Network Analysis of Symptoms Co-Occurrence in Chronic Fatigue Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measures and Procedure
2.2.1. Chronic Fatigue Syndrome Diagnosis
2.2.2. Fatigue Measurements
2.2.3. Subjective Assessment of Autonomic and Cardiovascular Functions
2.2.4. Objective Assessment of Autonomic and Cardiovascular Function
2.2.5. Body Composition Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Group of Chemical Compound | Total Sample (n = 110) |
|---|---|
| Occurrence | |
| None | 63 |
| Diet supplements | 23 |
| Hormones | 5 |
| PPI | 2 |
| Contraception | 4 |
| Painkillers | 4 |
| Beta-blockers | 1 |
| Antiallergic | 4 |
| Other | 14 |
| Antidepresants | 7 |
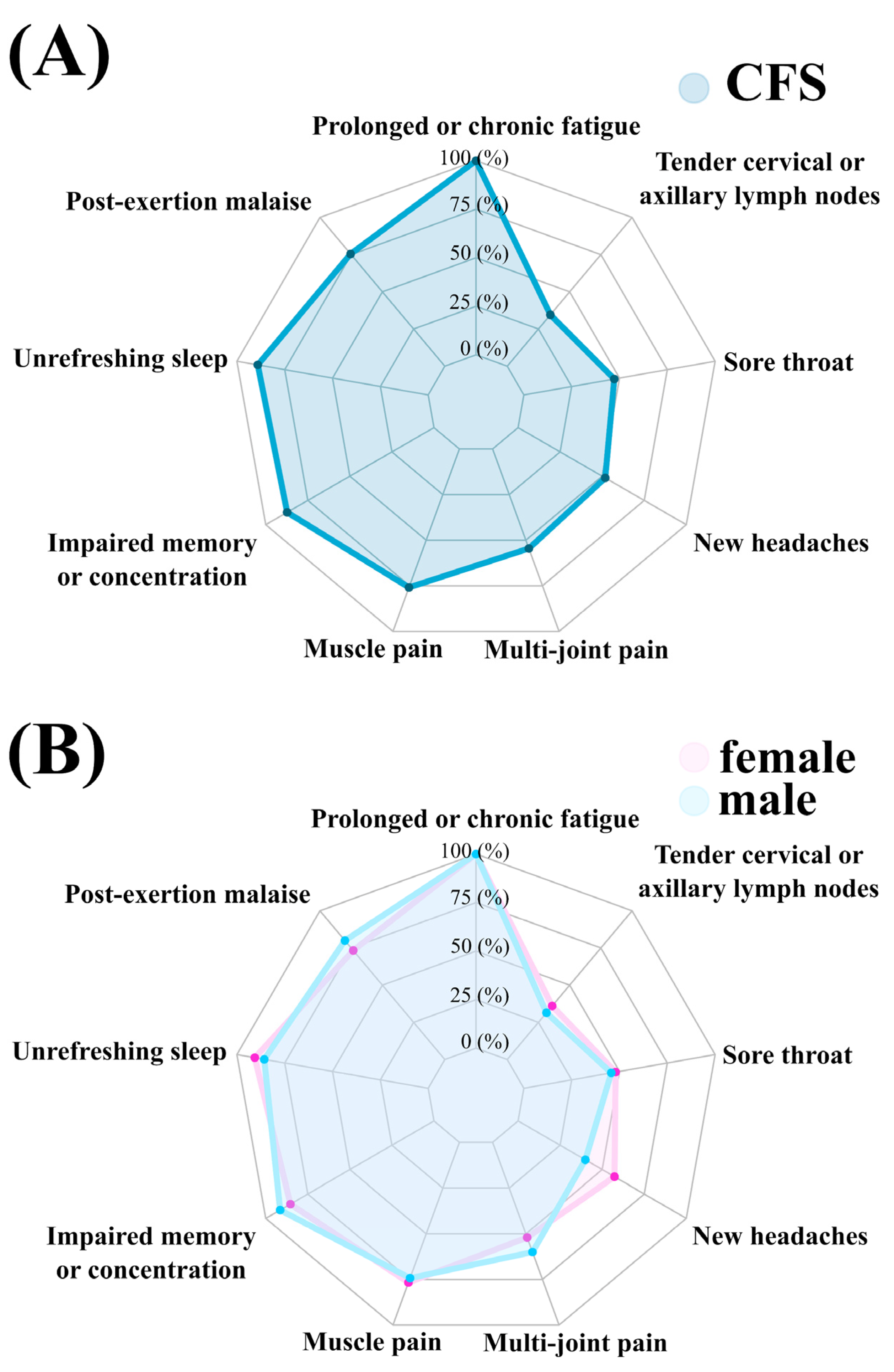
| Fukuda Criteria Symptom | Total Sample (n = 110) | Male (n = 35) | Female (n = 75) |
|---|---|---|---|
| Occurrence (Percent) | Occurrence (Percent) | Occurrence (Percent) | |
| Prolonged or chronic fatigue | 110 (100) | 35 (100) | 75 (100) |
| PEM | 83 (75.45) | 28 (80) | 55 (73.33) |
| Unrefreshing sleep | 98 (89.09) | 30 (85.71) | 68 (90.67) |
| Impaired memory or concentration | 96 (87.27) | 32 (91.43) | 64 (85.33) |
| Muscle pain | 82 (75.93) | 26 (74.29) | 56 (76.71) |
| Multi-joint pain | 60 (54.55) | 21 (60) | 39 (52) |
| New headaches | 57 (51.82) | 14 (40) | 43 (57.33) |
| Sore throat | 52 (47.27) | 16 (45.71) | 36 (48) |
| Tender cervical or axillary lymph nodes | 38 (34.55) | 11 (31.43) | 27 (36) |
References
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Brurberg, K.G.; Fønhus, M.S.; Larun, L.; Flottorp, S.; Malterud, K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. BMJ Open 2014, 4, e003973. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.C.; Archard, L.C.; Banatvala, J.E.; Borysiewicz, L.K.; Clare, A.W.; David, A.; Edwards, R.H.; Hawton, K.E.; Lambert, H.P.; Lane, R.J. A report--chronic fatigue syndrome: Guidelines for research. J. R. Soc. Med. 1991, 84, 118–121. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Toogood, P.L.; Clauw, D.J.; Phadke, S.; Hoffman, D. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Where will the drugs come from? Pharmacol. Res. 2021, 165, 105465. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, H.; Muraki, K.; Eaton, N.; Balinas, C.; Staines, D.; Marshall-Gradisnik, S. Loss of Transient Receptor Potential Melastatin 3 ion channel function in natural killer cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients. Mol. Med. 2018, 24, 44. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, R.; Du Preez, S.; Eaton-Fitch, N.; Thapaliya, K.; Barnden, L.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. A systematic review of neurological impairments in myalgic encephalomyelitis/ chronic fatigue syndrome using neuroimaging techniques. PLoS ONE 2020, 15, e0232475. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G. The Chalder Fatigue Scale (CFQ 11). Occup. Med. 2015, 65, 86. [Google Scholar] [CrossRef] [Green Version]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994, 18 (Suppl. 1), S79–S83. [Google Scholar] [CrossRef]
