Transfer of Natural Radionuclides in Terrestrial Food Chains—A Review of Investigations in Finland
Abstract
1. Introduction
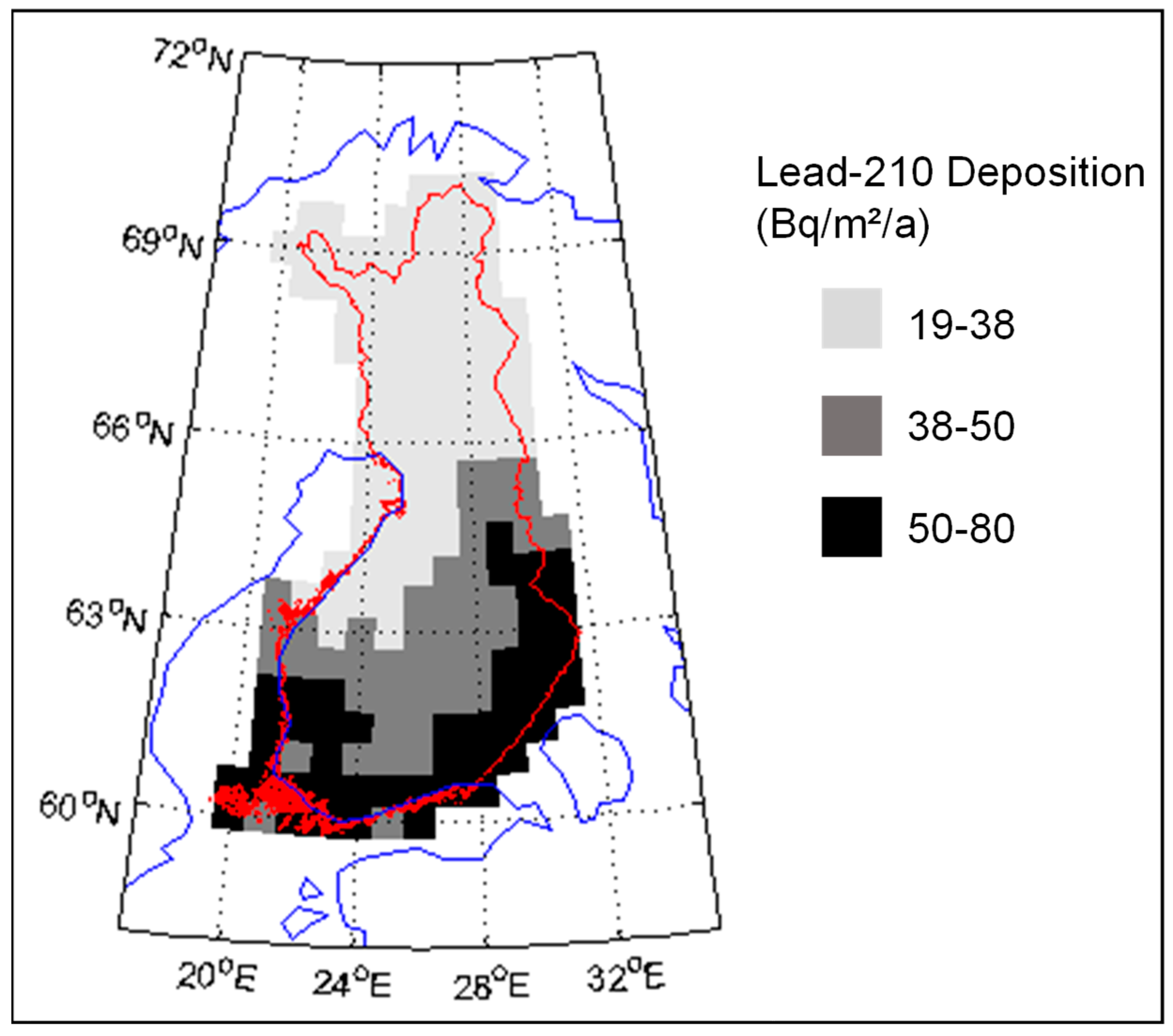
2. 210Pb and 210Po
3. Uranium Isotopes
4. Thorium Isotopes
5. Radiation Dose Due to 210Pb and 210Po from Consumption of Reindeer Meat
6. Summary of Highlights in Natural Radionuclide Studies in Terrestrial Environment in Finland in Recent Decades
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miettinen, J.K.; Jokelainen, A.; Roine, P.; Lidén, K.; Naversten, Y. 137Cs and potassium in people and diet—A study of finnish lapps. Ann. Acad. Sci. Fenn. Ser. A II Chemica 1963, 120, 1–46. [Google Scholar]
- Svensson, G.K.; Lidén, K. The transport of 137Cs from lichen to animal and man. Health Phys. 1965, 11, 1393–1400. [Google Scholar] [CrossRef]
- Hanson, W.C. Cesium-137 in Alaskan lichens, caribou and eskimos. Health Phys. 1967, 13, 383–389. [Google Scholar] [CrossRef]
- Baskaran, M.; Coleman, C.H.; Santschi, P.H. Atmospheric depositional fluxes of 7Be and 210Pb at Galveston and college station, Texas. J. Geophys. Res. 1993, 98, 20555–20571. [Google Scholar] [CrossRef]
- Turtiainen, T.; Kostiainen, E. Radiological hazards in finnish cereals: Comparison of man-made and natural sources. Cereal Res. Commun. 2013, 41, 366–375. [Google Scholar] [CrossRef]
- Kämäräinen, M.; Turtiainen, T.; Vaaramaa, K. Radioactivity of Meat and Eggs Produced in Finland. Ympäristön Säteilyvalvonnan Toimintaohjelma, Ympäristön Säteilyvalvonta ja Valmius; Radiation and Nuclear Safety Authority: Helsinki, Finland, 2017.
- Vetikko, V.; Turtiainen, T.; Leppänen, A.P.; Kämäräinen, M. Radioactivity of Timber in Finland. Ympäristön Säteilyvalvonnan Toimintaohjelma. Ympäristön Säteilyvalvonta; Radiation and Nuclear Safety Authority: Helsinki, Finland, 2015.
- Paatero, J.; Salminen-Paatero, S. Transfer of transuranium elements along the food chain lichen-reindeer-man—A review of investigations in Finnish Lapland. J. Environ. Radioact. 2020, 212, 106126. [Google Scholar] [CrossRef]
- Paatero, J.; Vaaramaa, K.; Buyukay, M.; Hatakka, J.; Lehto, J. Deposition of atmospheric 210Pb and total beta activity in Finland. J. Radioanal. Nucl. Chem. 2015, 303, 2413–2420. [Google Scholar] [CrossRef]
- Paatero, J.; Vesterbacka, K.; Makkonen, U.; Kyllönen, K.; Hellen, H.; Hatakka, J.; Anttila, P. Resuspension of radionuclides into the atmosphere due to forest fires. J. Radioanal. Nucl. Chem. 2009, 282, 473–476. [Google Scholar] [CrossRef]
