Plausible Role of Estrogens in Pathogenesis, Progression and Therapy of Lung Cancer
Abstract
:1. Introduction
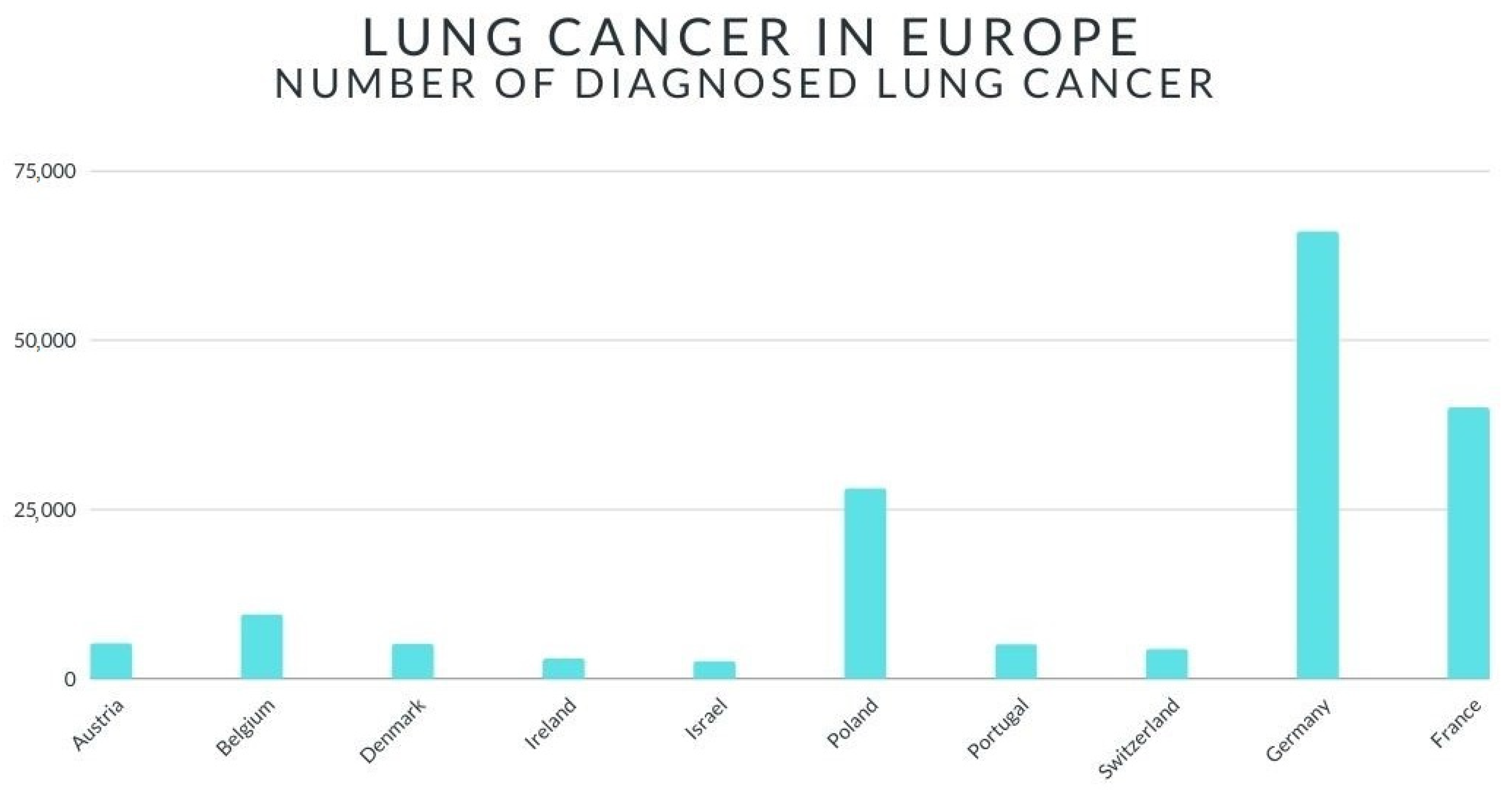
2. Lung Cancer—Short Review
3. Sex Differences in Lung Cancer
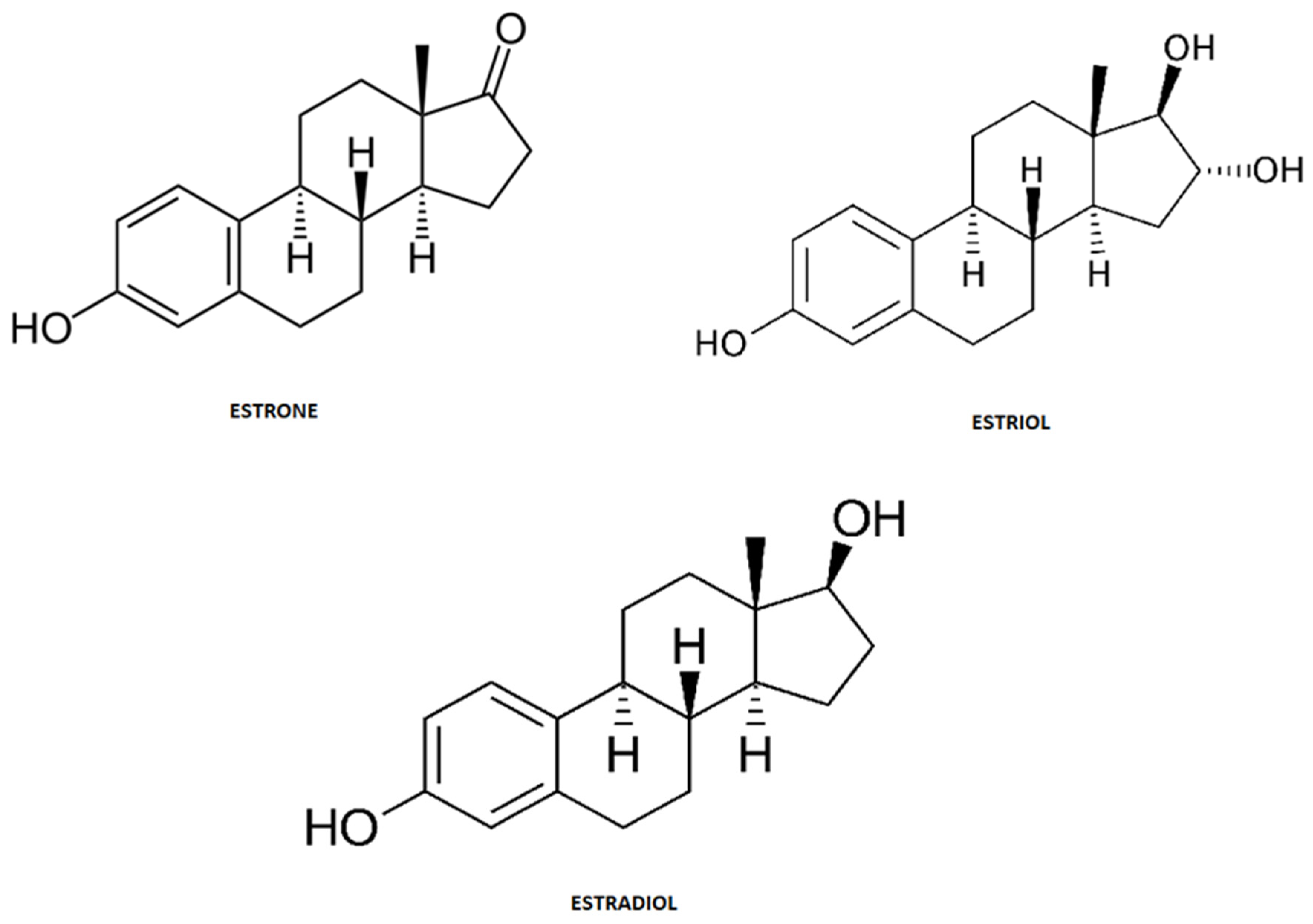
4. Estrogens Short Review
5. Estrogens in Etiopathogenesis and Therapy of Lung Cancer
6. Current Clinical Trials Registered for Non-Small Lung Cancer and Estrogens with Completed Status with Results
- Study Evaluating the Addition of Fulvestrant to Erlotinib in Stage IIIB/IV Non-Small Cell Lung Cancer—ClinicalTrials.gov Identifier: NCT00592007, disease entity: stage IIIb/IV NSCLC; drug treatment: fulvestrant, erlotinib; clinical trial is aimed at determining the effectiveness of the combination of fulvestrant which inhibits the access of estrogen to the tumor with erlotinib. Only patients who express estrogen are eligible for the study. Moreover, estrogen sensitivity was tested on previously removed tumor samples [99].
