Effects of Untreated Drinking Water at Three Indigenous Yaqui Towns in Mexico: Insights from a Murine Model
Abstract
1. Introduction
2. Materials and Methods
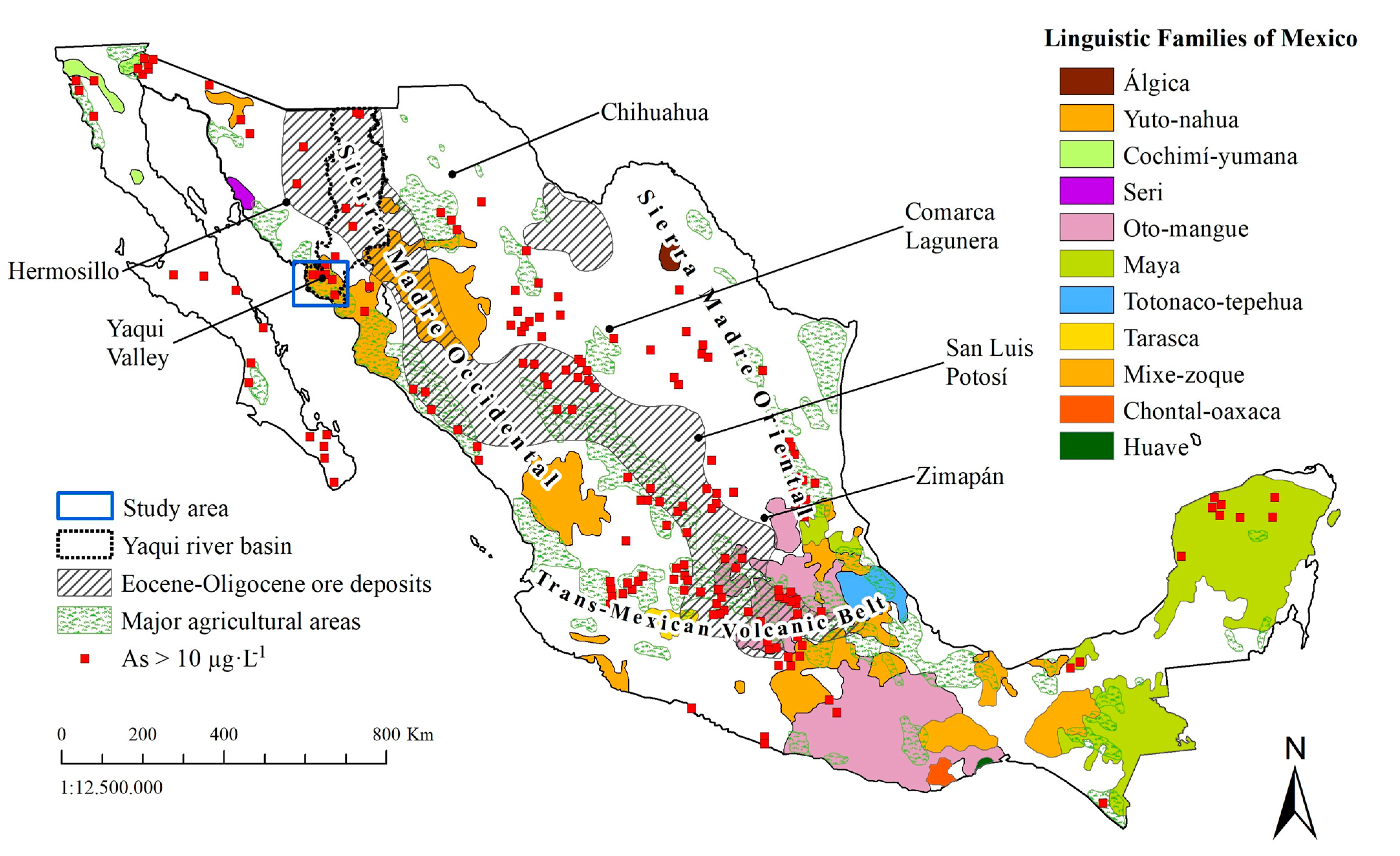
2.1. Study Area
2.2. Water Sample Collection and Analysis
2.3. Animals and Treatment
2.4. Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. Metal(Oid)s in Drinking Water Used in the Murine Model
3.2. The Murine Model
3.3. Histological Analysis
3.3.1. Brain Cortex
3.3.2. Liver
3.4. Hematological and Biochemical Analyses
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bundschuh, J.; Garcia, M.E.; Birkle, P.; Cumbal, L.H.; Bhattacharya, P.; Matschullat, J. Occurrence, health effects and remediation of arsenic in groundwaters of Latin America. In Natural Arsenic in Groundwaters of Latin American Arsenic in the Environmen; Taylor & Francis (CRC Press): London, UK, 2009. [Google Scholar]
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef]
- Agusa, T.; Trang, P.T.K.; Lan, V.M.; Anh, D.H.; Tanabe, S.; Viet, P.H.; Berg, M. Human exposure to arsenic from drinking water in Vietman. Sci. Total Environ. 2014, 488, 562–569. [Google Scholar] [CrossRef]
- Bardach, A.E.; Ciapponi, A.; Soto, N.; Chaparro, M.R.; Calderón, M.; Briatore, A.; Cadoppi, N.; Tassara, R.; Litter, M.I. Epidemiology of chronic disease related to arsenic in Argentina: A systematic review. Sci. Total Environ. 2015, 538, 802–816. [Google Scholar] [CrossRef]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Balmes, J.R.; Liaw, J.; Troncoso, P.; Dauphine, D.C.; Nardone, A.; Smith, A.H. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol. Appl. Pharmacol. 2016, 313, 10–15. [Google Scholar] [CrossRef]
- Mueller, B. Arsenic in groundwater in the southern lowlands of Nepal and its mitigation options: A review. Environ. Rev. 2017, 25, 296–305. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, M.; Dumat, C.; Khalid, S.; Khan, N.; Imran, M.; Bibi, I.; Ahmad, I.; Mohkum, H.; Ahmad, R. Arsenic level and risk assessment of groundwater in Vehari, Punjab Province, Pakistan. Expo. Health 2017, 10, 229–239. [Google Scholar] [CrossRef]
- Singh, C.K.; Kumar, A.; Bindal, S. Arsenic contamination in Rapti River Basin, Terai region of India. J. Geochem. Explor. 2018, 192, 120–131. [Google Scholar] [CrossRef]
- Grau-Pérez, M.; Navas-Acien, A.; Galan-Chilet, I.; Briongos-Figuero, L.S.; Morchon-Simon, D.; Bermudez, J.D.; Crainiceanu, C.M.; DeMarco, G.; Rentero-Garrido, P.; García-Barrera, T.; et al. Arsenic exposure, diabetes-related genes and diabetes prevalence in a general population from Spain. Environ. Poll. 2018, 235, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Chang, Y.T.; Cheng, H.L.; Shen, K.H.; Sung, J.M.; Guo, H.R. Associations between arsenic in drinking water and occurrence of end-stage renal disease with modifications by comorbidities: A nationwide population-based study in Taiwan. Sci. Total Environ. 2018, 626, 581–591. [Google Scholar] [CrossRef]