- Hansen, W.R.; Watters, R.L. Plant uptake of 210Po from soil. Radiat. Bot. 1970, 10, 371–375. [Google Scholar] [CrossRef]
- Vaaramaa, K.; Aro, L.; Solatie, D.; Lehto, J. Distribution of 210Pb and 210Po in boreal forest soil. Sci. Total Environ. 2010, 408, 6165–6171. [Google Scholar] [CrossRef]
- Thomas, P.A.; Gates, T.E. Radionuclides in the lichen-caribou-human food chain near uranium mining operations in Northern Saskatchewan, Canada. Environ. Health Persp. 1999, 107, 527–537. [Google Scholar] [CrossRef]
- Laaksovirta, K.; Olkkonen, H.; Alakuijala, P. Observations on the lead content of lichen and bark adjacent to a highway in Southern Finland. Environ. Pollut. 1976, 11, 247–255. [Google Scholar] [CrossRef]
- Kauranen, P.; Miettinen, J.K. 210Po and 210Pb in the Arctic food chain and the natural radiation exposure of lapps. Health Phys. 1969, 16, 287–295. [Google Scholar] [CrossRef]
- Solatie, D.; Junttila, M.; Vesterbacka, P. 210Po and 210Pb in the food chain lichen–reindeer–man. Radiochemistry 2006, 48, 632–633. [Google Scholar] [CrossRef]
- Vaaramaa, K.; Solatie, D.; Aro, L. Distribution of 210Pb and 210Po concentrations in wild berries and mushrooms in boreal forest ecosystems. Sci. Total Environ. 2009, 408, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Mussalo-Rauhamaa, H.; Jaakkola, T.; Miettinen, J.K.; Laiho, K. Plutonium in Finnish Lapps. An estimate of the gastrointestinal absorption of plutonium by man based on a comparison of the plutonium content of Lapps and Southern Finns. Health Phys. 1984, 46, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.R. 210Pb-Atmospheric deposition in lichen-carpets in northern Sweden during 1961–1969. Tellus 1970, 22, 564–571. [Google Scholar] [CrossRef][Green Version]
- Turpeinen, R.; Salminen, J.; Kairesalo, T. Mobility and bioavailability of lead in contaminated boreal forest soil. Environ. Sci. Technol. 2000, 34, 5152–5156. [Google Scholar] [CrossRef]
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef]
- Paatero, J.; Dauvalter, V.; Derome, J.; Lehto, J.; Pasanen, J.; Vesala, T.; Miettinen, J.; Makkonen, U.; Kyrö, E.-M.; Jernström, J.; et al. Effects of Kola Air Pollution on the Environment in the Western Part of the Kola Peninsula and Finnish Lapland—Final Report; Finnish Meteorological Institute: Helsinki, Finland, 2008.