- Fulvestrant and Anastrozole as Consolidation Therapy in Postmenopausal Women With Advanced Non-small Cell Lung Cancer—ClinicalTrials.gov Identifier: NCT00932152; target audience: postmenopausal women, NSCLC; Drug: fulvestrant (Faslodex), anastrozole (Arimidex), bevacizumab (Avastin), best supportive care; clinical trail included the assessment of 17β-estradiol, VEGF, E-selectin, thrombospondin-1 and IGF-1 levels and other plasma biomarkers. Evaluation of biomarkers such as ERα, ERβ, PR, VEGF and aromatase expression. Archiving of tumor tissue was also used in the study [100].
- Alisertib in Adults With Nonhematological Malignancies, Followed by Alisertib in Lung, Breast, Head and Neck or Gastroesophageal Malignancies—ClinicalTrials.gov Identifier: NCT01045421; disease: advanced nonhematological malignancies, non-small cell lung cancer, small cell lung cancer, metastatic breast cancer, head and neck squamous cell carcinoma, gastroesophageal adenocarcinoma, drug: MLN8237 (Alisertib); in lung cancer, the chemo-sensitive, chemo-resistant population was analyzed, in breast cancer, ER2 and ER2 were analyzed. HR + = positive estrogen or progesterone receptor, both SCLC and NSCLC patients received 50 mg of MLN8237 orally twice daily for 7 days, consecutively 14 days off [101].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Urman, A.; Hosgood, D. Lung Cancer Risk, Genetic Variation, and Air Pollution. EBioMedicine 2015, 2, 491–492. [Google Scholar] [CrossRef] [Green Version]
- Alberg, A.J.; Samet, J.M. Epidemiology of lung cancer. Chest 2003, 123, 21–49. [Google Scholar] [CrossRef] [Green Version]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.H. Lung Cancer: European Commission: Series for General Practitioners; Springer: Berlin, Germany, 1990; pp. 1–48. [Google Scholar]
- Siegel, R.; Ward, E.; Brawley, O. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart; International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Addario, B.J. Lung cancer is a global epidemic and requires a global effort. Ann. Transl. Med. 2015, 3, 26. [Google Scholar]
- Lung Cancer in Europe. Available online: https://www.astrazeneca.com/content/dam/az/our-focus-areas/Oncology/2020/lungcancer/Lung%20Cancer%20in%20Europe%20Backgrounder_APPROVED_MAY2020.pdf (accessed on 22 December 2020).
- Lung Cancer Statistics. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/lung-cancer-statistics (accessed on 22 December 2020).
