Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Animals and Diets
2.3. Measurement of Blood Biochemical Parameters and Hepatic TG Content
2.4. Hematoxylin and Eosin (H&E) Staining and Adipocyte Size Measurement
2.5. Total Polyphenol and Flavonoid Contents of Ginger Extract
2.6. Cell Culture and Cell Viability Assay
2.7. Analysis of Messenger RNA (mRNA) by Real-Time Polymerase Chain Reaction (RT-PCR)
2.8. Protein Isolation and Western Blotting
2.9. Statistics
3. Results
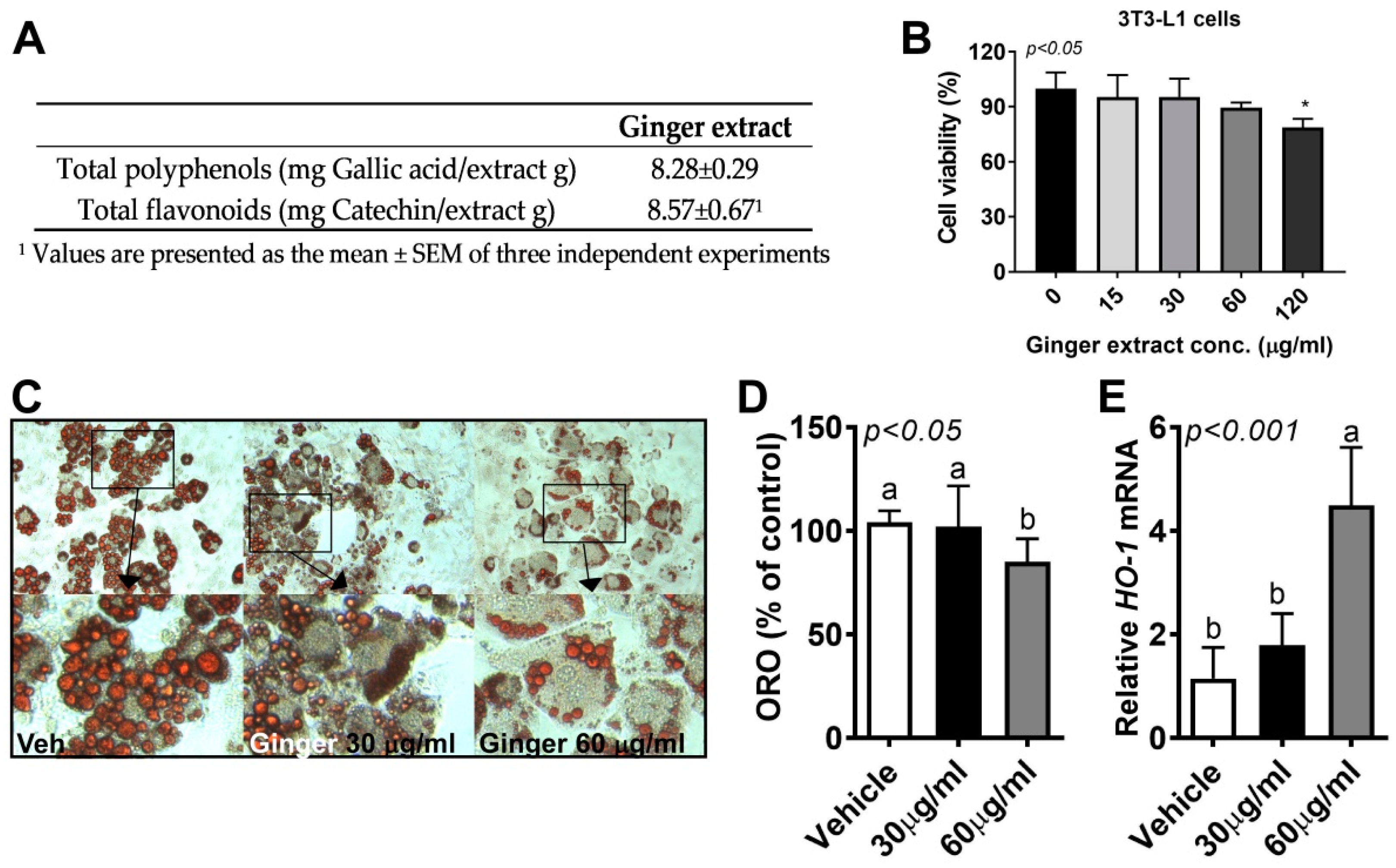
3.1. Ginger Reduced Lipid Accumulation in 3T3-L1 Adipocytes
3.2. Ginger Supplementation Ameliorated HF-Diet-Induced Metabolic Parameters without a Change in Food Intake
3.3. Ginger Supplementation Reduced HF-Diet-Induced Hepatic Lipid Accumulation
3.4. Ginger Supplementation Attenuated HF-Induced Adipocyte Hypertrophy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grundy, S.M. Metabolic Complications of Obesity. Endocrine 2000, 13, 155–165. [Google Scholar] [CrossRef]
- Rasouli, N.; Kern, P.A. Adipocytokines and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93, s64–s73. [Google Scholar] [CrossRef] [Green Version]
- Romieu, I.; On behalf of the IARC working group on Energy Balance and Obesity; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, High-fat Diet–induced Metabolic Syndrome and Cardiovascular Remodeling in Rats: Erratum. J. Cardiovasc. Pharmacol. 2011, 57, 610. [Google Scholar] [CrossRef] [PubMed]
- Putti, R.; Sica, R.; Migliaccio, V.; Lionetti, L. Diet impact on mitochondrial bioenergetics and dynamics. Front. Physiol. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blüher, M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr. Opin. Lipidol. 2010, 21, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [Green Version]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: Key players in immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Luo, Y.; Guo, H.; Ren, F. Purification, characterization, and milk coagulating properties of ginger proteases. J. Dairy Sci. 2011, 94, 2259–2269. [Google Scholar] [CrossRef] [Green Version]
- Kota, N.; Panpatil, V.V.; Kaleb, R.; Varanasi, B.; Polasa, K. Dose-dependent effect in the inhibition of oxidative stress and anticlastogenic potential of ginger in STZ induced diabetic rats. Food Chem. 2012, 135, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Misawa, K.; Hashizume, K.; Yamamoto, M.; Minegishi, Y.; Hase, T.; Shimotoyodome, A. Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor δ pathway. J. Nutr. Biochem. 2015, 26, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-Oxidative and Anti-Inflammatory Effects of Ginger in Health and Physical Activity: Review of Current Evidence. Int. J. Prev. Med. 2013, 4, S36–S42. [Google Scholar] [PubMed]
