Diagnosis of Dental Fluorosis Using Micro-Raman Spectroscopy Applying a Principal Component-Linear Discriminant Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Fluorosis Classification
2.3. Sample Preparation
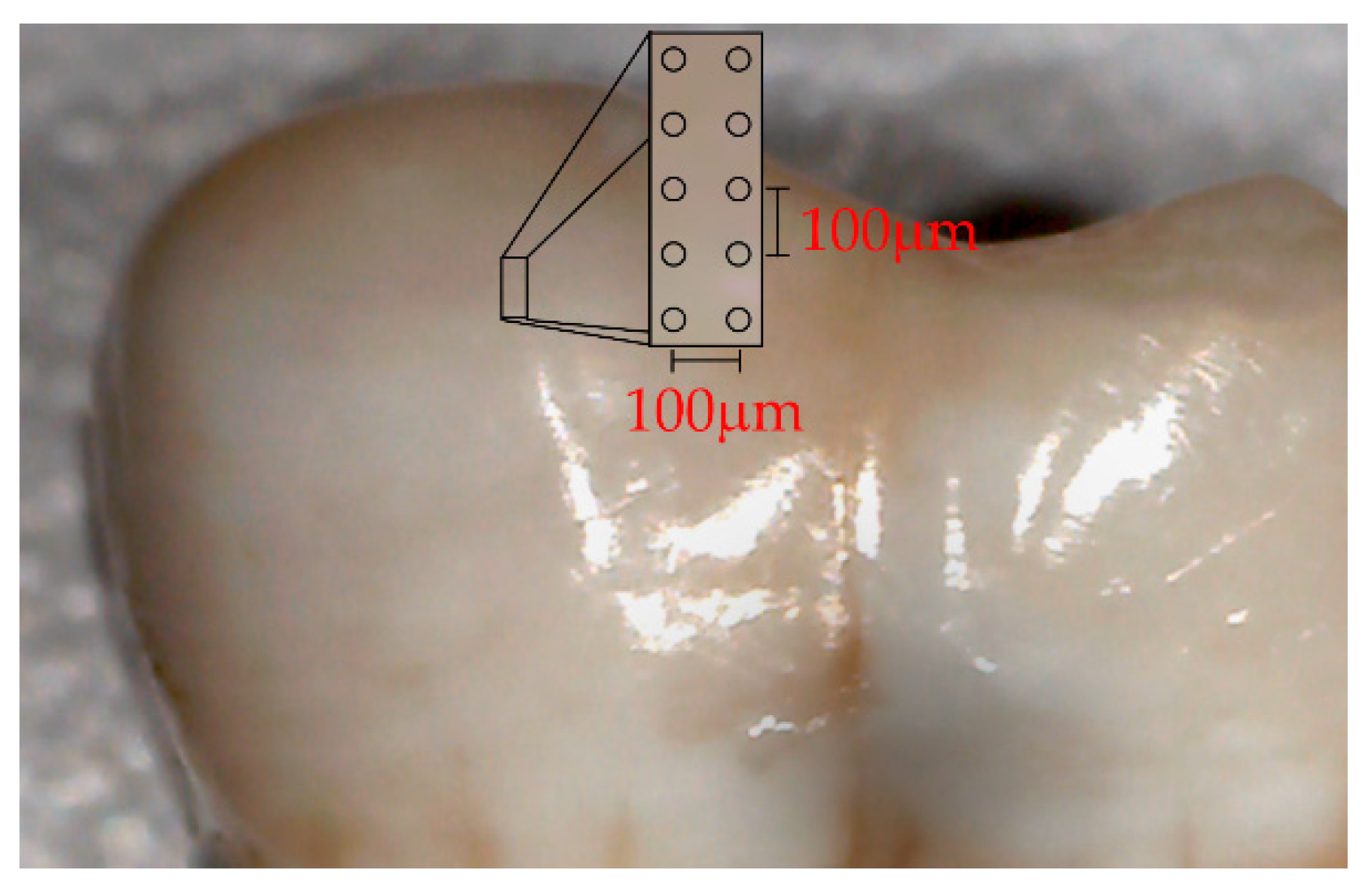
2.4. Micro-Raman Spectroscopy
2.5. Statistical Analysis
3. Results
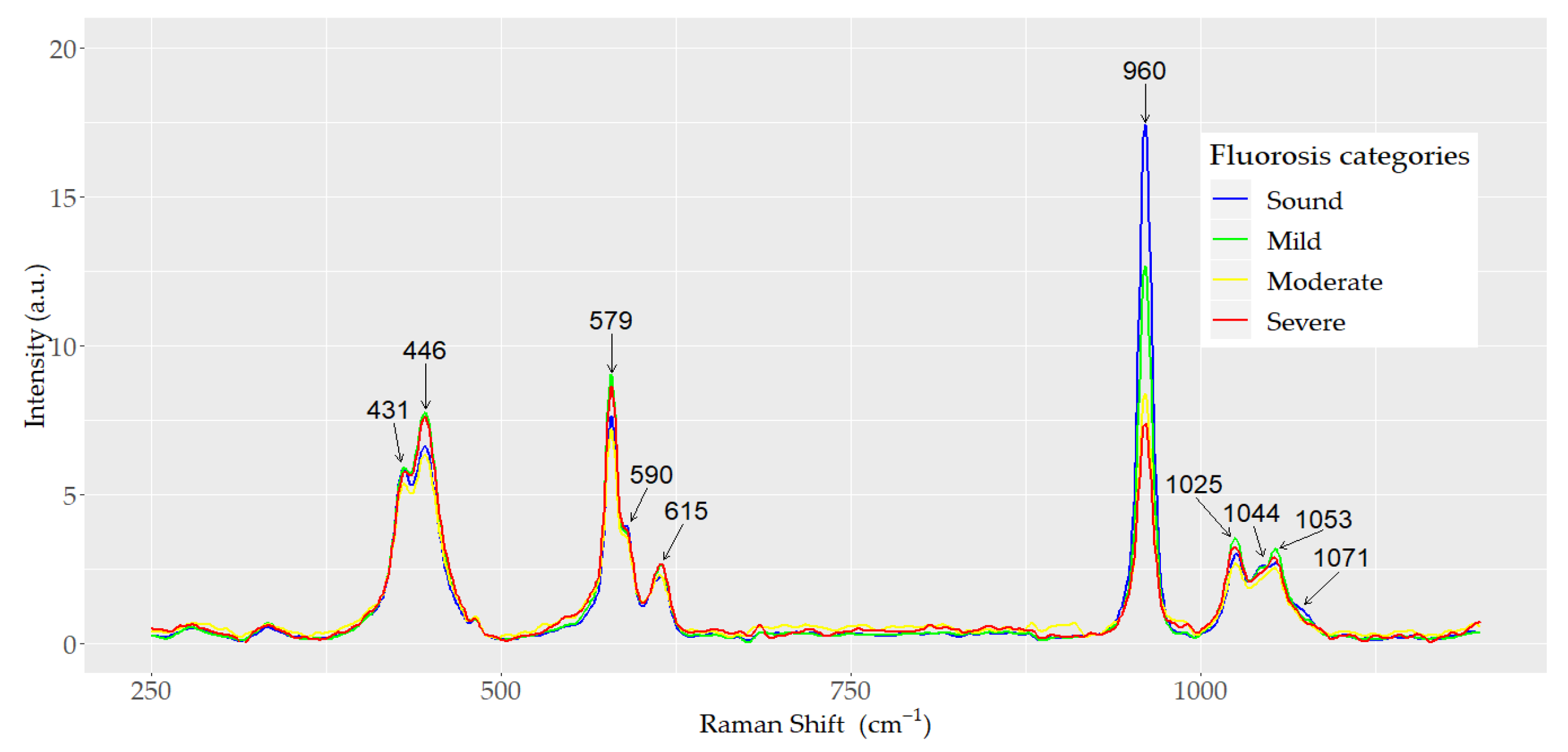
3.1. Principal Components Analysis and Linear Discriminant Analysis
3.2. Linear Discriminant Analysis and cross-Validation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aoba, T.; Fejerskov, O. Dental fluorosis: Chemistry and biology. Crit. Rev. Oral Biol. Med. 2002, 13, 155–170. [Google Scholar] [CrossRef]
- World Health Organization. Water Sanitation. Water Related Diseases. Fluorosis. Available online: https://www.who.int/water_sanitation_health/diseases-risks/diseases/fluorosis/en/ (accessed on 22 June 2020).
