, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, L.; Zhang, M.; Zhou, C.; Lin, N. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: A mechanism for uric acid-induced endothelial dysfunction. Int. J. Mol. Med. 2016, 37, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Braun, S.; Haase, H.-U.; Schulz, S.; Ranftl, S.; Hadamitzky, M.; Mehilli, J.; Schömig, A.; Kastrati, A.; Schulz-Schüpke, S. Prognostic Value of Uric Acid in Patients With Acute Coronary Syndromes. Am. J. Cardiol. 2012, 109, 1260–1265. [Google Scholar] [CrossRef]
- Wang, J.-G.; Staessen, J.A.; Fagard, R.H.; Birkenhäger, W.H.; Gong, L.; Liu, L. Prognostic Significance of Serum Creatinine and Uric Acid in Older Chinese Patients With Isolated Systolic Hypertension. Hypertension 2001, 37, 1069–1074. [Google Scholar] [CrossRef]
- Okura, T.; Higaki, J.; Kurata, M.; Irita, J.; Miyoshi, K.-I.; Yamazaki, T.; Hayashi, D.; Kohro, T.; Nagai, R. The JCAD Study Investigators Elevated Serum Uric Acid is an Independent Predictor for Cardiovascular Events in Patients With Severe Coronary Artery Stenosis: Subanalysis of the Japanese Coronary Artery Disease (JCAD) Study. Circ. J. 2009, 73, 885–891. [Google Scholar] [CrossRef]
- Yiginer, O.; Ozcelik, F.; Inanc, T.; Aparci, M.; Ozmen, N.; Cingozbay, B.Y.; Kardesoglu, E.; Suleymanoglu, S.; Şener, G.; Cebeci, B.S. Allopurinol improves endothelial function and reduces oxidant-inflammatory enzyme of myeloperoxidase in metabolic syndrome. Clin. Res. Cardiol. 2007, 97, 334–340. [Google Scholar] [CrossRef]
- Dogan, A.; Yarlioglues, M.; Kaya, M.G.; Karadag, Z.; Dogan, S.; Ardic, I.; Dogdu, O.; Kilinc, Y.; Zencir, C.; Akpek, M.; et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2010, 20, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.P.; Ang, D.S.; Gandy, S.J.; Nadir, M.A.; Houston, J.G.; Lang, C.; Struthers, A.D. Allopurinol Benefits Left Ventricular Mass and Endothelial Dysfunction in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, N.S.; Ireland, S.; George, J.; Belch, J.J.; Lang, C.C.; Struthers, A.D. Mechanistic Insights Into the Therapeutic Use of High-Dose Allopurinol in Angina Pectoris. J. Am. Coll. Cardiol. 2011, 58, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Rekhraj, S.; Gandy, S.J.; Szwejkowski, B.R.; Nadir, M.A.; Noman, A.; Houston, J.G.; Lang, C.C.; George, J.; Struthers, A.D. High-Dose Allopurinol Reduces Left Ventricular Mass in Patients With Ischemic Heart Disease. J. Am. Coll. Cardiol. 2013, 61, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Schoene, N.; Rauchhaus, M.; Leyva-Leon, F.; Pavitt, D.V.; Reaveley, D.A.; Schuler, G.; Coats, A.J.; Anker, S.D.; Hambrecht, R. Effects of Xanthine Oxidase Inhibition With Allopurinol on Endothelial Function and Peripheral Blood Flow in Hyperuricemic Patients With Chronic Heart Failure: Results from 2 placebo-controlled studies. Circulation 2002, 105, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Guthikonda, S.; Sinkey, C.; Barenz, T.; Haynes, W.G. Xanthine Oxidase Inhibition Reverses Endothelial Dysfunction in Heavy Smokers. Circulation 2003, 107, 416–421. [Google Scholar] [CrossRef]