- Attari, V.E.; Mahdavi, A.M.; Javadivala, Z.; Mahluji, S.; Vahed, S.Z.; Ostadrahimi, A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother. Res. 2018, 32, 577–585. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Kim, C.-Y.; Jang, J.-H. [6]-Gingerol attenuates β-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2011, 49, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guan, S.; Shen, X.; Qian, W.; Huang, G.; Deng, X.; Xie, G. Immunosuppressive Activity of 8-Gingerol on Immune Responses in Mice. Molecules 2011, 16, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-I.; Lee, J.-K.; Youn, H.-S. Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol. Cells 2009, 27, 211–215. [Google Scholar] [CrossRef]
- Sahebkar, A. Potential efficacy of ginger as a natural supplement for nonalcoholic fatty liver disease. World J. Gastroenterol. 2011, 17, 271–272. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017, 226, 79–88. [Google Scholar] [CrossRef]
- Suk, S.; Kwon, G.T.; Lee, E.; Jang, W.J.; Yang, H.; Kim, J.H.; Thimmegowda, N.R.; Chung, M.-Y.; Kwon, J.Y.; Yang, S.; et al. Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet-fed mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Irii, H.; Tahara, Y.; Ishii, H.; Hirao, A.; Udagawa, H.; Hiramoto, M.; Yasuda, K.; Takanishi, A.; Shibata, S.; et al. Synthesis of a New [6]-Gingerol Analogue and Its Protective Effect with Respect to the Development of Metabolic Syndrome in Mice Fed a High-Fat Diet. J. Med. Chem. 2011, 54, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, M.; Hadi, A.; Rafie, N.; Najafgholizadeh, A.; Mohammadi, H.; Rouhani, M.H. The effect of ginger supplementation on lipid profile: A systematic review and meta-analysis of clinical trials. Phytomedicine 2018, 43, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Navaei, R.; Roozbeh, F.; Saravi, M.; Pouramir, M.; Jalali, F.; Moghadamnia, A.A. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Saudi Med. J. 2008, 29, 1280–1284. [Google Scholar] [PubMed]
- Park, H.J.; Jo, S.-M.; Seo, S.H.; Lee, M.; Lee, Y.; Kang, I. Anti-Inflammatory Potential of Cultured Ginseng Roots Extract in Lipopolysaccharide-Stimulated Mouse Macrophages and Adipocytes. Int. J. Environ. Res. Public Health 2020, 17, 4716. [Google Scholar] [CrossRef]
- Judge, M.K.; Zhang, J.; Tumer, N.; Carter, C.; Daniels, M.J.; Scarpace, P.J. Prolonged hyperphagia with high-fat feeding contributes to exacerbated weight gain in rats with adult-onset obesity. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R773–R780. [Google Scholar] [CrossRef] [Green Version]