- Beltrán-Aguilar, E.D.; Griffin, S.O.; Lockwood, S.A. Prevalence and Trends in Enamel Fluorosis in the United States from the 1930s to the 1980s. J. Am. Dent. Assoc. 2002, 133, 157–165. [Google Scholar] [CrossRef]
- Molina-Frechero, N.; Nevarez-Rascón, M.; Nevarez-Rascón, A.; González-González, R.; Irigoyen-Camacho, M.E.; Sánchez-Pérez, L.; López-Verdin, S.; Bologna-Molina, R. Impact of Dental Fluorosis, Socioeconomic Status and Self-Perception in Adolescents Exposed to a High Level of Fluoride in Water. Int. J. Environ. Res. Public Health. 2017, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Wiener, R.C.; Shen, C.; Findley, P.; Tan, X.; Sambamoorthi, U. Dental Fluorosis over Time: A Comparison of National Health and Nutrition Examination Survey Data from 2001–2002 and 2011–2012. J. Dent. Hyg. 2018, 92, 23–29. [Google Scholar] [PubMed]
- Gevera, P.; Mouri, H.; Maronga, G. Occurrence of Fluorosis in a Population Living in a High-Fluoride Groundwater Area: Nakuru Area in the Central Kenyan Rift Valley. Environ. Geochem. Health 2019, 41, 829–840. [Google Scholar] [CrossRef]
- Riordan, P.J. Perceptions of Dental Fluorosis. J. Dent. Res. 1993, 72, 1268–1274. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. The Great Public Health Achivements—United States, 1900–1999. Morbidity and Mortality Weekly Report. 1999. Available online: https://www.cdc.gov/mmwr/pdf/wk/mm4812.pdf (accessed on 6 October 2021).
- Marthaler, T.M. Salt fluoridation and oral health. Acta Med. Acad. 2013, 42, 140–155. [Google Scholar] [CrossRef] [PubMed]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; Petersen, P.E.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and Oral Health. Community Dent. Health 2016, 33, 69–99. [Google Scholar]
- García-Pérez, Á.; Irigoyen-Camacho, M.E.; Borges-Yáñez, S.A.; Zepeda-Zepeda, M.A.; Bolona-Gallardo, I.; Maupomé, G. Impact of Caries and Dental Fluorosis on Oral Health-Related Quality of Life: A Cross-Sectional Study in Schoolchildren Receiving Water Naturally Fluoridated at above-Optimal Levels. Clin. Oral Investig. 2017, 21, 2771–2780. [Google Scholar] [CrossRef]
- Pandya, M.; Diekwisch, T.G.H. Enamel Biomimetics—Fiction or Future of Dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S.J.; Natarajan, A.K.; Clark, A.S.S.; Drummond, B.K.; Gordon, K.C. A Raman Spectroscopic Study of Teeth Affected with Molar-Incisor Hypomineralisation. J. Raman Spectrosc. 2015, 46, 202–210. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman Spectral Study of the PO4 and CO3 Vibrational Modes in Synthetic and Biological Apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef]
- Elliot, J.C.; Wilson, R.M.; Dowker, S. Apatite Structures. Adv. X-ray Anal. 2002, 45, 172–181. [Google Scholar]
- Thomas, D.B.; Fordyce, R.E.; Frew, R.D.; Gordon, K.C. A Rapid, Non-Destructive Method of Detecting Diagenetic Alteration in Fossil Bone Using Raman Spectroscopy. J. Raman Spectrosc. 2007, 38, 1533–1537. [Google Scholar] [CrossRef]
