The Upper Extremity Functional Index: Reliability and Validity in Patients with Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Procedure
2.3. Outcome Measures
2.3.1. Upper Extremity Functional Index (UEFI)
2.3.2. St. George’s Respiratory Questionnaire (SGRQ)
2.3.3. Lung Function
2.3.4. Global Rating of Change Scale (GRC)
2.4. Statistical Analysis
2.4.1. Floor and Ceiling Effects
2.4.2. Internal Consistency
2.4.3. Test–Retest Reliability and Measurement Error
2.4.4. Construct Validity Using Hypothesis Testing
2.4.5. Sample Size Estimation
3. Results
3.1. Floor and Ceiling Effects
3.2. Internal Consistency
3.3. Test–Retest Reliability and Measurement Error
3.4. Construct Validity Using Hypothesis Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Bendixen, H.J.; Waehrens, E.E.; Wilcke, J.T.; Sorensen, L.V. Self-reported quality of ADL task performance among patients with COPD exacerbations. Scand. J. Occup. Ther. 2014, 21, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Janson, C.; Marks, G.; Buist, S.; Gnatiuc, L.; Gislason, T.; McBurnie, M.A.; Nielsen, R.; Studnicka, M.; Toelle, B.; Benediktsdottir, B.; et al. The impact of COPD on health status: Findings from the BOLD study. Eur. Respir. J. 2013, 42, 1472–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Gutierrez, F.J.; Miravitlles, M.; Calle, M.; Gobartt, E.; Lopez, F.; Martin, A.; Grupo de Estudio, E. [Impact of chronic obstructive pulmonary disease on activities of daily living: Results of the EIME multicenter study]. Arch. Bronconeumol. 2007, 43, 64–72. [Google Scholar] [CrossRef]
- Marques, A.; Jacome, C.; Gabriel, R.; Figueiredo, D. Comprehensive ICF core set for obstructive pulmonary diseases: Validation of the activities and participation component through the patient’s perspective. Disabil. Rehabil. 2013, 35, 1686–1691. [Google Scholar] [CrossRef]
- Marques, A.; Jacome, C.; Goncalves, A.; Silva, S.; Lucas, C.; Cruz, J.; Gabriel, R. Validation of the Comprehensive ICF Core Set for obstructive pulmonary diseases from the patient’s perspective. Int. J. Rehabil. Res. 2014, 37, 152–158. [Google Scholar] [CrossRef]
- Vaes, A.W.; Wouters, E.F.M.; Franssen, F.M.E.; Uszko-Lencer, N.; Stakenborg, K.H.P.; Westra, M.; Meijer, K.; Schols, A.; Janssen, P.P.; Spruit, M.A. Task-related oxygen uptake during domestic activities of daily life in patients with COPD and healthy elderly subjects. Chest 2011, 140, 970–979. [Google Scholar] [CrossRef]
- Meijer, K.; Annegarn, J.; Lima Passos, V.; Savelberg, H.H.; Schols, A.M.; Wouters, E.F.; Spruit, M.A. Characteristics of daily arm activities in patients with COPD. Eur. Respir. J. 2014, 43, 1631–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucki, A.; Stoll, T.; Cieza, A.; Weigl, M.; Giardini, A.; Wever, D.; Kostanjsek, N.; Stucki, G. ICF Core Sets for obstructive pulmonary diseases. J. Rehabil. Med. 2004, 36, 114–120. [Google Scholar] [CrossRef]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; ZuWallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef]
- Janaudis-Ferreira, T.; Beauchamp, M.K.; Robles, P.G.; Goldstein, R.S.; Brooks, D. Measurement of activities of daily living in patients with COPD: A systematic review. Chest 2014, 145, 253–271. [Google Scholar] [CrossRef]
- Monjazebi, F.; Dalvandi, A.; Ebadi, A.; Khankeh, H.R.; Rahgozar, M.; Richter, J. Functional Status Assessment of COPD Based on Ability to Perform Daily Living Activities: A Systematic Review of Paper and Pencil Instruments. Glob. J. Health Sci. 2015, 8, 210–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratford, P.W.; Binkley, J.M.; Stratford, D.M. Development and initial validation of the Upper Extremity Functional Index. Physiother. Can. 2001, 53, 259–267. [Google Scholar]
- Chesworth, B.M.; Hamilton, C.B.; Walton, D.M.; Benoit, M.; Blake, T.A.; Bredy, H.; Burns, C.; Chan, L.; Frey, E.; Gillies, G.; et al. Reliability and validity of two versions of the upper extremity functional index. Physiother. Can. 2014, 66, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytar, A.; Yuruk, Z.O.; Tuzun, E.H.; Baltaci, G.; Karatas, M.; Eker, L. The Upper Extremity Functional Index (UEFI): Cross-cultural adaptation, reliability, and validity of the Turkish version. J. Back Musculoskelet. Rehabil. 2015, 28, 489–495. [Google Scholar] [CrossRef]
- Lehman, L.A.; Sindhu, B.S.; Shechtman, O.; Romero, S.; Velozo, C.A. A comparison of the ability of two upper extremity assessments to measure change in function. J. Hand Ther. 2010, 23, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yang, Z.; Xia, X.; Wang, S. The reliability and validity of the Chinese version of the upper extremity functional index. Chin. J. Phys. Med. Rehabil. 2012, 12, 903–906. [Google Scholar]
- Binkley, J.M.; Stratford, P.; Kirkpatrick, S.; Farley, C.R.; Okoli, J.; Gabram, S. Estimating the Reliability and Validity of the Upper Extremity Functional Index in Women after Breast Cancer Surgery. Clin. Breast Cancer 2018, 18, e1261–e1267. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, 2021 Report. Available online: http://goldcopd.org/ (accessed on 3 January 2021).