- Bernier, M.; Mitchell, S.J.; Wahl, D.; Diaz, A.; Singh, A.; Seo, W.; Wang, M.; Ali, A.; Kaiser, T.; Price, N.L.; et al. Disulfiram Treatment Normalizes Body Weight in Obese Mice. Cell Metab. 2020, 32, 203–214.e4. [Google Scholar] [CrossRef]
- Kang, I.; Espín, J.C.; Carr, T.P.; Tomás-Barberán, F.A.; Chung, S. Raspberry seed flour attenuates high-sucrose diet-mediated hepatic stress and adipose tissue inflammation. J. Nutr. Biochem. 2016, 32, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.H.; Jo, S.-M.; Kim, J.; Lee, M.; Lee, Y.; Kang, I. Peanut Sprout Extracts Attenuate Triglyceride Accumulation by Promoting Mitochondrial Fatty Acid Oxidation in Adipocytes. Int. J. Mol. Sci. 2019, 20, 1216. [Google Scholar] [CrossRef] [Green Version]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W.; DeFuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte Death, Adipose Tissue Remodeling, and Obesity Complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef] [Green Version]
- Sayed, S.M.; Ahmed, M.M.; El-Shehawi, A.M.; Alkafafy, M.; Alotaibi, S.S.; El-Sawy, H.; Farouk, S.; Elshazly, S.A. Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats. Foods 2020, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger prevents obesity through regulation of energy metabolism and activation of browning in high-fat diet-induced obese mice. J. Nutr. Biochem. 2019, 70, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Li, D.; Hu, X.; Chen, F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, B.; Mun, E.-G.; Jeong, S.-Y.; Cha, Y.-S. The antioxidant activity of steamed ginger and its protective effects on obesity induced by high-fat diet in C57BL/6J mice. Nutr. Res. Pract. 2018, 12, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Jung, S.-J.; Choi, E.-K.; Ha, K.-C.; Baek, H.-I.; Park, Y.-K.; Han, K.-H.; Jeong, S.-Y.; Oh, J.-H.; Cha, Y.-S.; et al. The effects of steamed ginger ethanolic extract on weight and body fat loss: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Biotechnol. 2020, 29, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; Mcmahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, A.; Misra, A. Hepatic Steatosis, Insulin Resistance, and Adipose Tissue Disorders. J. Clin. Endocrinol. Metab. 2002, 87, 3019–3022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H.-L. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Britton, K.A.; Fox, C.S. Ectopic Fat Depots and Cardiovascular Disease. Circulation 2011, 124, e837–e841. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Dong, L.; Hu, X.; Feng, F.; Chen, F. 6-Gingerol, a Functional Polyphenol of Ginger, Promotes Browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2019, 67, 14056–14065. [Google Scholar] [CrossRef]