- Tiankao, W.; Chotpantarat, S. Risk assessment of arsenic from contaminated soils to shallow groundwater in Ong Phra Sub-District, Suphan Buri Province, Thailand. J. Hydrol. Reg. S 2018, 19, 80–96. [Google Scholar] [CrossRef]
- Rahman, M.; Sohel, N.; Yunus, F.M.; Alam, N.; Nahar, Q.; Streatfield, P.K.; Yunus, M. Arsenic exposure and young adult’s mortality risk: A 13-year follow-up study in Matlab, Bangladesh. Environ. Int. 2019, 123, 358–367. [Google Scholar] [CrossRef]
- Osuna-Martínez, C.C.; Armienta, M.A.; Bergés-Tiznado, M.E.; Paéz-Osuna, F. Arsenic in waters, soils, sediments, and biota from Mexico: An environmental review. Sci. Total Environ. 2021, 752, 142062. [Google Scholar] [CrossRef]
- Jiménez-Córdova, M.I.; Sánchez-Peña, L.C.; Barreda-Hernández, A.; González-Horta, C.; Barbier, O.C.; Del Razo, L.M. Fluoride exposure is associated with altered metabolism of arsenic in an adult Mexican population. Sci. Total Environ. 2019, 684, 621–628. [Google Scholar] [CrossRef]
- Currier, J.M.; Ishida, M.C.; González-Horta, C.; Sánchez-Ramírez, B.; Ballinas-Casarrubias, L.; Gutiérrez-Torres, D.S.; Hernandez-Cerón, R.; Viniegra-Morales, D.; Baeza-Terrazas, F.A.; Del Razo, L.M.; et al. Associations between Arsenic Species in Exfoliated Urothelial Cells and Prevalence of Diabetes among Residents of Chihuahua, Mexico. Environ. Health Perspect. 2014, 122, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Recio-Vega, R.; González-Cortes, T.; Olivas-Calderon, E.; Lantz, R.C.; Gandolfi, A.J.; González-De Alba, C. In utero and early childhood exposure to arsenic decreases lung function in children. J. Appl. Toxicol. 2015, 35, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vázquez, M.S.; Ochoa-Martínez, A.C.; Ruiz-Vera, T.; Araiza-Gamboa, Y.; Pérez-Maldonado, I.N. Evaluation of epigenetic alterations (mir-126 and mir-155 expression levels) in Mexican children exposed to inorganic arsenic via drinking water. Environ. Sci. Pollut. Res. 2017, 24, 28036–28045. [Google Scholar] [CrossRef]
- Díaz-Barriga, F.; Santos, M.A.; Mejia, J.D.; Batres, L.; Yanez, L.; Carrizales, L.; Vera, E.; Delrazo, L.M.; Cebrian, M.E. Children living near a smelter complex in San Luis Potosi, Mexico. Environ. Res. 1993, 62, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas González, M.; Osorio-Yáñez, C.; Gaspar-Ramírez, O.; Pavkovic, M.; Ochoa-Martínez, A.; López-Ventura, D.; Medeiros, M.; Barbier, O.C.; Pérez-Maldonado, I.N.; Sabbisetti, V.S.; et al. Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ. Res. 2016, 150, 653–662. [Google Scholar] [CrossRef]
