Melanin: Production from Cheese Bacteria, Chemical Characterization, and Biological Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Source, Isolation, and Identification
2.2. Bacterial Growth and Melanin Production
2.3. Melanin Extraction, Purification, and Quantification
2.4. Melanin Characterization
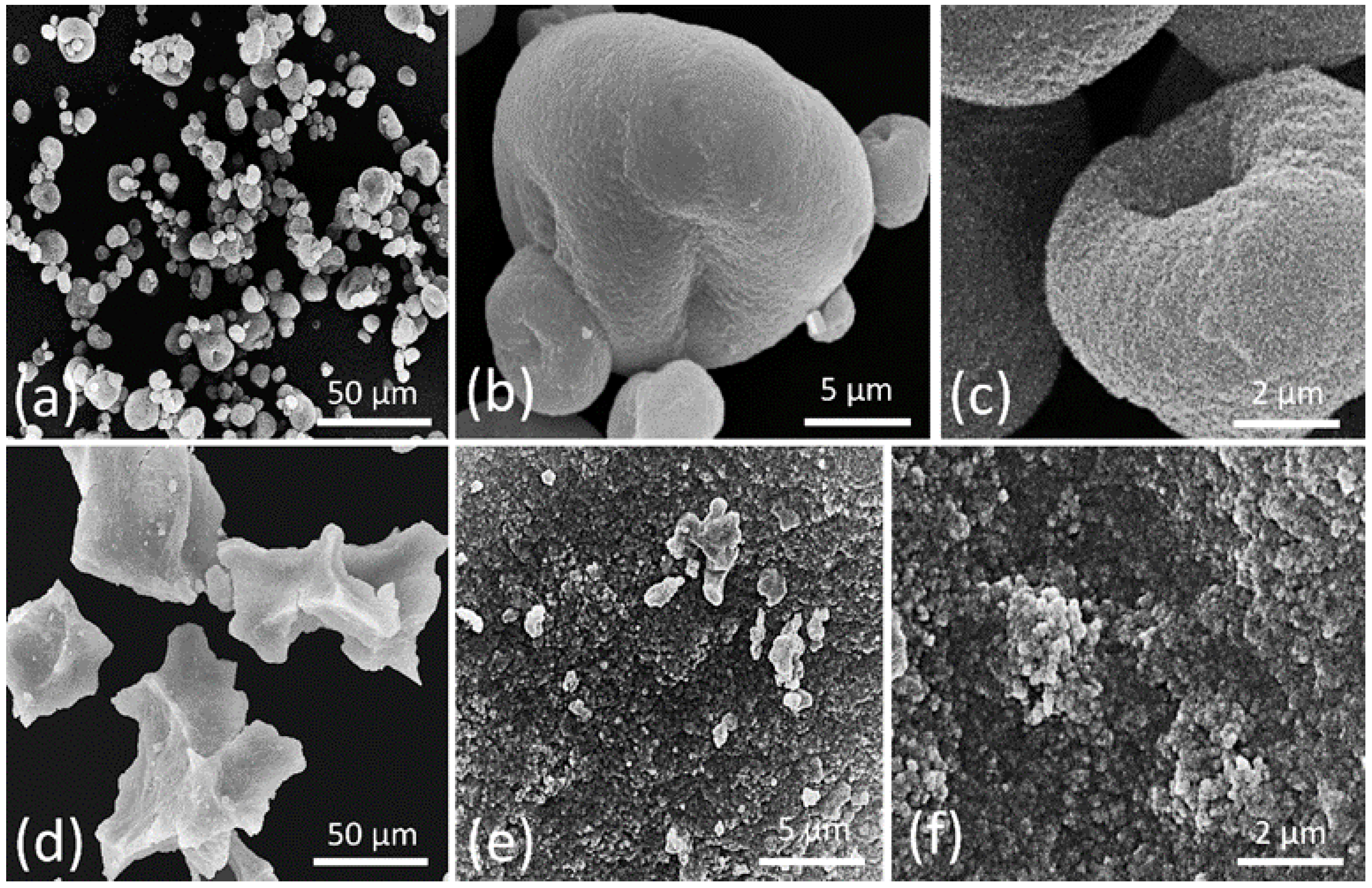
2.4.1. Scanning Electron Microscopy (SEM)
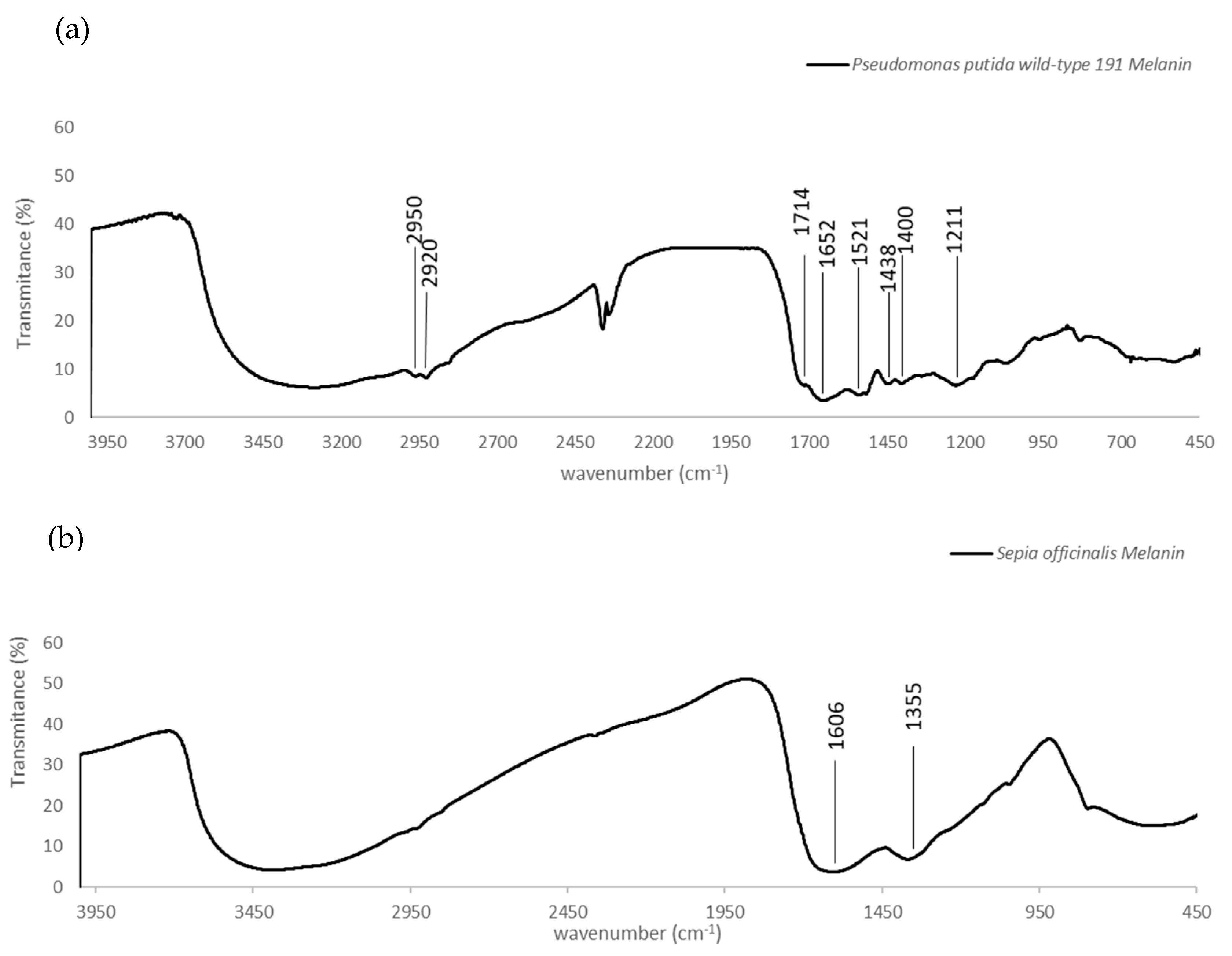
2.4.2. Fourier Transform IR Analysis (FTIR)
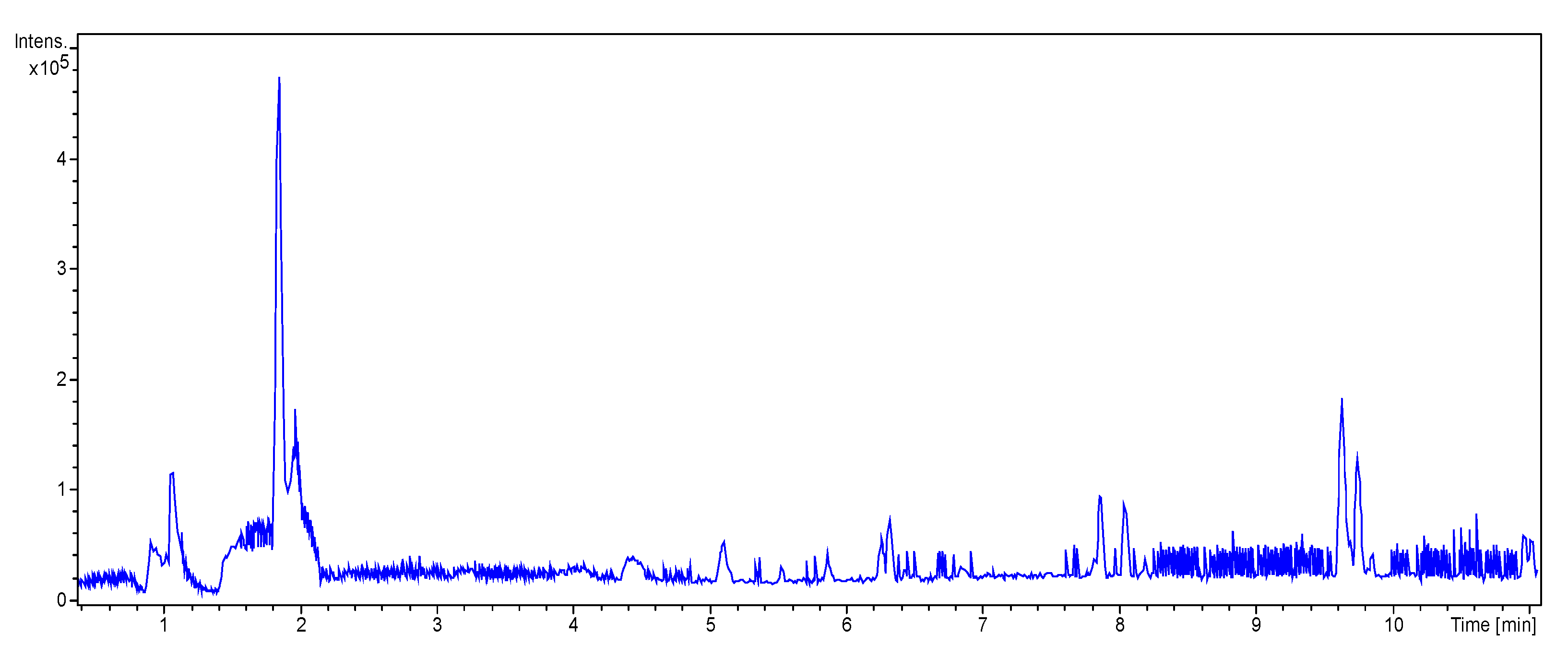
2.4.3. UHPLC-HRMS/MS Mass Spectrometry
2.5. Biological Activities
2.5.1. Antioxidant Activity
2.5.2. Acetylcholinesterase Inhibitory Activity