- Vlahopoulos, S.; Adamaki, M.; Khoury, N.; Zoumpourlis, V.; Boldogh, I. Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer. Pharmacol. Ther. 2019, 194, 59–72. [Google Scholar] [CrossRef]
- Alegre, E.; Fusco, J.P.; Restituto, P.; Salas-Benito, D.; Rodríguez-Ruiz, M.E.; Andueza, M.P.; Gonzalez, A. Total and mutated EGFR quantification in cell free DNA from non-small cell lung cancer patients detects tumor heterogeneity and presents prognostic value. Tumour Biol. 2016, 37, 13687–13694. [Google Scholar] [CrossRef]
- Brenner, D.R.; Fanidi, A.; Grankvist, K.; Muller, D.C.; Brennan, P.; Manjer, J.; Johansson, M. Inflammatory Cytokines and Lung Cancer Risk in 3 prospective Studies. Am. J. Epidemiol. 2017, 185, 86–95. [Google Scholar] [CrossRef]
- Perera, F.P.; Mooney, L.A.; Stampfer, M.; Phillips, D.H.; Bell, D.A.; Rundle, A.; Cho, S.; Tsai, W.-Y.; Ma, J.; Blackwood, A.; et al. Associations between carcinogen–DNA damage, glutathione S-transferase genotypes, and risk of lung cancer in the prospective Physicians’ Health Cohort Study. Carcinogenesis 2002, 23, 1641–1646. [Google Scholar] [CrossRef] [Green Version]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef]
- Schnoll, R.A.; Martinez, E.; Tatum, K.L.; Weber, D.M.; Kuzla, N.; Glass, M.; Ridge, J.A.A.; Langer, C.; Miyamoto, C.; Wileyto, E.P.; et al. A bupropion smoking cessation clinical trial for cancer patients. Cancer Causes Control 2010, 21, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, J.K.; Dubey, S.; Prochaska, J.J. Smoking Cessation: An Integral Part of Lung Cancer Treatment. Oncology 2010, 78, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.H.; Hsiao, C.F.; Chang, G.C. Interactive effect of cigarette smoking with human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) polymorphisms on the risk of lung cancer: A case–control study in Taiwan. Am. J. Epidemiol. 2009, 170, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Denissenko, M.F.; Pao, A.; Tang, M.; Pfeifer, G.P. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 1996, 274, 430–432. [Google Scholar] [CrossRef] [Green Version]
- El-Telbany, A.; Ma, P.C. Cancer genes in lung cancer: Racial disparities: Are there any? Genes Cancer 2012, 3, 467–480. [Google Scholar] [CrossRef]
- Porebska, I.; Wyrodek, E.; Kosacka, M.; Adamiak, J.; Jankowska, R.; Harłozińska-Szmyrka, A. Apoptotic markers p53, Bcl-2 and Bax in primary lung cancer. In Vivo 2006, 20, 599–604. [Google Scholar]
- Stark, A.M.; Hugo, H.H.; Tscheslog, H.; Mehdorn, H.M. p53, BCL-2 and BAX in non-small cell lung cancer brain metastases: A comparison of real-time RT-PCR, ELISA and immunohistochemical techniques. Neurol. Res. 2007, 29, 435–440. [Google Scholar] [CrossRef]
- Samet, J.M.; Avila-Tong, E.; Boffetta, P. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, E.; Negoescu, A.; Gazzeri, S.; Lantuejoul, S.; Moro, D.; Brambilla, C.; Coll, J.L. Apoptosis-related factors p53, Bcl2, and Bax in neuroendocrine lung tumors. Am. J. Pathol. 1996, 149, 1941–1952. [Google Scholar]
- Wang, S.; Zimmermann, S.; Parikh, K.; Mansfield, A.S.; Adjei, A.A. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin. Proc. 2019, 94, 1599–1622. [Google Scholar] [CrossRef]
- Byers, L.A.; Rudin, C.M. Small cell lung cancer: Where do we go from here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Page, N.; Morgensztern, D. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006, 24, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Fruh, M.; De Ruysscher, D.; Popat, S. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Skarlos, D.V.; Samantas, E.; Kosmidis, P. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer: A Hellenic Co-operative Oncology Group study. Ann. Oncol. 1994, 5, 601–607. [Google Scholar] [CrossRef]
- Shoji, T.; Kikuchi, E.; Kikuchi, J. Evaluating the immunoproteasome as a potential therapeutic target in cisplatin-resistant small cell and non-small cell lung cancer. Cancer Chemother. Pharmacol. 2020, 85, 843–853. [Google Scholar] [CrossRef]