- Kushiyama, A.; Okubo, H.; Sakoda, H.; Kikuchi, T.; Fujishiro, M.; Sato, H.; Kushiyama, S.; Iwashita, M.; Nishimura, F.; Fukushima, T.; et al. Xanthine Oxidoreductase Is Involved in Macrophage Foam Cell Formation and Atherosclerosis Development. Arter. Thromb. Vasc. Biol. 2012, 32, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Loeb, J.N. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972, 15, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wang, J.; Liu, H.; Kwong, J.S.-W.; Chen, H.; Li, L.; Chung, S.-C.; Shah, A.; Chen, Y.; et al. Diagnosis and treatment for hyperuricemia and gout: A systematic review of clinical practice guidelines and consensus statements. BMJ Open 2019, 9, e026677. [Google Scholar] [CrossRef]
- Kellgren, J.; Jeffrey, M.R.; Ball, J. The Epidemiolgy of Chronic Rheumatism; Blackwell Scientific Publications: Oxford, UK, 1963. [Google Scholar]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merrill, J.T.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Rheum. 2012, 64, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, P.; FitzGerald, J.D.; Singh, M.K.; Bae, S.; Neogi, T.; Pillinger, M.H.; Merrill, J.T.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: Therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Rheum. 2012, 64, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.D.; Mikuls, T.R.; Neogi, T.; Singh, J.A.; Robbins, M.; Khanna, P.P.; Turner, A.S.; Myslinski, R.; Suter, L.G. Development of the American College of Rheumatology Electronic Clinical Quality Measures for Gout. Arthritis Rheum. 2018, 70, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Inoue, T.; Iseki, K.; Touma, T.; Kinjo, K.; Ohya, Y.; Takishita, S. Hyperuricemia as a Predictor of Hypertension in a Screened Cohort in Okinawa, Japan. Hypertens. Res. 2004, 27, 835–841. [Google Scholar] [CrossRef]
- Al-Arfaj, A. Hyperuricemia in Saudi Arabia. Rheumatol. Int. 2001, 20, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, H.; Xia, W.; Chang, X.; Wang, M.; Chang, X. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Klemp, P.; Stansfield, S.A.; Castle, B.; Robertson, M.C. Gout is on the increase in New Zealand. Ann. Rheum. Dis. 1997, 56, 22–26. [Google Scholar] [CrossRef]
- Major, T.J.; Topless, R.K.; Dalbeth, N.; Merriman, T.R. Evaluation of the diet wide contribution to serum urate levels: Meta-analysis of population based cohorts. BMJ 2018, 363, k3951. [Google Scholar] [CrossRef]
- Campion, E.W.; Glynn, R.J.; Delabry, L.O. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am. J. Med. 1987, 82, 421–426. [Google Scholar] [CrossRef]
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 2018, 5, 160. [Google Scholar] [CrossRef]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Richette, P.; Bardin, T. Gout. Lancet 2010, 375, 318–328. [Google Scholar] [CrossRef]
- McAdams-DeMarco, M.A.; Maynard, J.W.; Baer, A.N.; Gelber, A.C.; Young, J.H.; Alonso, A.; Coresh, J. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: The Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2011, 64, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Loenen, H.M.; Eshuis, H.; Löwik, M.R.; Schouten, E.G.; Hulshof, K.F.; Odink, J.; Kok, F.J. Serum uric acid correlates in elderly men and women with special reference to body composition and dietary intake (Dutch nutrition surveillance system). J. Clin. Epidemiol. 1990, 43, 1297–1303. [Google Scholar] [CrossRef]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.; Chudek, J. Prevalence and factors of hyperuricemia in older adults. Gerontol. Polska 2020, 28, 38–44. [Google Scholar]