2.5.3. Cytotoxicity
2.5.4. Cholesterol Permeation Studies
2.6. Cholesterol Quantification by HPLC-DAD
2.7. Data Analysis
3. Results
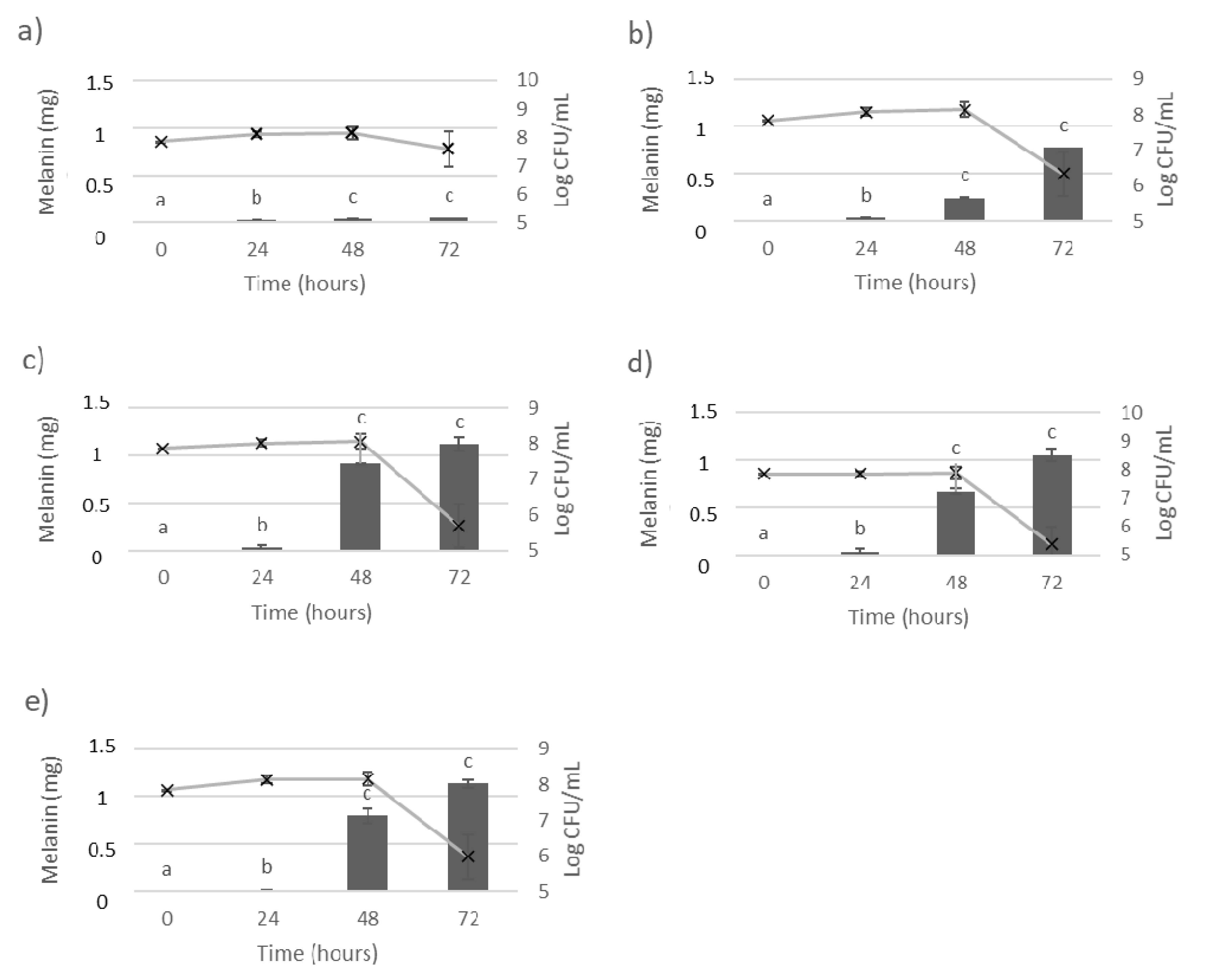
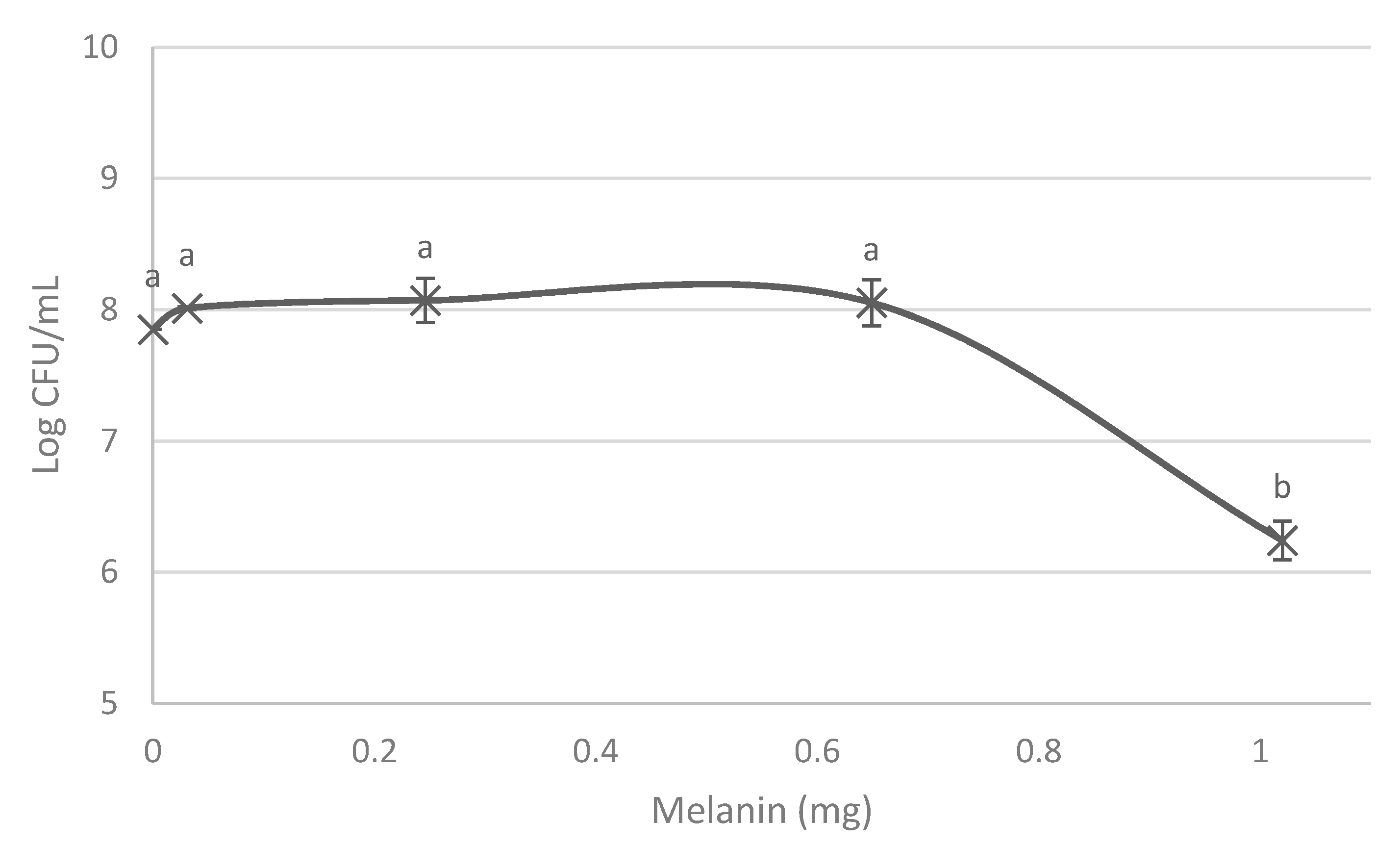
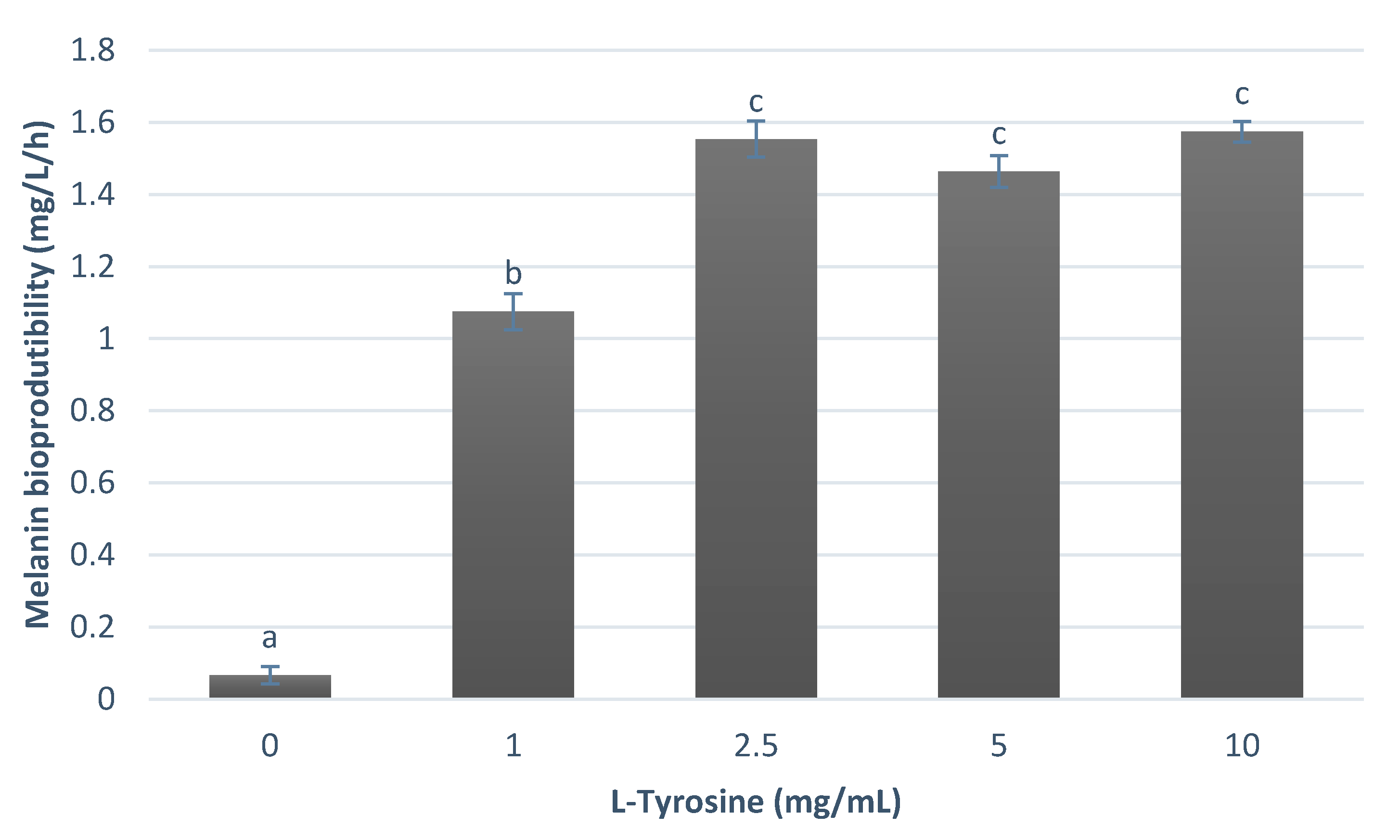
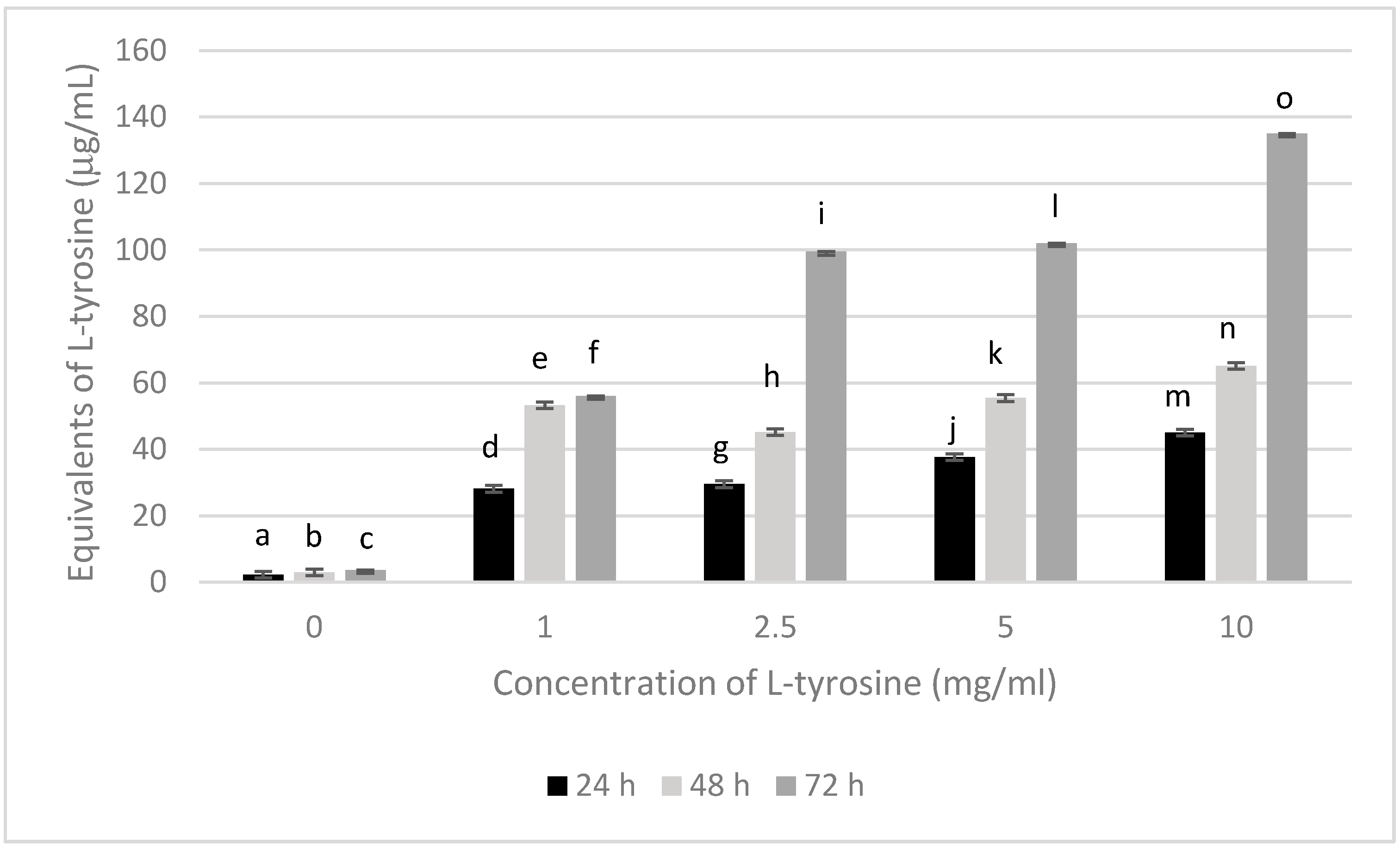
3.1. Melanin Bioproduction under the Influence of L-Tyrosine
3.2. Melanin Morphologic Analysis by SEM
3.3. Melanin Structural Identification
3.3.1. FTIR Analysis
3.3.2. UHPLC-HRMS/MS Mass Spectrometry
3.4. Melanin Bioactivities
3.4.1. AChE and Antioxidant Activities
3.4.2. Cytotoxicity
3.4.3. Cholesterol In Vitro Intestinal Permeation
4. Discussion
4.1. Production and Structural Analysis
4.2. Bioactivities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucey, J. How to Avoid Color Defects in Cheese. Dairy Food 2017. Available online: https://www.dairyfoods.com/articles/92229-how-to-avoid-color-defects-in-cheese (accessed on 16 July 2021).