- Torres-Arellano, J.M.; Osorio-Yáñez, C.; Sánchez-Peña, L.C.; Ayllon-Vergara, J.C.; Arreola-Mendoza, L.; Aguilar-Madrid Del Razo, L.M. Natriuretic peptides and echocardiographic parameters in Mexican children environmentally exposed to arsenic. Toxicol. Appl. Pharmacol. 2020, 403, 115164. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.J.; Fimbres, C.; Romo, L.; Méndez, R.O.; Grijalva, M. Incidence of Heavy Metal Contamination in Water Supplies in Northern Mexico. Environ. Res. 1998, 76, 114–119. [Google Scholar] [CrossRef]
- Meza, M.M.; Kopplin, M.J.; Burgess, J.L.; Gandolfi, A.J. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ. Res. 2004, 96, 119–126. [Google Scholar] [CrossRef]
- García-Rico, L.; Meza-Figueroa, D.; Gandolfi, A.J.; Del Rivero, C.I.; Martinez-Cinco, M.A.; Meza-Montenegro, M.M. Health risk assessment and urinary excretion of children exposed to arsenic through drinking water and soils in Sonora, Mexico. Biol. Trace Elem. Res. 2019, 187, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Flores-Casillas, L. Determinación de plaguicidas organoclorados en muestras de agua en el Valle del Yaqui y Mayo, Sonora. Bachelor’s Thesis, Biotechnology Engineering, Sonora Technological Institute, Sonora, México, December 2008. [Google Scholar]
- Maldonado-Escalante, J.F.; Meza-Figueroa, D.; Dévora-Figueroa, A.; García-Rico, L.; Burgess, J.L.; Lantz, R.C.; Yañez Estrada, L.; Martinez-Cinco, M.A.; Balderas-Cortés, J.; Mondaca-Fernández, I.; et al. An integrated health risk assessment of indigenous children exposed to arsenic in Sonora, Mexico. Hum. Ecol. Risk Assess. 2018, 25, 706–721. [Google Scholar] [CrossRef]
- Meza, M.M.; Yu, L.; Rodriguez, Y.Y.; Guild, M.; Thompson, D.; Gandolfi, A.J.; Klimecki, W.T. Developmentally restricted genetic determinants of human arsenic metabolism: Association between urinary methylated arsenic and CYT19 polymorphisms in children. Environ. Health Perspect. 2005, 113, 775–781. [Google Scholar] [CrossRef]
- Meza, M.M.; Kopplin, M.J.; Burgess, J.L.; Gandolfi, A.J. Urinary arsenic methylation profile in children exposed to low arsenic levels through drinking water. Toxicol. Environ. Chem. 2008, 90, 957–970. [Google Scholar] [CrossRef]
- Andrew, A.S.; Burgess, J.L.; Meza, M.M.; Demidenko, E.; Waugh, M.G.; Hamilton, J.W.; Karagas, M.R. Arsenic exposure is associated with deceased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ. Health Perspect. 2006, 114, 1193–1198. [Google Scholar] [CrossRef]