- Wang, Y.; Zong, K.; Jiang, L.; Sun, J.; Ren, Y.; Sun, Z.; Wen, C.; Chen, X.; Cao, S. Characterization of an Arabidopsis cadmium-resistant mutant cdr3-1D reveals a link between heavy metal resistance as well as seed development and flowering. Planta 2011, 233, 697–706. [Google Scholar] [CrossRef]
- Yu, G.; Ma, J.; Jiang, P.; Li, J.; Gao, J.; Qiao, S.; Zhao, Z. The mechanism of plant resistance to heavy metal. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 052004. [Google Scholar] [CrossRef]
- Soininen, L.; Mussalo-Rauhamaa, H. Cancer incidence of finnish sami in the light of exposure to radioactive fallout. Int J. Environ. Res. Public Health 2021, 18, 8186. [Google Scholar] [CrossRef]
- Muikku, M.; Heikkinen, T.; Solatie, D.; Vesterbacka, P. Natural variation in 210Po and 210Pb activity concentrations in the urine of Finnish population groups. Radiat. Environ. Biophys. 2011, 50, 531–538. [Google Scholar] [CrossRef]
- Holm, E.; Persson, B.R.R. Comparative studies of natural and artificial α-emitters (actinides) in the lichen-reindeer-man food chain. In Actinides in Man and Animals, Proceedings of the Snowbird Actinide Workshop; 15–17 October 1979, Wrenn, M.E., Ed.; RD Press: Salt Lake City, UT, USA, 1979; pp. 525–537. [Google Scholar]
- Appleby, P.G.; Oldfield, F.; Thompson, R.; Huttunen, P.; Tolonen, K. 210Pb dating of annually laminated lake sediments from Finland. Nature 1979, 280, 53–55. [Google Scholar] [CrossRef]
- El-Daoushy, F.; Tolonen, K.; Rosenberg, R. Lead 210 and moss-increment dating of two Finnish Sphagnum hummocks. Nature 1982, 296, 429–431. [Google Scholar] [CrossRef]
- El-Daoushy, F.; Tolonen, K. Lead-210 and heavy metal contents in dated ombrotrophic peat hummocks from Finland. Nucl. Instrum. Methods Phys. Res. 1984, 223, 392–399. [Google Scholar] [CrossRef]
- Tolonen, M. Vegetational history in coastal SW Finland studied on a lake and a peat bog by pollen and charcoal analysis. In Annales Botanici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 1987; Volume 24, pp. 353–370. [Google Scholar]
- Reinikainen, P.; Meriläinen, J.J.; Virtanen, A.; Veijola, H.; Äystö, J. Accuracy of 210Pb dating in two annually laminated lake sediments with high Cs background. Appl. Radiat. Isot. 1997, 48, 1009–1019. [Google Scholar] [CrossRef]
- Rausch, N.; Nieminen, T.; Ukonmaanaho, L.; Le Roux, G.; Krachler, M.; Cheburkin, A.K.; Bonani, G.; Shotyk, W. Comparison of atmospheric deposition of copper, nickel, cobalt, zinc, and cadmium recorded by finnish peat cores with monitoring data and emission records. Environ. Sci. Technol. 2005, 39, 5989–5998. [Google Scholar] [CrossRef]
- Ukonmaanaho, L.; Nieminen, T.M.; Rausch, N.; Cheburkin, A.; Le Roux, G.; Shotyk, W. Recent organic matter accumulation in relation to some climatic factors in ombrotrophic peat bogs near heavy metal emission sources in Finland. Glob. Planet Change 2006, 53, 259–268. [Google Scholar] [CrossRef]
- Zhang, H.; Väliranta, M.; Piilo, S.; Amesbury, M.J.; Aquino-Lopez, M.A.; Roland, T.P.; Salminen-Paatero, S.; Paatero, J.; Lohila, A.; Tuittila, E.-S. Decreased carbon accumulation feedback driven by climate-induced drying of two southern boreal bogs over recent centuries. Glob. Change Biol. 2020, 26, 2435–2448. [Google Scholar] [CrossRef]