- Aljathlani, M.F.; Alshammari, M.O.; Alsuwaygh, M.A.; Al-Mutairi, M.S.; Aljassir, F.F.; Bindawas, S.M.; Alnahdi, A.H. Cross-cultural adaptation and validation of the Arabic version of the upper extremity functional index. Disabil. Rehabil. 2021, 1–7. [Google Scholar] [CrossRef]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M. The St George’s Respiratory Questionnaire. Respir. Med. 1991, 85 (Suppl. B), 25–31. [Google Scholar] [CrossRef]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M.; Littlejohns, P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992, 145, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Akiki, Z.; Hallit, S.; Layoun, N.; Cherfane, M.; Sacre, H.; Waked, M.; Salameh, P. Validation of the St George’s respiratory questionnaire and risks factors affecting the quality of life of Lebanese COPD and asthma patients. J. Asthma 2019, 56, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- El Rhazi, K.; Nejjari, C.; Benjelloun, M.C.; Bourkadi, J.; Afif, H.; Serhier, Z.; Tachfouti, N.; Berraho, M.; Barberger-Gateau, P. Validation of the St. George’s Respiratory Questionnaire in patients with COPD or asthma in Morocco. Int. J. Tuberc. Lung Dis. 2006, 10, 1273–1278. [Google Scholar]
- Khalladi, R.; Gargouri, I.; Mahjoub, M.; Belhareth, S.; Ben Saad, H. [Evaluation of quality of life (QOL) of Tunisians patients with COPD]. Rev. Pneumol. Clin. 2017, 73, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Culver, B.H.; Graham, B.L.; Coates, A.L.; Wanger, J.; Berry, C.E.; Clarke, P.K.; Hallstrand, T.S.; Hankinson, J.L.; Kaminsky, D.A.; MacIntyre, N.R.; et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am. J. Respir. Crit. Care Med. 2017, 196, 1463–1472. [Google Scholar] [CrossRef]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global rating of change scales: A review of strengths and weaknesses and considerations for design. J. Man. Manip. Ther. 2009, 17, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Prinsen, C.A.; Vohra, S.; Rose, M.R.; Boers, M.; Tugwell, P.; Clarke, M.; Williamson, P.R.; Terwee, C.B. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”—A practical guideline. Trials 2016, 17, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vet, H.C.W.; Terwee, C.B.; Mokkink, L.B.; Knol, D.L. Measurement in Medicine: A Practical Guide; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J. Clin. Epidemiol. 