- Burgess, J.L.; Kurzius-Spencer, M.; O’Rourke, M.K.; Littau, S.R.; Roberge, J.; Meza-Montenegro, M.M.; Gutierrez-Millan, L.E.; Harris, R.B. Environmental arsenic exposure and serum matrix metalloproteinase-9. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rubio, P.; Roberge, J.; Arendell, L.; Harris, R.B.; O´Rourke, M.K.; Chen, Z.; Cantu-Soto, E.; Meza-Montenegro, M.M.; Billheimer, D.; Lu, Z.; et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol. Appl. Pharmacol. 2011, 252, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.; Navarro, M.E.; Jimenez-Capdeville, M.E.; Santos-Díaz, M.A.; Golden, A.; Rodríguez-Leyva, I.; Borja-Aburto, V.; Díaz-Barriga, F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ. Res. 2001, 85, 69–76. [Google Scholar] [CrossRef]
- Watanabe, C.T.; Inaoka, T.; Kadono, T.; Nagano, M.; Nakamura, S.; Ushijima, K.; Murayama, N.; Miyazaki, K.; Ohtsuka, R. Males in rural Bangladesh communities are more susceptible to chronic arsenic poisoning than females: Analyses based on urinary arsenic. Environ. Health Perspec. 2001, 109, 1265–1270. [Google Scholar] [CrossRef]
- Rossman, T.G.; Uddin, A.N.; Burns, F.J.; Bosland, M.C. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: An animal model for arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 2001, 176, 64–71. [Google Scholar] [CrossRef]
- Santra, A.; Chowdhury, A.; Ghatak, S.; Biswas, A.; Dhali, G.K. Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by N-acetylcysteine. Toxicol. Appl. Pharmacol. 2007, 220, 146–155. [Google Scholar] [CrossRef]
- Waalkes, M.P.; Liu, J.; Ward, J.M.; Diwan, B.A. Animal models for arsenic carcinogenesis: Inorganic arsenic is a transplacental carcinogen in mice. Toxicol. Appl. Pharmacol. 2004, 198, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Smeester, L.; Fry, R.C. Long-term health effects and underlying biological mechanisms of developmental exposure to arsenic. Curr. Environ. Health Rep. 2018, 5, 134–144. [Google Scholar] [CrossRef]
- Garcia-Chavez, E.B.; Segura, B.; Merchant, H.; Jimenez, I.; Del Razo, L.M. Functional and morphological effects of repeated sodium arsenite exposure on rat peripheral sensory nerves. J. Neurol. Sci. 2007, 258, 104–110. [Google Scholar] [CrossRef]
- NOM-230-SSA1-2002, NORMA Oficial Mexicana: Salud Ambiental, Agua para uso y Consumo Humano, y Requisitos Sanitarios que se Deben Cumplir en los Sistemas de Abastecimiento Públicos y Privados Durante el Manejo de Agua. Procedimientos Sanitarios para el Muestreo. Available online: http://www.salud.gob.mx/unidades/cdi/nom/230ssa102.html (accessed on 17 January 2021).
- USEPA. Method 200.7. Revision 4.4. Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-atomic Emission Spectrometry; USEPA (US Environmental Protection Agency): Washington, DC, USA, 1994; pp. 1–59.
- NOM-062-ZOO-1999, NORMA Oficial Mexicana: Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 17 January 2021).