- Suksi, J.; Ruskeeniemi, T.; Lindberg, A.; Jaakkola, T. The distribution of natural radionuclides on fracture surfaces in Palmottu Analogue Study site in SW Finland. Radiochim. Acta 1991, 52–53, 367–372. [Google Scholar] [CrossRef]
- Suksi, J.; Rasilainen, K. On the role of alpha-recoil in uranium migration—Some findings from the Palmottu natural analogue site, SW Finland. Radiochim. Acta 1996, 74, 297–302. [Google Scholar] [CrossRef]
- Ahonen, L.; Ervanne, H.; Jaakkola, T.; Blomqvist, R. Redox chemistry in uranium-rich groundwater of Palmottu uranium deposit, Finland. Radiochim. Acta 1994, 66–67, 115–122. [Google Scholar] [CrossRef]
- Ervanne, H. Uranium oxidation states in allanite, fergusonite and monazite of pegmatites from Finland. Neues Jahrbuch für Mineralogie-Monatshefte 2004, 7, 289–301. [Google Scholar] [CrossRef]
- Yliruokanen, I. Uranium, thorium, lead, lanthanoids and yttrium in some plants growing on granitic and radioactive rocks. Bull. Geol. Soc. Finl. 1975, 47, 71–78. [Google Scholar] [CrossRef]
- Roivainen, P.; Makkonen, S.; Holopainen, T.; Juutilainen, J. Soil-to-plant transfer of uranium and its distribution between plant parts in four boreal forest species. Boreal Environ. Res. 2011, 16, 158–166. [Google Scholar]
- Kahlos, H.; Asikainen, M. Internal radiation doses from radioactivity of drinking water in Finland. Health Phys. 1980, 39, 108–111. [Google Scholar]
- Auvinen, A.; Kurttio, P.; Pekkanen, J.; Pukkala, E.; Ilus, T.; Salonen, L. Uranium and other natural radionuclides in drinking water and risk of leukemia: A case-cohort study in Finland. Cancer Causes Control 2002, 13, 825–829. [Google Scholar] [CrossRef]
- Kurttio, P.; Auvinen, A.; Salonen, L.; Saha, H.; Pekkanen, J.; Mäkeläinen, I.; Väisänen, S.B.; Penttilä, I.M.; Komulainen, H. Renal effects of uranium in drinking water. Environ. Health Perspect. 2002, 110, 337–342. [Google Scholar] [CrossRef]
- Vesterbacka, P.; Mäkeläinen, I.; Arvela, H. Natural radioactivity in drinking water in private wells in Finland. Radiat. Prot. Dosim. 2005, 113, 223–232. [Google Scholar] [CrossRef]
- Vesterbacka, P. Natural radioactivity in drinking water in Finland. Boreal Env. Res. 2007, 12, 11–16. [Google Scholar]
- Kurttio, P.; Salonen, L.; Ilus, T.; Pekkanen, J.; Pukkala, E.; Auvinen, A. Well water radioactivity and risk of cancers of the urinary organs. Environ. Res. 2006, 102, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Turtiainen, T.; Muikku, M.; Vesterbacka, P.; Heikkinen, T. Uranium and 226Ra in drinking water supplied by Finnish waterworks. Radioprotection 2011, 46, S255–S263. [Google Scholar] [CrossRef]
- Anke, M.; Seeber, O.; Müller, R.; Schäfer, U.; Zerull, J. Uranium transfer in the food chain from soil to plants, animals and man. Geochemistry 2009, 69, 75–90. [Google Scholar] [CrossRef]
- Nieminen, K.; Yliruokanen, I. Trace element analysis of granitic and radioactive rocks by spark source mass spectrometry with electrical detection. Bull. Geol. Soc. Finl. 1974, 46, 167–176. [Google Scholar] [CrossRef]