- Thylstrup, A.; Fejerskov, O. Clinical Appearance of Dental Fluorosis in Permanent Teeth in Relation to Histologic Changes. Community Dent. Oral Epidemiol. 1978, 6, 315–328. [Google Scholar] [CrossRef]
- Zavala-Alonso, V.; Loyola-Rodríguez, J.P.; Terrones, H.; Patiño-Marín, N.; Martínez-Castañón, G.A.; Anusavice, K. Analysis of the Molecular Structure of Human Enamel with Fluorosis Using Micro-Raman Spectroscopy. J. Oral Sci. 2012, 54, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Development of a new approach to diagnosis of the early fluorosis forms by means of FTIR and Raman microspectroscopy. Sci. Rep. 2020, 10, 20891. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman Spectroscopy in Cancer Diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef] [Green Version]
- Frost, J.; Ludeman, L.; Hillaby, K.; Gornall, R.; Lloyd, G.; Kendall, C.; Shore, A.C.; Stone, N. Raman Spectroscopy and Multivariate Analysis for the Non Invasive Diagnosis of Clinically Inconclusive Vulval Lichen Sclerosus. Analyst 2017, 142, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Neto, L.P.M.; e Silva, L.F.D.C.; Dos Santos, L.; Soto, C.A.T.; de Azevedo Canevari, R.; de Oliveira Santos, A.B.; Mello, E.S.; Pereira, M.A.; Cernea, C.R.; Brandao, L.G.; et al. Micro-Raman Spectroscopic Study of Thyroid Tissues. Photodiagnosis Photodyn. Ther. 2017, 17, 164–172. [Google Scholar] [CrossRef]
- Zheng, X.; Lv, G.; Zhang, Y.; Lv, X.; Gao, Z.; Tang, J.; Mo, J. Rapid and Non-Invasive Screening of High Renin Hypertension Using Raman Spectroscopy and Different Classification Algorithms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 244–248. [Google Scholar] [CrossRef]
- Sajda, P. Machine Learning for Detection and Diagnosis of Disease. Annu. Rev. Biomed. Eng. 2006, 8, 537–565. [Google Scholar] [CrossRef] [Green Version]
- Brauchle, E.; Schenke-Layland, K. Raman Spectroscopy in Biomedicine—Non-Invasive in Vitro Analysis of Cells and Extracellular Matrix Components in Tissues. Biotechnol. J. 2013, 8, 288–297. [Google Scholar] [CrossRef]
- Agrawal, G.; Samal, S.K. Raman Spectroscopy for Advanced Polymeric Biomaterials. ACS Biomater. Sci. Eng. 2018, 4, 1285–1299. [Google Scholar] [CrossRef]
- Salehi, H.; Collart-Dutilleul, P.Y.; Gergely, C.; Cuisinier, F.J. Confocal Raman microscopy to monitor extracellular matrix during dental pulp stem cells differentiation. J. Biomed. Opt. 2015, 20, 076013. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnaiah, R.; Rehman, G.U.; Basavarajappa, S.; Al Khuraif, A.A.; Durgesh, B.H.; Khan, A.S.; Rehman, I.U. Applications of Raman Spectroscopy in Dentistry: Analysis of Tooth Structure. Appl. Spectrosc. Rev. 2015, 50, 332–350. [Google Scholar] [CrossRef]
- Senado de la Republica/Comisiones Unidas. Punto de Acuerdo sobre el Consumo de Agua con Arsénico y Flúor. [Senate of the Republic/United Commissions. Point of Agreement on the Consumption of Water with Arsenic and Fluoride]. Available online: https://www.senado.gob.mx/64/gaceta_del_senado/documento/3616 (accessed on 12 October 2019).