- Mazza, A.; Lenti, S.; Schiavon, L.; Del Monte, A.; Townsend, D.M.; Ramazzina, E.; Rubello, D.; Casiglia, E. Asymptomatic hyperuricemia is a strong risk factor for resistant hypertension in elderly subjects from general population. Biomed. Pharmacother. 2016, 86, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.T.; Köttgen, A.; Law, A.; Windham, B.G.; Segev, D.; Baer, A.N.; Coresh, J.; McAdams-DeMarco, M.A. Physical Function, Hyperuricemia, and Gout in Older Adults. Arthritis Rheum. 2015, 67, 1730–1738. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bulló, M.; Babio, N.; Martínez-González, M.A.; Estruch, R.; Covas, M.-I.; Wärnberg, J.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Mediterranean Diet and Risk of Hyperuricemia in Elderly Participants at High Cardiovascular Risk. J. Gerontol. Ser. A 2013, 68, 1263–1270. [Google Scholar] [CrossRef]
- Yu, Q.; Shen, H.-C.; Hu, Y.-C.; Chen, Y.-F.; Tung, T.-H. Prevalence and Metabolic Factors of Hyperuricemia in an Elderly Agricultural and Fishing Population in Taiwan. Arch. Rheumatol. 2017, 32, 149–157. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hu, H.-Y.; Chou, Y.-J.; Huang, N.; Chou, Y.-C.; Lee, M.-S.; Li, C.-P.; Wu, C.; Mph, Y.C. High Serum Uric Acid Levels Are Associated with All-Cause and Cardiovascular, but Not Cancer, Mortality in Elderly Adults. J. Am. Geriatr. Soc. 2015, 63, 1829–1836. [Google Scholar] [CrossRef]
- Kumar AU, A.; Browne, L.D.; Li, X.; Adeeb, F.; Perez-Ruiz, F.; Fraser, A.D.; Stack, A.G. Temporal trends in hyperuricaemia in the Irish health system from 2006-2014: A cohort study. PLoS ONE 2018, 13, e0198197. [Google Scholar] [CrossRef] [PubMed]
- Bledowski, P.; Mossakowska, M.; Chudek, J.; Grodzicki, T.; Milewicz, A.; Szybalska, A.; Wieczorowska-Tobis, K.; Wiecek, A.; Bartoszek, A.; Dabrowski, A.; et al. Medical, psychological and socioeconomic aspects of aging in Poland: Assumptions and objectives of the PolSenior project. Exp. Gerontol. 2011, 46, 1003–1009. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Report on a WHO Consultation on Obesity; WHO: Geneva, Switzerland, 2000; ISBN 92-4-120894-5. [Google Scholar]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Zdrojewski, T.; Wizner, B.; Więcek, A.; Ślusarczyk, P.; Chudek, J.; Mossakowska, M.; Bandosz, P.; Bobak, M.; Kozakiewicz, K.; Broda, G.; et al. Prevalence, awareness, and control of hypertension in elderly and very elderly in Poland. J. Hypertens. 2016, 34, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Jialal, I. Hypercholesterolemia; StatPearls Publishing: Treasure Island, CA, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459188/ (accessed on 1 March 2020).
- Rygiel, K. Hypertriglyceridemia—Common Causes, Prevention and treatment Strategies. Curr. Cardiol. Rev. 2018, 14, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Chronic Kidney Disease Epidemiology Collaboration. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef]
- Chudek, J.; Wieczorowska-Tobis, K.; Zejda, J.E.; Broczek, K.; Skalska, A.; Zdrojewski, T.; Więcek, A. The prevalence of chronic kidney disease and its relation to socioeconomic conditions in an elderly Polish population: Results from the national population-based study PolSenior. Nephrol. Dial. Transplant. 2013, 29, 1073–1082. [Google Scholar] [CrossRef]
- Epstein, M. Aging and the Kidney. J. Urol. 1998, 7, 1103. [Google Scholar] [CrossRef]