- Shahandeh, H.; Hossner, L.R. Role of soil properties in phytoaccumulation of uranium. Water Air Soil Pollut. 2002, 141, 165–180. [Google Scholar] [CrossRef]
- Rodríguez, P.B.; Tomé, F.V.; Fernández, M.P.; Lozano, J.C. Linearity assumption in soil-to-plant transfer factors of natural uranium and radium in Helianthus annuus L. Sci. Total Environ. 2006, 361, 1–7. [Google Scholar] [CrossRef]
- Chang, P.; Kim, K.-W.; Yoshida, S.; Kim, S.-Y. Uranium accumulation of crop plants enhanced by citric acid. Environ. Geochem. Health 2005, 27, 529–538. [Google Scholar] [CrossRef]
- Shtangeeva, I. Uptake of uranium and thorium by native and cultivated plants. J. Environ. Radioact. 2010, 101, 458–463. [Google Scholar] [CrossRef]
- Soudek, P.; Petrová, Š.; Benešová, D.; Dvořáková, M.; Vaněk, T. Uranium uptake by hydroponically cultivated crop plants. J. Environ. Radioact. 2011, 102, 598–604. [Google Scholar] [CrossRef]
- Olszewski, G.; Boryło, A.; Skwarzec, B. A study on possible use of Urtica dioica (Common nettle) plants as uranium (234U, 238U) contamination bioindicator near phosphogypsum stockpile. J. Radioanal. Nucl. Chem. 2016, 308, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Reimann, C.; Koller, F.; Frengstad, B.; Kashulina, G.; Niskavaara, H.; Englmaier, P. Comparison of the element composition in several plant species and their substrate from a 1,500,000-km2 area in Northern Europe. Sci. Total Environ. 2001, 278, 87–112. [Google Scholar] [CrossRef]
- Muuronen, S. Distribution and Concentration of Thorium Isotopes in Lichen, Reindeer Bone and Human Bone. Radiochemistry Research Project Report; Laboratory of Radiochemistry, University of Helsinki: Helsinki, Finland, 1980. [Google Scholar]
- Pietrzak-Flis, Z.; Suplinska, M.M.; Rosiak, L. The dietary intake of 238U, 234U, 230Th, 232Th, 228Th and 226Ra from food and drinking water by inhabitants of the Walbrzych region. J. Radioanal. Nucl. Chem. 1997, 222, 189–193. [Google Scholar] [CrossRef]
- Sathyapriya, R.S.; Nair, S.; Baskaran, K.; Prabhath, R.; Nair, M.; Acharya, R.; Rao, D. Estimation of thorium intake due to consumption of vegetables by inhabitants of high background radiation area by INAA. J. Radioanal. Nucl. Chem. 2013, 294, 387–390. [Google Scholar] [CrossRef]
- Frindik, O. Thorium und Uran in Lebensmitteln tierischer Herkunft [Thorium amd uranium in food of animal origin]. Z. Lebensm. Unters. Forsch. 1992, 194, 377–380. [Google Scholar] [CrossRef]
- Lauria, D.D.C.; Rochedo, E.R.R.; Godoy, M.L.D.P.; Santos, E.E.; Hacon, S.S. Naturally occurring radionuclides in food and drinking water from a thorium-rich area. Radiat. Environ. Biophys. 2012, 51, 367–374. [Google Scholar] [CrossRef]
- Wrenn, M.E.; Singh, N.P.; Ibrahim, S.A.; Cohen, N.; Saccomanno, G. Thorium in human tissues. In Proceedings of the Conference on natural radiation environment III, Houston, TX, USA, 23–28 April 1978; Gesell, T.F., Lowder, W.M., Eds.; Department of Energy, Technical Information Center: Oak Ridge, TN, USA, 1980; pp. 783–799. [Google Scholar]