2010, 63, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolk, J.J.; Janssen, R.P.A.; Prinsen, C.; van der Steen, M.M.C.; Bierma Zeinstra, S.M.A.; Reijman, M. Measurement properties of the OARSI core set of performance-based measures for hip osteoarthritis: A prospective cohort study on reliability, construct validity and responsiveness in 90 hip osteo-arthritis patients. Acta Orthop. 2019, 90, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terwee, C.B.; Mokkink, L.B.; Knol, D.L.; Ostelo, R.W.; Bouter, L.M.; de Vet, H.C. Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Qual. Life Res. 2012, 21, 651–657. [Google Scholar] [CrossRef] [Green Version]
- McKeough, Z.J.; Alison, J.A.; Bye, P.T. Arm exercise capacity and dyspnea ratings in subjects with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2003, 23, 218–225. [Google Scholar] [CrossRef]
- Hefford, C.; Abbott, J.H.; Arnold, R.; Baxter, G.D. The patient-specific functional scale: Validity, reliability, and responsiveness in patients with upper extremity musculoskeletal problems. J. Orthop. Sports Phys. Ther. 2012, 42, 56–65. [Google Scholar] [CrossRef]
- Razmjou, H.; Bean, A.; van Osnabrugge, V.; MacDermid, J.C.; Holtby, R. Cross-sectional and longitudinal construct validity of two rotator cuff disease-specific outcome measures. BMC Musculoskelet. Disord. 2006, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Abbott, J.H.; Schmitt, J.S. The Patient-Specific Functional Scale was valid for group-level change comparisons and between-group discrimination. J. Clin. Epidemiol. 2014, 67, 681–688. [Google Scholar] [CrossRef]
- Horner, A.; Burghuber, O.C.; Hartl, S.; Studnicka, M.; Merkle, M.; Olschewski, H.; Kaiser, B.; Wallner, E.M.; Lamprecht, B. Quality of Life and Limitations in Daily Life of Stable COPD Outpatients in a Real-World Setting in Austria—Results from the CLARA Project. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Van der Molen, T.; Willemse, B.W.; Schokker, S.; ten Hacken, N.H.; Postma, D.S.; Juniper, E.F. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual. Life Outcomes 2003, 1, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
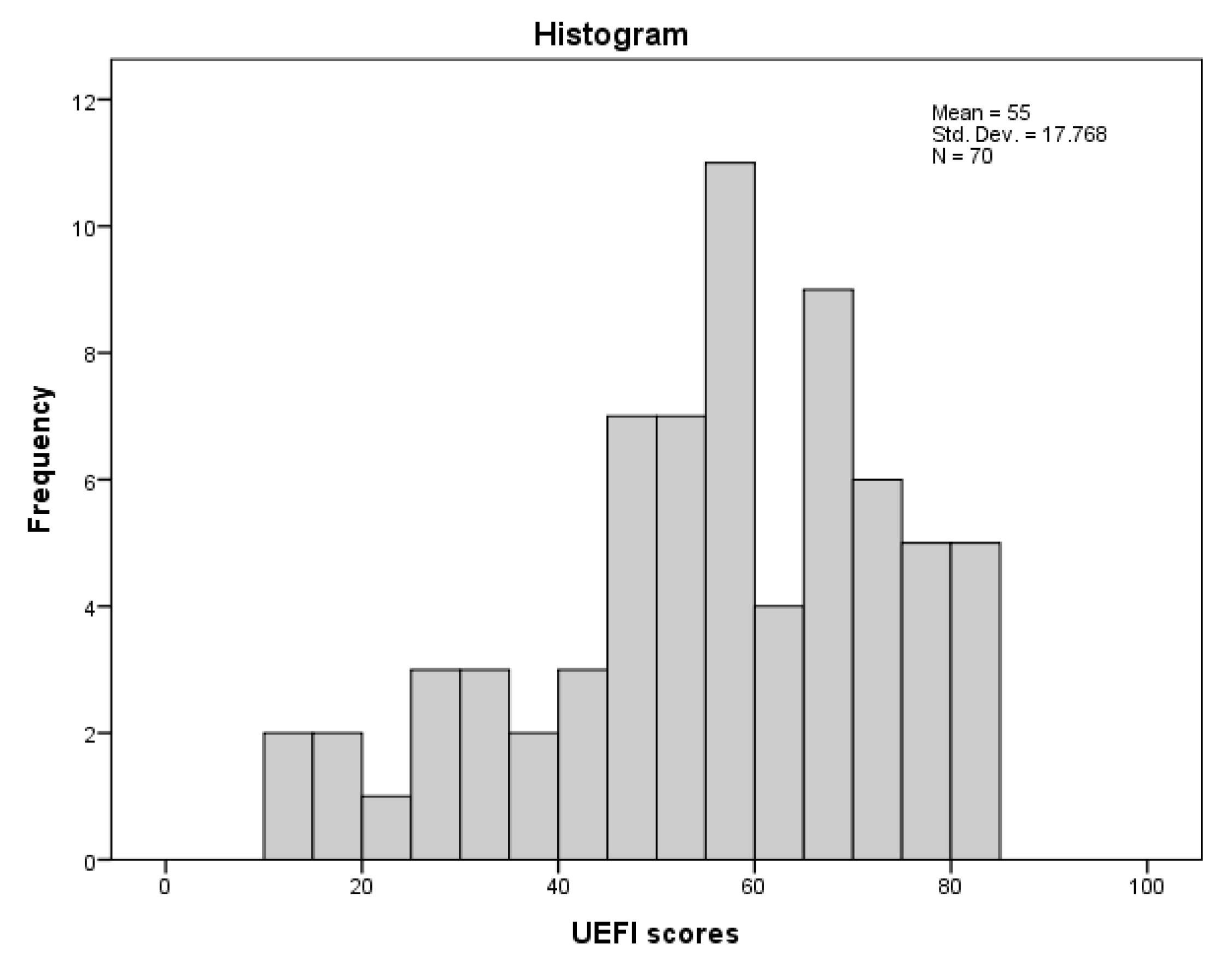

| Variable | Mean ± SD or Median (IQR; Q1, Q3) or N (%) |
|---|---|
| Age (year) | 63.23 ± 11.10 |
| Sex | |
| Male | 56 (80) |
| Female | 14 (20) |
| Height (m) | 1.65 ± 0.08 |
| Mass (Kg) | 78.88 ± 15.26 |
| Body mass index (Kg/m2) | 29.05 ± 5.35 |
| Smoking Status | |