- Mossakowska, M.; Więcek, A.; Błędowski, P. Aspekty Medyczne, Psychologiczne, Socjologiczne i Ekonomiczne Starzenia się Ludzi w Polsce; Termedia: Poznan, Poland, 2012; ISBN 978-83-62138-81-4. [Google Scholar]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- Xiong, Z.-J.; Zhu, C.-S.; Qian, X.; Zhu, J.; Wu, Z.; Chen, L. Serum uric acid is associated with dietary and lifestyle factors in elderly women in Suburban Guangzhou in Guangdong Province of South China. J. Nutr. Health Aging 2012, 17, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Bartels, E.M.; Henriksen, M.; Wæhrens, E.E.; Gudbergsen, H.; Bliddal, H.; Astrup, A.; Knop, F.K.; Carmona, L.; Taylor, W.J.; et al. Weight loss for overweight and obese individuals with gout: A systematic review of longitudinal studies. Ann. Rheum. Dis. 2017, 76, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Barlow, K.A. Hyperlipidemia in primary gout. Metabolism 1968, 17, 289–299. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Brzozowska, A.; Roszkowski, W. Energy and nutrients intake of elderly people living in the Warsaw region, Poland. J. Nutr. Health Aging 2001, 5, 248–252. [Google Scholar]
- Tiit, E.-M.; Saks, K. Body mass index of elderly Europeans. Pap. Anthr. 2015, 24, 114–128. [Google Scholar] [CrossRef][Green Version]
- Krishnan, E. Interaction of Inflammation, Hyperuricemia, and the Prevalence of Hypertension Among Adults Free of Metabolic Syndrome: NHANES 2009–2010. J. Am. Hear. Assoc. 2014, 3. [Google Scholar] [CrossRef]
- Sah, S.K.; Khatiwada, S.; Pandey, S.; Kc, R.; Das, B.K.L.; Baral, N.; Lamsal, M. Association of high-sensitivity C-reactive protein and uric acid with the metabolic syndrome components. SpringerPlus 2016, 5, 269. [Google Scholar] [CrossRef]
- Aronson, D.; Bartha, P.; Zinder, O.; Kerner, A.; Markiewicz, W.; Avizohar, O.; Brook, G.J.; Levy, Y. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int. J. Obes. 2004, 28, 674–679. [Google Scholar] [CrossRef]
- Owczarek, A.J.; Choręza, P.; Arabzada, H.; Chudek, J.; Wojnicz, R. Kidney Function, Nutritional Status, and the Left Ventricle Dysfunction Are Associated with Serum Uric Acid Levels in Patients with Heart Failure with Reduced Ejection Fraction. Ann. Clin. Lab. Sci. 2018, 48, 608–613. [Google Scholar]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart Disease and Stroke Statistics—2013 Update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, C.; Slim, R.; Fathallah, N.; Hmouda, H. Drug-induced hyperuricaemia and gout. Rheumatology 2016, 56, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Neogi, T.; Chen, C.; Chaisson, C.; Hunter, D.J.; Choi, H. Low-dose aspirin use and recurrent gout attacks. Ann. Rheum. Dis. 2014, 73, 385–390. [Google Scholar] [CrossRef] [PubMed]
- De Vera, M.A.; Marcotte, G.; Rai, S.; Galo, J.; Bhole, V. Medication Adherence in Gout: A Systematic Review. Arthritis Rheum. 2014, 66, 1551–1559. [Google Scholar] [CrossRef]
- Solomon, D.H.; Avorn, J.; Levin, R.; Brookhart, M.A. Uric acid lowering therapy: Prescribing patterns in a large cohort of older adults. Ann. Rheum. Dis. 2007, 67, 609–613. [Google Scholar] [CrossRef]
- Jennings, C.G.; MacKenzie, I.S.; Flynn, R.; Ford, I.; Nuki, G.; De Caterina, R.; Riches, P.L.; Ralston, S.H.; Macdonald, T.M. Up-titration of allopurinol in patients with gout. Semin. Arthritis Rheum. 2014, 44, 25–30. [Google Scholar] [CrossRef]

| All | Men N = 2101 (52.2%) | Women N = 1927 (47.8%) | |
|---|---|---|---|
| Age (years) | 79 ± 9 | 79 ± 8 | 79 ± 9 |