- Kaler, S.; Dhar, P.; Bhattacharya, A.; Mehra, R.D. Preliminary morphological and immunohistochemical changes in rat hippocampus following postnatal exposure to sodium arsenite. Toxicol. Int. 2013, 20, 160–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, H.; Zhao, W.; Ye, L.; Chen, Z.; Cui, Y. Postnatal low-concentration arsenic exposure induces autism-like behavior and affects frontal cortex neurogenesis in rats. Environ. Toxicol. Pharmacol. 2018, 62, 188–198. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Htway, S.M.; Sein, M.T.; Nohara, K.; Win-Shwe, T.T. Effects of developmental arsenic exposure on the social behavior and related gene expression in C3H adult male mice. Int. J. Environ. Res. Public Health 2019, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Tolins, M.; Ruchiwarat, M.; Landrigan, P. The developmental neurotoxicity of arsenic: Cognitive and behavioral consequences of early exposure. Ann. Glob. Health 2014, 80, 303–314. [Google Scholar] [CrossRef]
- Moreno Avila, C.L.; Limon-Pacheco, J.H.; Giordano, M.; Rodríguez, V.M. Chronic exposure to arsenic in drinking water causes alterations in locomotor activity and decreases striatal mRNA for the D2 dopamine receptor in CD1 male mice. J. Toxicol. 2016, 2016, 4763434. [Google Scholar] [CrossRef]
- Manthari, R.K.; Tikka, C.; Ommati, M.M.; Niu, R.; Sun, Z.; Wang, J.; Zhang, J.; Wang, J. Arsenic induces authophagy in developmental mouse cerebral cortex and hippocampus by inhibiting PI3K/Akt/mTOR signaling pathway: Involvement of blood-brain barrier´s tight junction proteins. Arch. Toxicol. 2018, 92, 3255–3275. [Google Scholar] [CrossRef]
- Yi, Y.; Gao, S.; Xia, J.; Li, C.; Zhao, Y.; Zhang, Y.; Liang, A.; Ji, S. Study of the accumulation and distribution of arsenic species and association with arsenic toxicity in rats after 30 days or oral realgar administration. J. Ethnopharmacol. 2020, 247, 111576. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Del Razo, L.M.; Limon-Pacheco, J.H.; Giordano, M.; Sanchez-Pena, L.C.; Uribe-Querol, E.; Gutierrez-Ospina, G.; Gonsebatt, M.E. Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol. Sci. 2005, 84, 157–166. [Google Scholar] [CrossRef]
- Del Razo, L.M.; Quintanilla-Vega, B.; Brambila-Colombres, E.; Calderon-Aranda, E.S.; Manno, M.; Albores, A. Stress proteins induced by arsenic. Toxicol. Appl. Pharmacol. 2001, 177, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Piao, F.; Ma, Y.; Hiraku, Y.; Murata, M.; Oikawa, S.; Cheng, F.; Zhong, L.; Yamauchi, T.; Kawanishi, S.; Yokoyama, K. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J. Occup. Health 2005, 47, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Chen, Y.M. Oxidative stress and neurodegenerative disorders. J. Biomed. Sci. 1998, 5, 401–414. [Google Scholar] [CrossRef]
- Cebrián, M.E. Chronic arsenic poisoning in humans: The case of Mexico. In Arsenic in the Environment, Part II: Human Health and Ecosystem Effects; John Wiley & Sons: New York, NY, USA, 1994; pp. 93–107. [Google Scholar]
- Aktar, S.; Jahan, M.; Alam, S.; Mohanto, N.C.; Arefin, A.; Rahman, A.; Haque, A.; Himeno, S.; Hossain, K.; Saud, Z.A. Individual and combined effects of arsenic and lead on behavioral and biochemical changes in mice. Biol. Trace Elem. Res. 2017, 177, 288–296. [Google Scholar] [CrossRef]