- Arslan, S.; Eyi, A.; Özdemir, F. Spoilage Potentials and Antimicrobial Resistance of Pseudomonas spp. Isolated from Cheeses. J. Dairy Sci. 2011, 94, 5851–5856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulkadir, N. Bacterial Pigments and Its Significance. MOJ Bioequiv. Bioavailab. 2017, 4, 285–288. [Google Scholar] [CrossRef] [Green Version]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The Realm of Microbial Pigments in the Food Color Market. Front. Sustain. Food Syst. 2021, 5, 54. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Nielsen, K.F.; Larsen, T.O.; Frisvad, J.C.; Meyer, A.S.; Thrane, U. Exploring Fungal Biodiversity for the Production of Water-Soluble Pigments as Potential Natural Food Colorants. Curr. Opin. Biotechnol. 2005, 16, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Cristea, D.; Vilarem, G. Improving Light Fastness of Natural Dyes on Cotton Yarn. Dyes Pigments 2006, 70, 238–245. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial Production of Melanin and Its Various Applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- López-Serrano, D.; Solano, F.; Sanchez-Amat, A. Identification of an Operon Involved in Tyrosinase Activity and Melanin Synthesis in Marinomonas mediterranea. Gene 2004, 342, 179–187. [Google Scholar] [CrossRef]
- Hill, H.Z. The Function of Melanin or Six Blind People Examine an Elephant. Bioessays 1992, 14, 49–56. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Review: Innovative Technology Advanced Chemical Methods in Melanin Determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Ohyama, A. Characterization of ‘Pyomelanin’-Producing Strains of Pseudomonas aeruginosa. Int. J. Syst. Bacteriol. 1972, 22, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Kotob, S.I.; Coon, S.L.; Quintero, E.J.; Weiner, R.M. Homogentisic Acid Is the Primary Precursor of Melanin Synthesis in Vibrio cholerae, a Hyphomonas Strain, and Shewanella colwelliana. Appl. Environ. Microbiol. 1995, 61, 1620–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, A.V.; Buccafusco, J.J. The Cholinergic Hypothesis of Age and Alzheimer’s Disease-Related Cognitive Deficits: Recent Challenges and Their Implications for Novel Drug Development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.Z.; Gazioglu, I.; Dag, A.; Salmas, R.E.; Kayık, G.; Durdagi, S.; Sonmez, F. Synthesis, Anticholinesterase Activity and Molecular Modeling Study of Novel Carbamate-Substituted Thymol/Carvacrol Derivatives. Bioorg. Med. Chem. 2017, 25, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, T.R.; Gevorkyan, O.V.; Chavushyan, V.A.; Meliksetyan, I.B.; Hovsepyan, A.S.; Manvelyan, L.R. Effects of Bacterial Melanin on Motor Recovery and Regeneration after Unilateral Destruction of Substantia Nigra Pars Compacta in Rats. Neuropeptides 2014, 48, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ressaissi, A.; Attia, N.; Pacheco, R. Aqueous Extracts from Nopal (Opuntia ficus-indica): Antiacetylcholinesterase and Antioxidant Activity from Phenolic Bioactive Compounds. Int. J. Green Herb. Chem. 2016, 5, 337–348. [Google Scholar]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P. European Cardiovascular Disease Statistics 2017. Eur. Hear. Netw. 2017, 94–100. Available online: https://ehnheart.org/images/CVD-statistics-report-August-2017.pdf (accessed on 16 July 2021).
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Frazão, M.F.N.; Serralheiro, M.L.M. Studies on the Molecular Mechanism of Cholesterol Reduction by Fraxinus angustifolia, Peumus boldus, Cynara cardunculus and Pterospartum tridentatum Infusions. J. Med. Plants Res. 2014, 8, 9–17. [Google Scholar]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M. From Extraction to Advanced Analytical Methods: The Challenges of Melanin Analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [Green Version]
- André, R.; Guedes, L.; Melo, R.; Ascensão, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on in Vitro Bioactivities and Chemical Components of Fucus Vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef]
- Seshagiri, R.; Jalmi, P.; Bodke, P. A Process for Production of Water Soluble Melanin Using a Strain of the Fungus Gliocepha-Lotrichum. International Application Published under the Patent Cooperation Treaty (pct). Patent WO 2010/064262/A2, 10 June 2010. [Google Scholar]