- Schabath, M.B.; Wu, X.; Vassilopoulou-Sellin, R.; Vaporciyan, A.A.; Spitz, M.R. Hormone replacement therapy and lung cancer risk: A case–control analysis. Clin. Cancer Res. 2004, 1, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Ramnath, N.; Menezes, R.J.; Loewen, G. Hormone replacement therapy as a risk factor for non-small cell lung cancer: Results of a case–control study. Oncology 2007, 10, 305–310. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Pinto, J.A.; Vallejos, C.S.; Raez, L.E. Gender and outcomes in non-small cell lung cancer: An old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018, 3, 000344. [Google Scholar] [CrossRef] [Green Version]
- Pujol, J.L.; Pirker, R.; Lynch, T.J.; Butts, C.A.; Rosell, R.; Shepherd, F.A.; Vansteenkiste, J.; O’Byrne, K.J.; de Blas, B.; Heighway, J.; et al. Meta-analysis of individual patient data from randomized trials of chemotherapy plus cetuximab as first-line treatment for advanced non-small cell lung cancer. Lung Cancer 2014, 83, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Mauguen, A.; Reck, M.; Sandler, A.B.; Saijo, N.; Johnson, D.H.; Burcoveanu, D.; Fukuoka, M.; Besse, B.; Pignon, J.P.; et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2013, 24, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Adami, H.O.; Persson, I.; Hoover, R.; Schairer, C.; Bergkvist, L. Risk of cancer in women receiving hormone replacement therapy. Int. J. Cancer 1989, 44, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Devesa, S.S.; Bray, F.; Vizcaino, A.P.; Parkin, D.M. International lung cancer trends by histologic type: Male:female differences diminishing and adenocarcinoma rates rising. Int. J. Cancer 2005, 117, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Zang, E.A.; Wynder, E.L. Differences in lung cancer risk between men and women: Examination of the evidence. J. Natl. Cancer Inst. 1996, 88, 183–192. [Google Scholar] [CrossRef]
- Dresler, C.M.; Fratelli, C.; Babb, J.; Everley, L.; Evans, A.A.; Clapper, M.L. Gender differences in genetic susceptibility for lung cancer. Lung Cancer 2000, 30, 153–160. [Google Scholar] [CrossRef]
- Kreuzer, M.; Boffetta, P.; Whitley, E.; Ahrens, W.; Gaborieau, V.; Heinrich, J.; Jockel, K.H.; Kreienbrock, L.; Mallone, S.; Merletti, F.; et al. Gender differences in lung cancer risk by smoking: A multicentre case-control study in Germany and Italy. Br. J. Cancer 2000, 82, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liu, X.; Farkas, A.M.; Parwani, A.V.; Lathrop, K.L.; Lenzner, D.; Land, S.R.; Srinivas, H. Land, Harish Srinivas, Estrogen Receptor β Functions through Nongenomic Mechanisms in Lung Cancer Cells. Mol. Endocrinol. 2009, 23, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Prabavathy, D.; Swarnalatha, Y.; Ramadoss, N. Lung cancer stem cells-origin, characteristics and therapy. Stem Cell Investig. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Ganti, A.K.; Marr, A.; Batra, S.K. Lung cancer in women: Role of estrogens. Expert Rev. Respir. Med. 2010, 4, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henschke, C.I.; Miettinen, O.S. Women’s susceptibility to tobacco carcinogens. Lung Cancer 2004, 43, 1–5. [Google Scholar] [CrossRef]
- Liang, H.; Pan, Z.; Cai, X.; Wang, W.; Guo, C.; He, J.; Chen, Y.; Liu, Z.; Wang, B.; He, J.; et al. AME Lung Cancer Cooperative Group. The association between human papillomavirus presence and epidermal growth factor receptor mutations in Asian patients with non-small cell lung cancer. Transl. Lung Cancer Res. 2018, 7, 397–403. [Google Scholar] [CrossRef]
- Kanwal, M.; Ding, X.J.; Cao, Y. Familial risk for lung cancer. Oncol. Lett. 2017, 13, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.; Feskanich, D.; Speizer, F.E.; Thun, M.; Hertzmark, E.; Rosner, B.A.; Colditz, G.A. Lung cancer rates in men and women with comparable histories of smoking. J. Natl. Cancer Inst. 2004, 96, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Mollerup, S.; Ryberg, D.; Hewer, A.; Phillips, D.H.; Haugen, A. Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 1999, 59, 3317–3320. [Google Scholar]