- Chang, C.; Guo, H.R.; Tsai, W.C.; Yang, K.L.; Lin, L.C.; Cheng, T.J.; Chuu, J.J. Subchronic arsenic exposure induces anxiety-like behaviors in normal mice and enhances depression-like behaviors in the chemically induced mouse model of depression. Biomed. Res. Int. 2015, 2015, 159015. [Google Scholar] [CrossRef]
- Selim, S.A.; Selim, A.O.; Askar, E.M. Harmful effects of arsenic on the cerebral cortex of adult male albino rats: Light and electron microscopic studies. Egypt. J. Histol. 2012, 35, 249–258. [Google Scholar] [CrossRef]
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A. Manganese-induced neurotoxicity: New insights into protein misfolding, mitochondrial impairment, and neuroinflammation. Front. Neurosci. 2019, 13, 654. [Google Scholar] [CrossRef]
- Roy, A.; Kordas, K.; Lopez, P.; Rosado, J.L.; Cebrian, M.E.; Vargas, G.G.; Ronquillo, D.; Stoltzfus, R.J. Association between arsenic exposure and behavior among first-graders from Torreon, Mexico. Environ. Res. 2011, 111, 670–676. [Google Scholar] [CrossRef]
- Centeno, J.A.; Mullick, F.G.; Martinez, L.; Page, N.P.; Gibb, H.; Longfellow, D.; Thompson, C.; Ladich, E.R. Pathology related to chronic arsenic exposure. Environ. Health Persp. 2002, 110, 883–886. [Google Scholar] [CrossRef]
- Thomas, D.J.; Styblo, M.; Lin, S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol. 2001, 176, 127–144. [Google Scholar] [CrossRef]
- Amuno, S.; Jamwal, A.; Grahn, B.; Niyogi, S. Chronic arsenicosis and cadmium exposure in wild snowshoe hares (Lepus americanus) breeding near Yellowknife, Northwest Territories (Canada), part 1: Evaluation of oxidative stress, antioxidant activities and hepatic damage. Sci. Total Environ. 2018, 618, 916–926. [Google Scholar] [CrossRef]
- Chiu, H.F.; Ho, S.C.; Wang, L.Y.; Wu, T.N.; Yang, C.Y. Does arsenic exposure increase the risk for liver cancer? J. Toxicol. Environ. Health A 2004, 67, 1491–1500. [Google Scholar] [CrossRef]
- Lin, H.J.; Sung, T.I.; Chen, C.Y.; Guo, H.R. Arsenic levels in drinking water and mortality of liver cancer in Taiwan. J. Hazard. Mater. 2013, 262, 1132–1138. [Google Scholar] [CrossRef]
- Bashir, S.Y.; Sharma, M.; Irshad, M.; Gupta, S.D.; Dogra, T.D. Arsenic-induced cell death in liver and brain of experimental rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 38–43. [Google Scholar] [CrossRef]
- Biswas, S.; Banna, H.; Jahan, M.; Anjum, A.; Siddique, A.E.; Roy, A.; Nikkon, F.; Salam, K.A.; Haque, A.; Himeno, S. In vivo evaluation of arsenic-associated behavioral and biochemical alterations in F0 and F1 mice. Chemosphere 2020, 245, 125619. [Google Scholar] [CrossRef] [PubMed]
- Ferzand, R.; Gadahi, J.; Saleha, S.; Ali, Q. Histological and haematological disturbance caused by arsenic toxicity in mice model. Pak. J. Biol. Sci. 2008, 11, 1405–1413. [Google Scholar] [CrossRef] [PubMed]





| Element | R2 | Cócorit | Vícam | Pótam | WHO Limit |
|---|---|---|---|---|---|
| Al | 1 | 13 | 11 | 24 | 100 |
| Sb | 0.9999 | <DL | <DL | <DL | 20 |
| As | 0.9984 | 6 | 12 | 75 | 10 |
| Ba | 1 | 35 | 57 | 14 | 1300 |
| Be | 1 | <DL | <DL | <DL | - |
| Cd | 1 | <DL | <DL | <DL | 3 |
| Cr | 1 | <DL | <DL | <DL | 50 |
| Co | 1 | <DL | <DL | <DL | - |
| Cu | 1 | 17 | 7 | 7 | 2000 |
| Fe | 1 | <DL | <DL | 19 | 300 |
| Pb | 1 | <DL | <DL | <DL | 10 |
| Mn | 1 | <DL | 7 | 12 | 100 |
| Mo | 1 | 12 | 56 | 34 | - |
| Ni | 0.9989 | <DL | <DL | <DL | 70 |
| Se | 0.9994 | 7 | 10 | 6 | 40 |
| Ag | 1 | <DL | <DL | <DL | - |
| Sr | 1 | 56 | 819 | 121 | - |