| 65–69 years, N (%) | 683 (16.9) | 327 (15.6) | 356 (18.5) |
| 70–74 years, N (%) | 778 (19.3) | 399 (19.0) | 379 (19.7) |
| 75–79 years, N (%) | 696 (17.3) | 372 (17.7) | 324 (16.8) |
| 80–84 years, N (%) | 627 (15.6) | 338 (16.1) | 289 (15.0) |
| 85–89 years, N (%) | 684 (17.0) | 387 (18.4) | 297 (15.4) |
| ≥90 years, N (%) | 560 (13.9) | 278 (13.2) | 282 (14.6) |
| Rural area residence, N (%) | 2395 (59.4) | 1254 (59.7) | 1141 (59.2) |
| Blue-collar, N (%) | 1365 (33.9) | 821 (39.1) | 544 (28.2) |
| White-collar, N (%) | 326 (8.1) | 216 (10.4) | 110 (5.8) |
| Living alone, N (%) | 816 (20.2) | 279 (13.4) | 537 (28.2) |
| Personal income | |||
| • Low, N (%) | 1433 (40.5) | 491 (26.9) | 942 (55.1) |
| • Average, N (%) | 1753 (49.6) | 1042 (57.0) | 711 (41.6) |
| • High, N (%) | 351 (9.9) | 294 (16.1) | 57 (3.3) |
| Vegetarian diet, N (%) | 57 (1.4) | 31 (1.5) | 26 (1.3) |
| Alcohol consumers “drinking”, N (%) | 628 (15.6) | 520 (25.1) | 108(5.7) |
| BMI (kg/m2) | 28.1 ± 5.0 | 27.4 ± 4.4 | 29.0 ± 5.5 |
| Overweight, N (%) | 1570 (41.2) | 912 (45.3) | 658 (36.5) |
| Obesity, N (%) | 1230 (32.2) | 512 (25.4) | 718 (39.8) |
| Visceral obesity, N (%) | 3158 (81.2) | 1505 (73.4) | 1653 (89.8) |
| Diabetes, N (%) | 925 (23.0) | 447 (21.3) | 478 (24.8) |
| Hypertension, N (%) | 2945 (73.4) | 1446 (69.1) | 1499 (78.1) |
| Coronary artery disease, N (%) | 865 (21.5) | 492 (23.4) | 373 (19.4) |
| Heart failure, N (%) | 243 (6.2) | 140 (6.8) | 103 (5.5) |
| Hypercholesterolemia, N (%) | 2973 (73.8) | 1408 (67.0) | 1565 (81.2) |
| Hypertriglyceridemia, N (%) | 1038 (25.8) | 446 (21.2) | 592 (30.7) |
| eGFR (mL/min/1.73m2) | 65.7 ± 18.4 | 67.5 ± 18.7 | 63.6 ± 17.8 |
| 45–59.9 mL/min/1.73m2, N (%) | 976 (24.2) | 466 (22.2) | 510 (26.5) |
| 30–44.9 mL/min/1.73m2, N (%) | 397 (9.9) | 187 (8.9) | 210 (10.9) |
| <30 mL/min/1.73m2, N (%) | 97 (2.4) | 39 (1.9) | 58 (3.0) |
| hs-CRP (mg/dL) | 2.36 (1.12–4.94) | 2.28 (1.02–5.01) | 2.44 (1.21–4.86) |
| >3 mg/dL, N (%) | 1664 (41.6) | 854 (40.9) | 810 (42.3) |
| Hydrochlorothiazide, N (%) | 243 (6.0) | 109 (5.2) | 134 (7.0) |
| Thiazide-like, N (%) | 623 (15.5) | 257 (12.2) | 366 (20.0) |
| Loop diuretics, N (%) | 387 (9.6) | 202 (9.6) | 185 (9.6) |
| Spironolactone, N (%) | 469 (11.6) | 226 (10.8) | 243 (12.6) |
| Aspirin, N (%) | 1367 (33.9) | 725 (34.5) | 642 (33.3) |
| Variable | β | SE (β) | OR | ±95% CI | p |
|---|---|---|---|---|---|
| Female gender | 0.1789 | 0.0715 | 1.196 | 1.039–1.376 | <0.05 |
| Age (per 5 years) | 0.1205 | 0.0041 | 1.128 | 1.119–1.137 | <0.001 |
| Age ≥80 years | 0.3886 | 0.0717 | 1.475 | 1.281–1.698 | <0.001 |
| Rural area residence | 0.1614 | 0.0734 | 1.175 | 1.017–1.357 | <0.05 |
| White-collar | −0.1417 | 0.1351 | 0.868 | 0.666–1.131 | 0.29 |
| Living alone | 0.0416 | 0.0885 | 1.047 | 0.880–1.246 | 0.60 |
| Income—average vs. low | 0.1409 | 0.0815 | 1.151 | 0.981–1.351 | 0.08 |
| Income–high vs. low | 0.3509 | 0.1302 | 1.420 | 1.100–1.833 | <0.01 |
| Vegetarian diet | −0.3031 | 0.3269 | 0.738 | 0.389–1.402 | 0.35 |
| Alcohol consumption | −0.0093 | 0.0988 | 0.991 | 0.816–1.202 | 0.92 |
| BMI (kg/m2) | 0.0900 | 0.0075 | 1.094 | 1.078–1.110 | <0.001 |
| Overweight vs. normal weight | 0.4328 | 0.1027 | 1.542 | 1.261–1.885 | <0.001 |
| Obesity vs. normal weight | 1.0488 | 0.1026 | 2.854 | 2.334–3.490 | <0.001 |
| Visceral obesity | 0.6444 | 0.1056 | 1.905 | 1.549–2.343 | <0.001 |