- André, R.; Catarro, J.; Freitas, D.; Pacheco, R.; Oliveira, M.C.; Serralheiro, M.L.; Falé, P.L. Action of Euptox A from Ageratina adenophora Juice on Human Cell Lines: A Top-down Study Using FTIR Spectroscopy and Protein Profiling. Toxicol. Vitr. 2019, 57, 217–225. [Google Scholar] [CrossRef]
- Magarelli, M.; Passamonti, P.; Renieri, C. Purification, Characterization and Analysis of Sepia Melanin from Commercial Sepia Ink (Sepia officinalis) Purificación, Caracterización y Análisis de La Melanina de Sepia a Partir de La Tinta de Sepia (Sepia officinalis) Resumen. Rev. CES Med. Vet. Zootec. 2010, 5, 18–28. [Google Scholar]
- Falé, P.L.; Borges, C.; Madeira, P.J.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L.M. Rosmarinic Acid, Scutellarein 4′-Methyl Ether 7-O-Glucuronide and (16S)-Coleon E Are the Main Compounds Responsible for the Antiacetylcholinesterase and Antioxidant Activity in Herbal Tea of Plectranthus barbatus (“Falso Boldo”). Food Chem. 2009, 114, 798–805. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Cleto, P.; Madeira, P.J.A.; Florêncio, M.H.; Frazatilde, F.N.; Serralheiro, M.L.M. Antioxidant and Anti-Acetylcholinesterase Activity of Commercially Available Medicinal Infusions after in Vitro Gastrointestinal Digestion. J. Med. Plants Res. 2013, 7, 1370–1378. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Maruzzella, F.; Helena Florêncio, M.; Frazão, F.N.; Serralheiro, M.L.M. Evaluation of Cholesterol Absorption and Biosynthesis by Decoctions of Annona cherimola Leaves. J. Ethnopharmacol. 2013, 150, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Mbonyiryivuze, A.; Mwakikunga, B.; Dhlamini, S.M.; Maaza, M. Fourier Transform Infrared Spectroscopy for Sepia Melanin. Phys. Mater. Chem. 2015, 3, 25–29. [Google Scholar] [CrossRef]
- Tarangini, K.; Mishra, S. Production, Characterization and Analysis of Melanin from Isolated Marine Pseudomonas Sp. Using Vegetable Waste. Res. J. Eng. Sci. 2013, 2, 40–46. [Google Scholar]
- Banerjee, A.; Supakar, S.; Banerjee, R. Melanin from the Nitrogen-Fixing Bacterium Azotobacter chroococcum: A Spectroscopic Characterization. PLoS ONE 2014, 9, e84574. [Google Scholar] [CrossRef] [Green Version]
- Araujo, M.; Xavier, J.R.; Nunes, C.D.; Vaz, P.D.; Humanes, M. Marine Sponge Melanin: A New Source of an Old Biopolymer. Struct. Chem. 2012, 23, 115–122. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Mario, G.F.; Ningsanond, S. Antioxidant Activity and Cytotoxicity of Rang Chuet (Thunbergia laurifolia Lindl.) Extracts. Asian J. Food Agro-Ind. 2008, 1, 116–128. [Google Scholar]
- Müller, M.; Ausländer, S.; Ausländer, D.; Kemmer, C.; Fussenegger, M. A Novel Reporter System for Bacterial and Mammalian Cells Based on the Non-Ribosomal Peptide Indigoidine. Metab. Eng. 2012, 14, 325–335. [Google Scholar] [CrossRef]
- Carreira, A.; Paloma, L.; Loureiro, V. Pigment Producing Yeasts Involved in the Brown Surface Discoloration of Ewes’ Cheese. Int. J. Food Microbiol. 1998, 41, 223–230. [Google Scholar] [CrossRef]
- Pavan, M.E.; López, N.I.; Pettinari, M.J. Melanin Biosynthesis in Bacteria, Regulation and Production Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 1357–1370. [Google Scholar] [CrossRef]
- Claus, H.; Decker, H. Bacterial Tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.A.; El-Ewasy, S.M. Bioproduction, Characterization, Anticancer and Antioxidant Activities of Extracellular Melanin Pigment Produced by Newly Isolated Microbial Cell Factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial Melanin: Recent Advances in Biosynthesis, Extraction, Characterization, and Applications. Biotechnol. Adv. 2021, 107773. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, V.; Smith, S.T.; Mikobi, E.; Raji, M.A. Acetylcholinesterase Inhibitors: Beneficial Effects on Comorbidities in Patients with Alzheimer’s Disease. Am. J. Alzheimers. Dis. Other Dement®. 2018, 33, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, M.F.; Falé, P.L.V.; Araújo, M.E.M.; Serralheiro, M.L.M. Acetylcholinesterase Inhibition and Antioxidant Activity of the Water Extracts of Several Hypericum Species. Food Chem. 2010, 120, 1076–1082. [Google Scholar] [CrossRef]
- Ece, A. Towards More Effective Acetylcholinesterase Inhibitors: A Comprehensive Modelling Study Based on Human Acetylcholinesterase Protein–Drug Complex. J. Biomol. Struct. Dyn. 2020, 38, 565–572. [Google Scholar] [CrossRef]