| Current smoker | 17 (24.3) |
| Previous smoker | 39 (55.7) |
| Never smoked | 14 (20) |
| Lung function | |
| FEV1 (L) | 1.57 ± 0.55 |
| FVC (L) | 2.81 (1.28; 2.22, 3.49) |
| FEV1/FVC ratio | 56.35 (21.45; 44.55, 66.0) |
| FEV1% predicted | 55.99 ± 14.98 |
| COPD severity * | |
| Mild (GOLD 1) | 4 (5.7) |
| Moderate (GOLD 2) | 36 (51.4) |
| Severe (GOLD 3) | 27 (38.6) |
| Very severe (GOLD 4) | 3 (4.3) |
| UEFI (0–80) | 55.0 ± 17.77 |
| UEFI in patients with mild to moderate disease (GOLD 1 and 2) | 58.90 ± 15.66 |
| UEFI in patients with severe to very severe disease (GOLD 3 and 4) | 49.80 ± 19.30 |
| SGRQ | |
| Symptoms (0–100) | 53.55 ± 23.46 |
| Activity (0–100) | 59.46 (38.93; 34.11, 73.04) |
| Impact (0–100) | 45.88 (47.25; 17.99, 65.25) |
| Total (0–100) | 49.14 ± 24.51 |
| Outcome Measure | Test Mean ± SD | Retest Mean ± SD | Mean Difference * (95% CI) | ICC2,1 (95% CI) | SEM | MDC90 |
|---|---|---|---|---|---|---|
| UEFI | 58.62 ± 16.52 | 57.25 ±16.12 | 1.36 (−0.49 to 3.22) | 0.91 (0.85 to 0.95) | 4.85 | 11.32 |
| Variable | rs (95% CI) | p |
|---|---|---|
| SGRQ Symptoms | −0.35 (−0.11 to −0.58) | 0.003 |
| SGRQ Activity | −0.66 (−0.46 to −0.80) | <0.001 |
| SGRQ Impact | −0.63 (−0.45 to −0.76) | <0.001 |
| SGRQ Total | −0.63 (−0.45 to −0.77) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnahdi, A.H.; Albarrati, A. The Upper Extremity Functional Index: Reliability and Validity in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Environ. Res. Public Health 2021, 18, 10608. https://doi.org/10.3390/ijerph182010608
Alnahdi AH, Albarrati A. The Upper Extremity Functional Index: Reliability and Validity in Patients with Chronic Obstructive Pulmonary Disease. International Journal of Environmental Research and Public Health. 2021; 18(20):10608. https://doi.org/10.3390/ijerph182010608
Chicago/Turabian StyleAlnahdi, Ali H., and Ali Albarrati. 2021. "The Upper Extremity Functional Index: Reliability and Validity in Patients with Chronic Obstructive Pulmonary Disease" International Journal of Environmental Research and Public Health 18, no. 20: 10608. https://doi.org/10.3390/ijerph182010608
APA StyleAlnahdi, A. H., & Albarrati, A. (2021). The Upper Extremity Functional Index: Reliability and Validity in Patients with Chronic Obstructive Pulmonary Disease. International Journal of Environmental Research and Public Health, 18(20), 10608. https://doi.org/10.3390/ijerph182010608