- Leiterer, A.; Berard, P.; Menetrier, F. Thorium and Health: State of the Art. Rapport CEA-R-6251; CEA Fontenay aux Roses, Direction des Sciences du Vivant, Unité Prositon: Fontenay aux Roses, France, 2010. [Google Scholar]
- Larivière, D.; Packer, A.P.; Marro, L.; Li, C.; Chen, J.; Cornett, R.J. Age dependence of natural uranium and thorium concentrations in bone. Health Phys. 2007, 92, 119–126. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Ayrault, S. Phytoextraction of thorium from soil and water media. Water Air Soil Pollut. 2004, 154, 19–35. [Google Scholar] [CrossRef]
- Salminen-Paatero, S.; Paatero, J. Total beta activity, 137Cs and 90Sr in surface air in northern Finland in 1963. Radiochim. Acta 2012, 100, 801–808. [Google Scholar] [CrossRef]






| Sample Type | A 210Pb (Bq/kg d.w.) | A 210Pb (Bq/kg w.w.) | Reference |
|---|---|---|---|
| Lichen (1961–1967) | 190–380 | [15] | |
| Lichen (1980) | 250 | [16] | |
| Lichen (2004) | 170 | [16] | |
| Tree leaves (1964–1965) | 11–42 | [15] | |
| Tree leaves (1989) | 20 | [16] | |
| Mushroom (2004) | 3 | [16] | |
| Mushrooms (2007) | 1.6–16 | [17] | |
| Berries (2004) | 2 | [16] | |
| Berries (2006–2007) | 0.09–0.45 | [17] | |
| Reindeer blood (1965–1967) | 1.5–3 | [15] | |
| Reindeer meat (1965–1967) | 0.12–0.30 | [15] | |
| Reindeer muscle (1984–2004) | 1.2–4.8 | [16] | |
| Reindeer bones (1964–1966) | 138–196 | [15] | |
| Reindeer bones (1988–2004) | 38–210 | [16] | |
| Reindeer kidney (1988–1989) | 62–85 | [16] | |
| Reindeer liver (1964–1967) | 10–56 | [15] | |
| Reindeer liver (2004) | 150 | [16] | |
| Human blood, reindeer herder (1966) | 0.23–0.27 | [15] | |
| Human blood, southern Finland (1966–1967) | 0.1 | [15] | |
| Placenta, Lapland (1966) | 0.02–0.12 | [15] | |
| Placenta, southern Finland (1966) | 0.01–0.05 | [15] | |
| Human kidney, southern Finland (~1966) | 0.12–0.24 | [15] | |
| Human teeth, Lapland (1966–1967) | 2.4–11 | [15] | |
| Human teeth, southern Finland (1966–1967) | 1.4–2.6 | [15] | |
| Human shinbone/tibia, southern Finland (~1966) | 1.0–1.5 | [15] | |
| Human ribs, Lapland (1977) | 0.85 and 1.5 | [18] | |
| Human liver, Lapland (1977–1979) | 0.13–1.0 (average 0.48) | [18] | |
| Human liver, southern Finland (~1966) | 0.08–0.11 | [15] | |
| Human liver, southern Finland (1976–1979) | 0.27 (average) | [18] |
| Sample Type | A 210Po (Bq/kg d.w.) | A 210Po (Bq/kg w.w.) | Reference |
|---|---|---|---|
| Lichen (1961–1967) | 170–350 | [15] | |
| Tree leaves (1964–1965) | 3–12 | [15] | |
| Tree leaves (1989) | 19 | [16] | |
| Mushroom (2004) | 140 | [16] | |
| Mushrooms (2007) | 9–2190 | [17] | |
| Berries (2004) | 2.2 | [16] | |
| Berries (2006–2007) | 0.3–1.0 | [17] | |
| Reindeer blood (1965–1967) | 4–18 | [15] | |
| Reindeer meat (1964–1967) | 3–12 | [15] | |
| Reindeer muscle (1984–2004) | 23–66 | [16] | |
| Reindeer bones (1964–1966) | 73–78 | [15] | |
| Reindeer bones (1988–2004) | 12–71 | [16] | |
| Reindeer kidney (1988–1989) | 120–160 | [16] | |
| Reindeer liver (1964–1967) | 38–174 | [15] | |
| Reindeer liver (2004) | 470 | [16] | |
| Human blood, reindeer herder (1966) | 0.24–0.57 | [15] | |