- Kligerman, S.; White, C. Epidemiology of lung cancer in women: Risk factors, survival, and screening. Am. J. Roentgenol. 2011, 196, 287–295. [Google Scholar] [CrossRef]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers—A different disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef]
- Mollerup, S.; Jorgensen, K.; Berge, G.; Haugen, A. Expression of estrogen receptors α and β in human lung tissue and cell lines. Lung Cancer 2002, 37, 153–159. [Google Scholar] [CrossRef]
- Thomas, L.; Doyle, L.A.; Edelman, M.J. Lung cancer in women: Emerging differences in epidemiology, biology, and therapy. Chest 2005, 128, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Kalita, K.; Lewandowski, S.; Skrzypczak, M.; Szymczak, S.; Tkaczyk, M.; Kaczmarek, L. Receptory Strogenowe, Receptory i Mechanizmy Przekazywania Sygnału; PWN: Warszawa, Poland, 2004; pp. 604–616. [Google Scholar]
- Anstead, G.M.; Carlson, K.E.; Katzenellenbogen, J.A. The estradiol pharmacophore: Ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 1997, 62, 268–303. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Sampson, J.N.; Falk, R.T.; Schairer, C.; Moore, S.C.; Fuhrman, B.J.; Dallal, C.M.; Bauer, D.C.; Dorgan, J.F.; Shu, X.O.; Zheng, W.; et al. Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer Res. 2017, 77, 918–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrman, B.J.; Xu, X.; Falk, R.T.; Dallal, C.M.; Veenstra, T.D.; Keefer, L.K.; Graubard, B.I.; Brinton, L.A.; Ziegler, R.G.; Gierach, G.L. Assay reproducibility and interindividual variation for 15 serum estrogens and estrogen metabolites measured by liquid chromatography-tandem mass spectrometry. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2649–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Roman, J.M.; Issaq, H.J.; Keefer, L.K.; Veenstra, T.D.; Ziegler, R.G. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal. Chem. 2007, 79, 7813–7821. [Google Scholar] [CrossRef]
- Xu, X.; Keefer, L.K.; Ziegler, R.G.; Veenstra, T.D. A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat. Protoc. 2007, 2, 1350–1355. [Google Scholar] [CrossRef]
- Konieczna, L.; Belka, M.; Okońska, M.; Pyszka, M.; Bączek, T. New 3D-printed sorbent for extraction of steroids from human plasma preceding LC–MS analysis. J. Chromatogr. A 2018, 1545, 1–11. [Google Scholar] [CrossRef]
- Belka, M.; Konieczna, L.; Okońska, M.; Pyszka, M.; Ulenberg, S.; Bączek, T. Application of 3D-printed scabbard-like sorbent for sample preparation in bioanalysis expanded to 96-wellplate high-throughput format. Anal. Chim. Acta 2019, 1081, 1–5. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Ren, L.; Liu, Y.; Dou, P.; Qian, K.; Chen, H.Y. On-line coupling of in-tube boronate affinity solid phase microextraction with high performance liquid chromatography-electrospray ionization tandem mass spectrometry for the determination of cis-diol biomolecules. Talanta 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Kang, J.S.; Jung, N.J.; Kim, S.; Kim, D.J.; Jang, D.D.; Yang, K.H. Downregulation of estrogen receptor alpha and beta expression in carcinogen-induced mammary gland tumors of rats. Exp. Oncol. 2004, 26, 31–35. [Google Scholar]
- Corcoran, M.P.; Lichtenstein, A.H.; Meydani, M.; Dillard, A.; Schaefer, E.J.; Lamon-Fava, S. The effect of 17β-estradiol on cholesterol content in human macrophages is influenced by the lipoprotein milieu. J. Mol. Endocrinol. 2011, 47, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, R.H., Jr.; Handa, R.J. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci. Lett. 2000, 288, 115–118. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, S.C.; Winuthayanon, W.; Korach, K.S. What’s new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 2016, 56, 55–71. [Google Scholar]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar]