- Gugnani, N.; Pandit, I.K.; Srivastava, N.; Gupta, M.; Sharma, M. International Caries Detection and Assessment System (ICDAS): A New Concept. Int. J. Clin. Pediatr. Dent. 2011, 4, 93–100. [Google Scholar] [CrossRef]
- Pendrys, D.G. The differential diagnosis of fluorosis. J. Public Health Dent. 1999, 59, 235–238. [Google Scholar] [CrossRef]
- Secretaría de Salud. PROYECTO de Norma Oficial Mexicana PROY-NOM-007-SSA3-2017, Para la Organización y Funcionamiento de los Laboratorios Clínicos. [Health Ministry. PROJECT of Official Mexican Standard PROY-NOM-007-SSA3-2017, For the Organization and Operation of Clinical Laboratories]. Available online: http://dof.gob.mx/nota_detalle.php?codigo=5511878&fecha=31/01/2018 (accessed on 10 November 2019).
- Beleites, C.; Sergo, V. HyperSpec: A Package to Handle Hyperspectral Data Sets in R. R Package Version 0.99-20180627. Available online: http://hyperspec.r_forge.r-project.org (accessed on 20 June 2020).
- Iqbal, Z.; Tomaselli, V.P.; Fahrenfeld, O.; Möller, K.D.; Ruszala, F.A.; Kostiner, E. Polarized Raman Scattering and Low Frequency Infrared Study of Hydroxyapatite. Phys. Chem. Solids. 1977, 32, 923. [Google Scholar] [CrossRef]
- De Asa, P.N.; Santos, C.; Pazo, A.; de Asa, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 1. Raman Spectrum of beta-Tricalcium Phosphate. Chem Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- González-Solís, J.L.; Martínez-Cano, E.; Magaña-López, Y. Early Detection of Dental Fluorosis Using Raman Spectroscopy and Principal Component Analysis. Lasers Med. Sci. 2015, 30, 1675–1681. [Google Scholar] [CrossRef]
- Buchwald, T.; Okulus, Z.; Szybowicz, M. Raman Spectroscopy as a Tool of Early Dental Caries Detection-New Insights. J. Raman Spectrosc. 2017, 48, 1094–1102. [Google Scholar] [CrossRef]
- Xu, C.; Reed, R.; Gorski, J.P.; Wang, Y.; Walker, M.P. The Distribution of Carbonate in Enamel and Its Correlation with Structure and Mechanical Properties. J. Mater. Sci. 2012, 47, 8035–8043. [Google Scholar] [CrossRef]
- Gong, B.; Oest, M.E.; Mann, K.A.; Damron, T.A.; Morris, M.D. Raman Spectroscopy Demonstrates Prolonged Alteration of Bone Chemical Composition Following Extremity Localized Irradiation. Bone 2013, 57, 252–258. [Google Scholar] [CrossRef] [Green Version]
- DenBesten, P.; Li, W. Chronic Fluoride Toxicity: Dental Fluorosis. Monogr. Oral Sci. 2011, 22, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Mier, E.A.; Shone, D.B.; Buckley, C.M.; Ando, M.; Lippert, F.; Soto-Rojas, A.E. Relationship between Enamel Fluorosis Severity and Fluoride Content. J. Dent. 2016, 46, 42–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suga, S. Enamel Hypomineralization Viewed from the Pattern of Progressive Mineralization of Human and Monkey Developing Enamel. Adv. Dent. Res. 1989, 3, 188–198. [Google Scholar] [CrossRef]
- Fejerskov, O.; Larsen, M.J.; Richards, A.; Baelum, V. Dental Tissue Effects of Fluoride. Adv. Dent. Res. 1994, 8, 15–31. [Google Scholar] [CrossRef]
- Sereda, V.; Ralbovsky, N.M.; Vasudev, M.C.; Naik, R.R.; Lednev, I.K. Polarized Raman Spectroscopy for Determining the Orientation of Di-D-Phenylalanine Molecules in a Nanotube. J. Raman Spectrosc. 2016, 47, 1056–1062. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Fluoride: Dose-Response Analysis for Non-Cancer Effects. Office of Water, Health and Ecological Criteria Division, Washington, DC. EPA Doc. 820R10019. 2010. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100N4S8.PDF?Dockey=P100N4S8.PDF (accessed on 3 October 2021).