| Tl | 0.9996 | <DL | 7 | <DL | - |
| Zn | 1 | 37 | 62 | 135 | - |
| V | 1 | <DL | <DL | <DL | - |
| Lesions | Group I (Control) | Group II (Cócorit) | Group III (Vícam) | Group IV (Pótam) |
|---|---|---|---|---|
| Brain | ||||
| Angulated cells | - | - | ++ | +++ |
| Satellitosis | - | - | +++ | ++++ |
| Gliosis | - | - | +++ | +++ |
| Axon retraction | - | - | + | ++ |
| Neuronal vacuolation | - | - | - | + |
| Liver | ||||
| Hepatocellular degeneration | - | - | + | ++ |
| Hepatocyte vacuolation | - | - | - | ++ |
| Necrosis | - | - | + | ++ |
| Binucleated cells | - | + | +++ | ++++ |
| Group I (Control) | Group II (Cócorit) | Group III (Vícam) | Group IV (Pótam) | |
|---|---|---|---|---|
| Hematic biometry | ||||
| Erythrocytes (×1000 × mm3) | 7.48 ± 0.54 | 7.97 ± 0.55 | 7.71 ± 0.21 | 7.16 ± 1.91 |
| Platelets (×1000 × mm3) | 1103.5 ± 698.20 | 1610.2 ± 257.68 | 925.5 ± 19.09 | 803.4 ± 490.52 |
| Leukocytes (×1000 × mm3) | 7.9 ± 2.29 | 5.74 ± 4.10 | 2.70 ± 0.14 | 6.08 ± 3.53 |
| Monocytes | 6.90 ± 6.89% | 5.34 ± 4.56% | 3.65 ± 2.05% | 6.25 ± 2.57% |
| Neutrophils | 11.77 ± 9.76% | 9.38 ± 5.43% | 11.95 ± 0.64% | 6.08 ± 1.37% |
| Lymphocytes | 81.03 ± 16.24% | 84.82 ± 4.18% | 83.65 ± 1.34% | 87.25 ± 3.26% |
| Hepatic profile | ||||
| Serum glutamate-oxaloacetate transaminase (IU·L−1) | 252.50 ± 179.37 | 214.00 ± 218.27 | 437.50 ± 340.53 | 420.00 ± 444.96 |
| Serum glutamate-pyruvate transaminase (IU·L−1) | 55.75 ± 30.24 | 55.80 ± 52.95 | 131.25 ± 83.30 | 90.00 ±87.25 |
| Alkaline phosphatase (IU·L−1) | 40.00 ± 13.54 | 34.33 ± 10.69 | 29.50 ± 5.97 | 23.20 ± 9.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Espinoza, S.; Angulo-Molina, A.; Meza-Figueroa, D.; López-Cervantes, G.; Meza-Montenegro, M.; Armienta, A.; Soto-Puebla, D.; Silva-Campa, E.; Burgara-Estrella, A.; Álvarez-Bajo, O.; et al. Effects of Untreated Drinking Water at Three Indigenous Yaqui Towns in Mexico: Insights from a Murine Model. Int. J. Environ. Res. Public Health 2021, 18, 805. https://doi.org/10.3390/ijerph18020805
Navarro-Espinoza S, Angulo-Molina A, Meza-Figueroa D, López-Cervantes G, Meza-Montenegro M, Armienta A, Soto-Puebla D, Silva-Campa E, Burgara-Estrella A, Álvarez-Bajo O, et al. Effects of Untreated Drinking Water at Three Indigenous Yaqui Towns in Mexico: Insights from a Murine Model. International Journal of Environmental Research and Public Health. 2021; 18(2):805. https://doi.org/10.3390/ijerph18020805
Chicago/Turabian StyleNavarro-Espinoza, Sofia, Aracely Angulo-Molina, Diana Meza-Figueroa, Guillermo López-Cervantes, Mercedes Meza-Montenegro, Aurora Armienta, Diego Soto-Puebla, Erika Silva-Campa, Alexel Burgara-Estrella, Osiris Álvarez-Bajo, and et al. 2021. "Effects of Untreated Drinking Water at Three Indigenous Yaqui Towns in Mexico: Insights from a Murine Model" International Journal of Environmental Research and Public Health 18, no. 2: 805. https://doi.org/10.3390/ijerph18020805
APA StyleNavarro-Espinoza, S., Angulo-Molina, A., Meza-Figueroa, D., López-Cervantes, G., Meza-Montenegro, M., Armienta, A., Soto-Puebla, D., Silva-Campa, E., Burgara-Estrella, A., Álvarez-Bajo, O., & Pedroza-Montero, M. (2021). Effects of Untreated Drinking Water at Three Indigenous Yaqui Towns in Mexico: Insights from a Murine Model. International Journal of Environmental Research and Public Health, 18(2), 805. https://doi.org/10.3390/ijerph18020805