- Hammersley, D.; Signy, M. Ezetimibe: An Update on Its Clinical Usefulness in Specific Patient Groups. Ther. Adv. Chronic Dis. 2017, 8, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| RT (Min) | Accuracy [M + H]+ (m/z) | Intensity | MS2 Fragment Ions [m/z, Attribution, (Intensity %)] | Proposed Compound | Chemical Structure |
|---|---|---|---|---|---|
| 1.9 | 182.0808 | 5.3 × 105 | 91.05 (100); 165.07 (58%); 136.07 (60.9); 123.04 (45.7); 119.04 (36.1); 65.03 (32.5); 95.04 (27.4); 77.03 (21.2) |  | C9H12NO3 Tyrosine Error = −0.2 |
| 2.4 | 195.1128 | 4.6 × 104 | 195.11 (100%); 167.00 (6.8%); 125.07 (11%); 98.06 (9%) 70.06 (36%) | 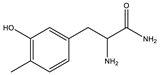 | C10H14N2O2 Error = −1 |
| 5.7 | 231.1124 | 4.6 × 104 | 214.08 (15%); 188.07 (17%); 158.09 (100%); 143.07 (56%); 130.06 (22%); 115.05 (11%); 74.02 (20%) | 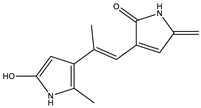 | C13H14N2O2 Error = 4 |
| 5.9 | 227.0818 | 8.5 × 104 | 199.08 (100%); 181.07 (40%); 154.06 (40%); 128.05 (10%) | 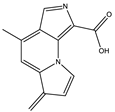 | C13H10N2O2 Error = 1.1 |
| 6.3 | 361.1051 | 9.5 × 104 | 315.09 (65%); 297.08 (11%); 269.09 (27%); 255.06 (53%); 237.05 (26%); 209.05 (31%); 191.04 (16%); 181.06 (16%); 163.05 (39%); 135.05 (26%); 107.04 (100%) | 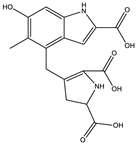 | C17H16N2O7 Error = 1.5 |
| 6.9 | 245.1307 | 4.1 × 104 | 154.07 (23%); 120.08 (41%); 98.06 (11%) | 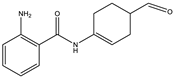 | C14H16N2O2 Error = 2 |
| 6.9 | 659.3416 | 1.1 × 104 | 614.17 (2%); 609.66 (2%); 521.70 (2%); 491.24 (6%); 344.18 (28%); 227.10 (42%); 217.13 (9%); 199.10 (37%); 182.13 (3%); 169.09 (35%) |  | C33H42N10O5 Error = 0 |
| 7.9 | 318.0970 | 7.1 × 104 | 272.09 (20%); 165.05 (27%); 147.04 (15%); 138.03 (11%); 123.04 (29%) | 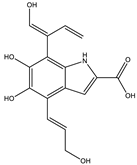 | C16H15NO6 Error = 0 |
| 8.0 | 558.1058 | 7.1 × 104 | 512.10 (94%); 498.08 (20%); 452.07 (48%); 346.03 (42%); 308.04 (81%); 289.03 (46%); 107.04 (20%) | 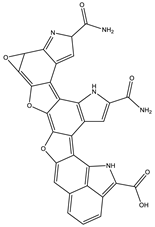 | C30H15N5O7 Error = −2.9 |
| 8.0 | 580.0888 | 5.5 × 103 | 536.10 (8%); 324.08 (15%); 276.02 (11%); 235.00 (21%); 107.04 (13%) |  | C31H17NO11 Error = −0.8 |
| 10.5 | 328.1414 | 1.1 × 105 | 298.13 (100%); 289.12 (19%); 257.11 (12%) 208.60 (16%) | 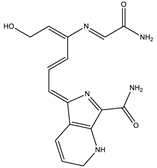 | C16H17N5O3 Error = −3.7 |
| 10.5 | 655.2763 | 2.1 × 104 | 637.26 (2%); 609.81 (1%); 596.26 (10%); 581.23 (2%); 551.23 (2%); 525.23 (1%) 445.04 (1%) | 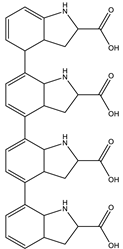 | C36H38N4O8 Error = −0.15 |
| Samples | AChE | DPPH |
|---|---|---|
| IC50 (µg/mL) | EC50 (µg/mL) | |
| P. putida ESACB 191 melanin | 575 ± 4.0 a | 74.2 ± 0.2 a |
| S. officinalis melanin | 1085 ± 8.7 b | 170.3 ± 0.5 b |
| Galantamine | 240 ± 6.0 c | - |
| BHT | - | 39.1 ± 0.3 c |
| Ascorbic acid | - | 4.4 ± 0.04 d |
| A375 | HeLa Kyoto | HepG2 | Caco2 | |
|---|---|---|---|---|
| Pseudomonas putida ESACB 191 melanin | 1.77 ± 0.09 a | 2.51 ± 0.06 b | 0.89 ± 0.14 d | 1.08 ± 0.09 d |
| Sepia officinalis melanin | 1.38 ± 0.03 a | 2.59 ± 0.05 c | - | - |
| Inhibition of Cholesterol Permeation | |
|---|---|
| Basolateral (%) | |
| Ezetimibe | 87.5 ± 1.7 a |
| Ezetimibe + melanin from P. putida ESACB 191 | 80.3 ± 1.2 b |
| Melanin from P. putida ESACB 191 | 17.0 ± 4.2 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, A.R.; Pacheco, R.; Vaz, P.D.; Pintado, C.S.; Ascensão, L.; Serralheiro, M.L. Melanin: Production from Cheese Bacteria, Chemical Characterization, and Biological Activities. Int. J. Environ. Res. Public Health 2021, 18, 10562. https://doi.org/10.3390/ijerph182010562
Ferraz AR, Pacheco R, Vaz PD, Pintado CS, Ascensão L, Serralheiro ML. Melanin: Production from Cheese Bacteria, Chemical Characterization, and Biological Activities. International Journal of Environmental Research and Public Health. 2021; 18(20):10562. https://doi.org/10.3390/ijerph182010562
Chicago/Turabian StyleFerraz, Ana Rita, Rita Pacheco, Pedro D. Vaz, Cristina S. Pintado, Lia Ascensão, and Maria Luisa Serralheiro. 2021. "Melanin: Production from Cheese Bacteria, Chemical Characterization, and Biological Activities" International Journal of Environmental Research and Public Health 18, no. 20: 10562. https://doi.org/10.3390/ijerph182010562
APA StyleFerraz, A. R., Pacheco, R., Vaz, P. D., Pintado, C. S., Ascensão, L., & Serralheiro, M. L. (2021). Melanin: Production from Cheese Bacteria, Chemical Characterization, and Biological Activities. International Journal of Environmental Research and Public Health, 18(20), 10562. https://doi.org/10.3390/ijerph182010562