- Spencer, A.J.; Do, L.G.; Mueller, U.; Baines, J.; Foley, M.; Peres, M.A. Understanding Optimum Fluoride Intake from Population-Level Evidence. Adv. Dent. Res. 2018, 29, 144–156. [Google Scholar] [CrossRef]
- Pramanik, S.; Saha, D. The genetic influence in fluorosis. Environ. Toxicol. Pharmacol. 2017, 56, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Doty, K.C.; Lednev, I.K. Raman spectroscopy for forensic purposes: Recent applications for serology and gunshot residue analysis. Trends Anal. Chem. 2018, 103, 215–222. [Google Scholar] [CrossRef]
- Mohanta, A. Dental Fluorosis—Revisited. Biomed. J. Sci. Tech. Res. 2018, 2, 2243–2247. [Google Scholar] [CrossRef] [Green Version]
- Susheela, A.K.; Toteja, G.S. Prevention & Control of Fluorosis & Linked Disorders: Developments in the 21st Century—Reaching out to Patients in the Community & Hospital Settings for Recovery. Indian J. Med. Res. 2018, 148, 539–547. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, H.; Han, H.; Wang, W.; Ji, X.; Liu, X.; Sun, D. The Relationships between Low Levels of Urine Fluoride on Children’s Intelligence, Dental Fluorosis in Endemic Fluorosis Areas in Hulunbuir, Inner Mongolia, China. J. Hazard. Mater. 2011, 186, 1942–1946. [Google Scholar] [CrossRef]




| Fluorosis Categories | Probability |
|---|---|
| Sound | 0.9667 |
| Mild | 0.9667 |
| Moderate | 0.8000 |
| Severe | 0.9333 |
| Fluorosis Categories | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| Severe | 93.3 | 94.4 | 94.2 |
| Severe + Moderate | 96.7 | 100.0 | 98.3 |
| Severe + Moderate + Mild | 98.9 | 96.7 | 98.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zepeda-Zepeda, M.A.; Picquart, M.; Irigoyen-Camacho, M.E.; Mejía-Gózalez, A.M. Diagnosis of Dental Fluorosis Using Micro-Raman Spectroscopy Applying a Principal Component-Linear Discriminant Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10572. https://doi.org/10.3390/ijerph182010572
Zepeda-Zepeda MA, Picquart M, Irigoyen-Camacho ME, Mejía-Gózalez AM. Diagnosis of Dental Fluorosis Using Micro-Raman Spectroscopy Applying a Principal Component-Linear Discriminant Analysis. International Journal of Environmental Research and Public Health. 2021; 18(20):10572. https://doi.org/10.3390/ijerph182010572
Chicago/Turabian StyleZepeda-Zepeda, Marco Antonio, Michel Picquart, María Esther Irigoyen-Camacho, and Adriana Marcela Mejía-Gózalez. 2021. "Diagnosis of Dental Fluorosis Using Micro-Raman Spectroscopy Applying a Principal Component-Linear Discriminant Analysis" International Journal of Environmental Research and Public Health 18, no. 20: 10572. https://doi.org/10.3390/ijerph182010572
APA StyleZepeda-Zepeda, M. A., Picquart, M., Irigoyen-Camacho, M. E., & Mejía-Gózalez, A. M. (2021). Diagnosis of Dental Fluorosis Using Micro-Raman Spectroscopy Applying a Principal Component-Linear Discriminant Analysis. International Journal of Environmental Research and Public Health, 18(20), 10572. https://doi.org/10.3390/ijerph182010572