| Human blood, southern Finland (1966–1967) | 0.03 | [15] | |
| Placenta, Lapland (1966) | 0.63–2.8 | [15] | |
| Placenta, southern Finland (1966) | 0.05–0.16 | [15] | |
| Human kidney, southern Finland (~1966) | 0.34–0.95 | [15] | |
| Human teeth, Lapland (1966–1967) | 2.3–10 | [15] | |
| Human teeth, southern Finland (1966–1967) | 1.4–2.5 | [15] | |
| Human shinbone/tibia, southern Finland (~1966) | 0.95–1.3 | [15] | |
| Human ribs, Lapland (1977) | 0.8 and 1.0 | [18] | |
| Human liver, Lapland (1977–1979) | 0.4–8.0 (average 3.2) | [18] | |
| Human liver, southern Finland (~1966) | 0.34–0.83 | [15] | |
| Human liver, southern Finland (1976–1979) | 0.57 (average) | [18] |
| Radionuclide | Activity Concentration (Bq/kg d.w.) | Relative Activity (Act. Conc. 238U = 1) |
|---|---|---|
| 210Po | 260 | 1300 |
| 234U | 0.2 | 1 |
| 235U | 0.007 | 0.035 |
| 238U | 0.2 | 1 |
| 228Th | 0.13 | 0.65 |
| 230Th | 0.06 | 0.3 |
| 232Th | 0.06 | 0.3 |
| 238Pu | 0.23 | 1.15 |
| 239+240Pu | 5.2 | 26 |
| 241Am | 1.1 | 5.5 |
| 237Np | 0.01 | 0.05 |
| 242Cm | 0.0002 | 0.001 |
| 244Cm | 0.002 | 0.01 |
| Sample Type | 238U-conc. (mg/kg d.w.) | 238U-conc. (Bq/kg d.w.) | Reference |
|---|---|---|---|
| Lichens Mosses Shrubs Trees (deciduous and conifers) | 0.06–0.57 0.16–47 0.05 0.03–0.51 | 0.75–7.1 2.0–584 0.62 0.37–6.3 | [40] |
| May lily, narrow buckler fern, rowan, Norway spruce | 0.01–0.02 (leaves and needles) 0.09–0.17 (coarse roots) | 0.12–0.25 1.1–2.1 | [41] |
| Sample Type | Act. conc. 232Th (Bq/kg) | Conc. 232Th (mg/kg) | Reference | |
|---|---|---|---|---|
| Lichens, mosses, dwarf shrubs, conifers | 0.24–4.1 (d.w.) | 0.06–1.0 (d.w.) | [40] | |
| Act. conc. 232Th (Bq/kg) | Act. conc. 230Th (Bq/kg) | Act. conc. 228Th (Bq/kg) | ||
| Lichen | 0.076–0.47 (d.w.) | 0.063–0.19 (d.w.) | 0.12–0.57 (d.w.) | [58] |
| Reindeer bone | 0.011–0.096 (d.w.) | 0.012–0.11 (d.w.) | 2.4–23 (d.w.) | [58] |
| Human bone | 0.002–0.003 (w.w.) | 0.004–0.005 (w.w.) | 0.010–0.013 (w.w.) | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salminen-Paatero, S.; Paatero, J. Transfer of Natural Radionuclides in Terrestrial Food Chains—A Review of Investigations in Finland. Int. J. Environ. Res. Public Health 2021, 18, 10577. https://doi.org/10.3390/ijerph182010577
Salminen-Paatero S, Paatero J. Transfer of Natural Radionuclides in Terrestrial Food Chains—A Review of Investigations in Finland. International Journal of Environmental Research and Public Health. 2021; 18(20):10577. https://doi.org/10.3390/ijerph182010577
Chicago/Turabian StyleSalminen-Paatero, Susanna, and Jussi Paatero. 2021. "Transfer of Natural Radionuclides in Terrestrial Food Chains—A Review of Investigations in Finland" International Journal of Environmental Research and Public Health 18, no. 20: 10577. https://doi.org/10.3390/ijerph182010577
APA StyleSalminen-Paatero, S., & Paatero, J. (2021). Transfer of Natural Radionuclides in Terrestrial Food Chains—A Review of Investigations in Finland. International Journal of Environmental Research and Public Health, 18(20), 10577. https://doi.org/10.3390/ijerph182010577