- Van Pelt, R.E.; Gavin, K.M.; Kohrt, W.M. Regulation of Body Composition and Bioenergetics by Estrogens. Endocrinol. Metab. Clin. N. Am. 2015, 44, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2016, 16, 4–20. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.; Roth, J.A. Induction of apoptosis in human lung cancer cells after wild-type p53 activation by methoxyestradiol. Oncogene 1997, 14, 379–384. [Google Scholar] [CrossRef] [Green Version]
- LaVallee, T.M.; Zhan, X.H.; Herbstritt, C.J.; Kough, E.C.; Green, S.J.; Pribluda, V.S. 2-Methoxyestradiol Inhibits Proliferation and Induces Apoptosis Independently of Estrogen Receptors α and β. Cancer Res. 2002, 62, 3691–3697. [Google Scholar]
- Schumacher, G. 2-Methoxyestradiol als Neue Substanz zur Behandlung Solider Tumore; Medizinischen Fakultät Charité der Humboldt-Universität zu: Berlin, Germany, 2004; pp. 1–109. [Google Scholar]
- Marino, M.; Galluzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, S.; Yang, F.; Wang, Y.; Shao, C.; Zava, D.T.; Ding, Q.; Shi, Y.E. 4-Hydroxy estrogen metabolite, causing genomic instability by attenuating the function of spindle-assembly checkpoint, can serve as a biomarker for breast cancer. Am. J. Transl. Res. 2019, 11, 4992–5007. [Google Scholar] [PubMed]
- Peng, J.; Meireles, S.I.; Xu, X.; Smith, W.E.; Slifker, M.J.; Riel, S.L.; Zhai, S.; Zhang, G.; Ma, X.; Kurzer, M.S.; et al. Estrogen metabolism in the human lung: Impact of tumorigenesis, smoke, sex and race/ethnicity. Oncotarget 2017, 8, 106778–106789. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.G.; Prysak, G.M.; Murphy, V.; Lonardo, F.; Pass, H.; Schwartz, J.; Brooks, S. Nuclear Estrogen Receptor β in Lung Cancer: Expression and Survival Differences by Sex. Clin. Cancer Res. 2005, 11, 7280–7287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietras, R.J.; Márquez-Garbán, D.C. Membrane-Associated Estrogen Receptor Signaling Pathways in Human Cancers. Clin. Cancer Res. 2007, 13, 4672–4676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.L.; Tsai, Y.M.; Lien, C.T.; Kuo, P.L.; Hung, A.J. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, J.A.; Benjamin, H.; Cholakh, H.; Chajut, A.; Clark, D.P.; Westra, W.H. Accurate classification of non-small cell lung carcinoma using a novel microRNA-based approach. Clin. Cancer Res. 2010, 16, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Lebanony, D.; Benjamin, H.; Gilad, S.; Ezagouri, M.; Dov, A.; Ashkenazi, K.; Gefen, N.; Izraeli, S.; Rechavi, G.; Pass, H. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J. Clin. Oncol. 2009, 27, 2030–2037. [Google Scholar] [CrossRef]
- Lujambio, A.; Ropero, S.; Ballestar, E.; Fraga, M.F.; Cerrato, C.; Setien, F.; Casado, S.; Suarez-Gauthier, A.; Sanchez-Cespedes, M.; Git, A. eGenetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007, 67, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.K.; Zhu, W.Y.; He, J.Y.; Chen, D.D.; Huang, Y.Y.; Le, H.B.; Liu, X.G. miRNAs expression profiling to distinguish lung squamous-cell carcinoma from adenocarcinoma subtypes. J. Cancer Res. Clin. Oncol. 2012, 138, 1641–1650. [Google Scholar] [CrossRef]
- Nishikawa, E.; Osada, H.; Okazaki, Y.; Arima, C.; Tomida, S.; Tatematsu, Y.; Taguchi, A.; Shimada, Y.; Yanagisawa, K.; Yatabe, Y. miR-375 Is Activated by ASH1 and Inhibits YAP1 in a Lineage-Dependent Manner in Lung Cancer. Cancer Res. 2011, 71, 6165–6173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demes, M.; Aszyk, C.; Bartsch, H.; Schirren, J.; Fisseler-Eckhoff, A. Differential miRNA-Expression as an Adjunctive Diagnostic Tool in Neuroendocrine Tumors of the Lung. Cancers 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadal, E.; Zhong, J.; Lin, J.; Reddy, R.M.; Ramnath, N.; Orringer, M.B.; Chang, A.C.; Beer, D.G.; Chen, G. A MicroRNA Cluster at 14q32 Drives Aggressive Lung Adenocarcinoma. Clin. Cancer Res. 2014, 20, 3107–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niikawa, H.; Suzuki, T.; Miki, Y.; Suzuki, S.; Nagasaki, S.; Akahira, J.; Honma, S.; Evans, D.B.; Hayash, S.; Kondo, T. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin. Cancer Res. 2008, 14, 4417–4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriegsmann, K.; Zgorzelski, C.; Muley, T.; Christopoulos, P.; von Winterfeld, M.; Herpel, E.; Goeppert, B.; Mechtersheimer, G.; Sinn, P.; Stenzinger, A.; et al. Immunohistological expression of oestrogen receptor, progesterone receptor, mammaglobin, human epidermal growth factor receptor 2 and GATA-binding protein 3 in non-small-cell lung cancer. Histopathology 2020, 77, 900–914. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, Z.; Liao, Y. 17β-estradiol upregulates IL6 expression through the ERβ pathway to promote lung adenocarcinoma progression. J. Exp. Clin. Cancer Res. 2018, 37, 133. [Google Scholar] [CrossRef]
- Gao, X.; Cai, Y.; Wang, Z.; He, W.; Cao, S.; Xu, R.; Chen, H. Estrogen receptors promote NSCLC progression by modulating the membrane receptor signaling network: A systems biology perspective. J. Transl. Med. 2019, 17, 308. [Google Scholar] [CrossRef]
- Rodriguez-Lara, V.; Hernandez-Martinez, J.M.; Arrieta, O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J. Thorac. Dis. 2018, 10, 482–497. [Google Scholar] [CrossRef] [Green Version]
- Smida, T.; Bruno, T.C.; Stabile, L.P. Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications. Front. Oncol. 2020, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Koutras, A.; Giannopoulou, E.; Kritikou, I. Antiproliferative effect of exemestane in lung cancer cells. Mol. Cancer 2009, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.J.; Li, J.; Hao, F.R. Dexamethasone suppresses the growth of human non-small cell lung cancer via inducing estrogen sulfotransferase and inactivating estrogen. Acta Pharmacol. Sin. 2016, 37, 845–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Liao, Y.; Zhang, C. Fulvestrant-mediated inhibition of estrogen receptor signaling slows lung cancer progression. Oncol. Res. 2014, 22, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Study Evaluating the Addition of Fulvestrant to Erlotinib in Stage IIIB/IV Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00592007?term=estrogen&rslt=With&cond=Lung+Cancer&draw=2&rank=1 (accessed on 20 December 2020).
- Fulvestrant and Anastrozole as Consolidation Therapy in Postmenopausal Women with Advanced Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00932152?term=estrogen&rslt=With&cond=Lung+Cancer&draw=2&rank=2 (accessed on 20 December 2020).
- Alisertib in Adults With Nonhematological Malignancies, Followed by Alisertib in Lung, Breast, Head and Neck or Gastroesophageal Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT01045421?term=estrogen&rslt=With&cond=Lung+Cancer&draw=2&rank=5 (accessed on 20 December 2020).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musial, C.; Zaucha, R.; Kuban-Jankowska, A.; Konieczna, L.; Belka, M.; Marino Gammazza, A.; Baczek, T.; Cappello, F.; Wozniak, M.; Gorska-Ponikowska, M. Plausible Role of Estrogens in Pathogenesis, Progression and Therapy of Lung Cancer. Int. J. Environ. Res. Public Health 2021, 18, 648. https://doi.org/10.3390/ijerph18020648
Musial C, Zaucha R, Kuban-Jankowska A, Konieczna L, Belka M, Marino Gammazza A, Baczek T, Cappello F, Wozniak M, Gorska-Ponikowska M. Plausible Role of Estrogens in Pathogenesis, Progression and Therapy of Lung Cancer. International Journal of Environmental Research and Public Health. 2021; 18(2):648. https://doi.org/10.3390/ijerph18020648
Chicago/Turabian StyleMusial, Claudia, Renata Zaucha, Alicja Kuban-Jankowska, Lucyna Konieczna, Mariusz Belka, Antonella Marino Gammazza, Tomasz Baczek, Francesco Cappello, Michal Wozniak, and Magdalena Gorska-Ponikowska. 2021. "Plausible Role of Estrogens in Pathogenesis, Progression and Therapy of Lung Cancer" International Journal of Environmental Research and Public Health 18, no. 2: 648. https://doi.org/10.3390/ijerph18020648