- Mohammad, Z.W. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef]



| Ingredients | LF | HF | HF + G |
|---|---|---|---|
| g/kg | g/kg | g/kg | |
| Casein | 200 | 200 | 200 |
| l-Cysteine | 3 | 3 | 3 |
| Sucrose | 100 | 68.8 | 68.8 |
| Corn starch | 397 | 0 | 0 |
| Maltodextrin 10 | 132 | 125 | 125 |
| Lard | 0 | 245 | 245 |
| Soybean oil | 70 | 25 | 25 |
| Cellulose | 50 | 50 | 0 |
| Mineral mix | 35 | 35 | 35 |
| Calcium phosphate | 3.4 | 3.4 | 3.4 |
| Vitamin mix | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2 | 2 |
| Ginger powder | 0 | 0 | 50 |
| Total | 1002.9 | 767.2 | 767.2 |
| kcal (%) | kcal (%) | kcal (%) | |
| Carbohydrate | 63 | 19 | 19 |
| Protein | 20 | 20 | 20 |
| Fat | 16 | 60 | 60 |
| Gene | Forward | Reverse |
|---|---|---|
| m36B4 | GGATCTGCTGCATCTGCTTG | GGCGACCTGGAAGTCCAACT |
| mACOX1 | CTTGGATGGTAGTCCGGAGA | TGGCTTCGAGTGAGGAAGTT |
| maP2 | AGCATCATAACCCTAGATGGCG | CATAACACATTCCACCACCAGC |
| mCPT1 | CCAGGCTACAGTGGGACATT | AAGGAATGCAGGTCCACATC |
| mFGF21 | GCTCTCTATGGATCGCCTCAC | GGCTTCAGACTGGTACACATT |
| mGPX1 | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
| mHO-1 | CAGGTGATGCTGACAGAGGA | TCTCTGCAGGGGCAGTATCT |
| mHPRT | TTGCTCGAGATGTCATGAAGGA | AGCAGGTCAGCAAAGAACTTATAGC |
| mMCP1 | AGGTCCCTGTCATGCTTCTG | GCTGCTGGTGATCCTCTTGT |
| mNRF1 | CCCTACTCACCCAGTCAGTATG | CATCGTGCGAGGAATGAGGA |
| mNRF2 | TCAGCGACGGAAAGAGTATGA | CCACTGGTTTCTGACTGGATGT |
| mPGC1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
| mPPARγ | GGCGATCTTGACAGGAAAGAC | CCCTTGAAAAATTCGGATGG |
| mSOD1 | AACCAGTTGTGTTGTCAGGAC | CCACCATGTTTCTTAGAGTGAGG |
| mSOD2 | CAGACCTGCCTTACGACTATGG | CTCGGTGGCGTTGAGATTGTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.H.; Fang, F.; Kang, I. Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice. Int. J. Environ. Res. Public Health 2021, 18, 631. https://doi.org/10.3390/ijerph18020631
Seo SH, Fang F, Kang I. Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice. International Journal of Environmental Research and Public Health. 2021; 18(2):631. https://doi.org/10.3390/ijerph18020631
Chicago/Turabian StyleSeo, Seok Hee, Feng Fang, and Inhae Kang. 2021. "Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice" International Journal of Environmental Research and Public Health 18, no. 2: 631. https://doi.org/10.3390/ijerph18020631
APA StyleSeo, S. H., Fang, F., & Kang, I. (2021). Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice. International Journal of Environmental Research and Public Health, 18(2), 631. https://doi.org/10.3390/ijerph18020631