| Diabetes | 0.5368 | 0.0808 | 1.710 | 1.460–2.004 | <0.001 |
| Hypertension | 0.4972 | 0.0870 | 1.644 | 1.386–1.950 | <0.001 |
| Coronary artery disease | 0.6130 | 0.0821 | 1.846 | 1.571–2.168 | <0.001 |
| Heart failure | 0.9940 | 0.1340 | 2.702 | 2.078–3.514 | <0.001 |
| Hypercholesterolemia | 0.0177 | 0.0814 | 1.018 | 0.868–1.194 | 0.83 |
| Hypertriglyceridemia | 0.7510 | 0.0775 | 2.118 | 1.820–2.466 | <0.001 |
| eGFR (per 10 mL/min/1.73 m2) | −0.6780 | 0.0028 | 0.508 | 0.505–0.510 | <0.001 |
| eGFR 45–59.9 vs. > 60 mL/min/1.73 m2 | 1.1669 | 0.0879 | 3.212 | 2.704–3.816 | <0.001 |
| eGFR 30–44.9 mL/min/1.73 m2 | 2.5986 | 0.1224 | 13.446 | 10.577–17.092 | <0.001 |
| eGFR < 30 mL/min/1.73 m2 | 3.6636 | 0.3034 | 39.001 | 21.520–70.682 | <0.001 |
| hs-CRP (mg/dL) | 0.0222 | 0.0041 | 1.022 | 1.014–1.031 | <0.001 |
| hs-CRP > 3 mg/dL vs | 0.6351 | 0.0725 | 1.887 | 1.637–2.176 | <0.001 |
| Medication: | |||||
| Hydrochlorothiazide | 1.0431 | 0.1325 | 2.838 | 2.189–3.679 | <0.001 |
| Thiazide-like | 0.8853 | 0.0888 | 2.424 | 2.036–2.885 | <0.001 |
| Loop diuretics | 1.7489 | 0.1113 | 5.748 | 4.621–7.150 | <0.001 |
| Spironolactone | 1.0010 | 0.0988 | 2.721 | 2.242–3.302 | <0.001 |
| Aspirin | 0.3012 | 0.0741 | 1.351 | 1.169–1.563 | <0.001 |
| Variable | LASSO | OR | ±95% CI | p |
|---|---|---|---|---|
| Obesity vs. normal weight | 0.2833 | 1.746 | 1.455–2.094 | <0.001 |
| Diabetes | 0.0141 | 1.163 | 0.954–1.418 | 0.13 |
| Coronary artery disease | 0.0185 | 1.303 | 1.068–1.589 | <0.05 |
| Heart failure | 0.1937 | 1.700 | 1.380–1.945 | <0.001 |
| Hypertriglyceridemia | 0.4191 | 1.884 | 1.565–2.268 | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 1.2297 | 4.096 | 3.445–4.869 | <0.001 |
| hs-CRP > 3 mg/dL | 0.2853 | 1.638 | 1.380–1.945 | <0.001 |
| Hydrochlorothiazide | 0.5706 | 2.965 | 2.181–4.030 | <0.001 |
| Thiazide-like diuretics | 0.7260 | 2.809 | 2.287–3.451 | <0.001 |
| Loop diuretics | 1.0688 | 4.203 | 3.213–5.496 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winder, M.; Owczarek, A.J.; Mossakowska, M.; Broczek, K.; Grodzicki, T.; Wierucki, Ł.; Chudek, J. Prevalence of Hyperuricemia and the Use of Allopurinol in Older Poles—Results from a Population-Based PolSenior Study. Int. J. Environ. Res. Public Health 2021, 18, 387. https://doi.org/10.3390/ijerph18020387
Winder M, Owczarek AJ, Mossakowska M, Broczek K, Grodzicki T, Wierucki Ł, Chudek J. Prevalence of Hyperuricemia and the Use of Allopurinol in Older Poles—Results from a Population-Based PolSenior Study. International Journal of Environmental Research and Public Health. 2021; 18(2):387. https://doi.org/10.3390/ijerph18020387
Chicago/Turabian StyleWinder, Mateusz, Aleksander J. Owczarek, Małgorzata Mossakowska, Katarzyna Broczek, Tomasz Grodzicki, Łukasz Wierucki, and Jerzy Chudek. 2021. "Prevalence of Hyperuricemia and the Use of Allopurinol in Older Poles—Results from a Population-Based PolSenior Study" International Journal of Environmental Research and Public Health 18, no. 2: 387. https://doi.org/10.3390/ijerph18020387
APA StyleWinder, M., Owczarek, A. J., Mossakowska, M., Broczek, K., Grodzicki, T., Wierucki, Ł., & Chudek, J. (2021). Prevalence of Hyperuricemia and the Use of Allopurinol in Older Poles—Results from a Population-Based PolSenior Study. International Journal of Environmental Research and Public Health, 18(2), 387. https://doi.org/10.3390/ijerph18020387