- Krupp, L.B.; La Rocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The Fatigue Severity Scale Application to Patients With Multiple Sclerosis and Systemic Lupus Erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Suarez, G.A.; Opfer-Gehrking, T.L.; Offord, K.P.; Atkinson, E.J.; O’Brien, P.C.; Low, P.A. The Autonomic Symptom Profile: A new instrument to assess autonomic symptoms. Neurology 1999, 52, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.M.; Mainardi, L.T.; Meloni, C.; Chierchiu, S.; Cerutti, S. Continuous monitoring of the sympatho-vagal balancethrough spectral analy-sis. IEEE Eng. Med. Biol. Mag. 1997, 16, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Klinger, T.; Wagner, C.; Sterner, H.; Madritsch, C.; Grullenberger, R. The task force monitor—A non-invasive beat-to-beat monitor for hemodynamic and autonomic function of the human body. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Austin, TX, USA, 1 November 1998. [Google Scholar]
- Fortin, J.; Marte, W.; Grullenberger, R. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput. Biol. Med. 2006, 36, 941–957. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Lim, E.J.; Kang, E.B.; Jang, E.S.; Son, C.G. The Prospects of the Two-Day Cardiopulmonary Exercise Test (CPET) in ME/CFS Patients: A Meta-Analysis. J. Clin. Med. 2020, 9, 4040. [Google Scholar] [CrossRef]
- Holtzman, C.S.; Bhatia, S.; Cotler, J.; Jason, L.A. Assessment of Post-Exertional Malaise (PEM) in Patients with Myalgic Encephalomyelitis (ME) and Chronic Fatigue Syndrome (CFS): A Patient-Driven Survey. Diagnostics 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Le Bon, O.; Fischler, B.; Hoffmann, G.; Murphy, J.R.; De Meirleir, K.; Cluydts, R.; Pelc, I. How significant are primary sleep disorders and sleepiness in the chronic fatigue syndrome? Sleep Res. Online 2000, 3, 43–48. [Google Scholar]
- De Becker, P.; McGregor, N.; De Meirleir, K. A definition-based analysis of symptoms in a large cohort of patients with chronic fatigue syndrome. J. Intern. Med. 2001, 250, 234–240. [Google Scholar] [CrossRef]
- Nisenbaum, R.; Jones, J.F.; Unger, E.R.; Reyes, M.; Reeves, W.C. A population-based study of the clinical course of chronic fatigue syndrome. Health Qual. Life Outcomes 2003, 1, 49. [Google Scholar] [CrossRef] [Green Version]
- Melinda, J.L.; Bruck, D. Sleep Abnormalities in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Review. J. Clin. Sleep Med. 2012, 8, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Nacul, L.C.; Lacerda, E.M.; Pheby, D.; Campion, P.; Molokhia, M.; Fayyaz, S.; Leite, J.C.; Poland, F.; Howe, A.; Drachler, M.L. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: A repeated cross-sectional study in primary care. BMC Med. 2011, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janal, M.N.; Ciccone, D.S.; Natelson, B.H. Sub-typing CFS patients on the basis of “minor” symptoms. Biol. Psychol. 2006, 73, 124–131. [Google Scholar] [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. In Review of the Evidence on Major ME/CFS Symptoms and Manifestations; National Academies Press (US): Washington, DC, USA, 2015.
- Klimas, N.G.; Salvato, F.R.; Morgan, R.; Fletcher, M.A. Immunologic abnormalities in chronic fatigue syndrome. J. Clin. Microbiol. 1990, 28, 1403–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton-Fitch, N.; du Preez, S.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. A systematic review of natural killer cells profile and cytotoxic function in myalgic encephalomyelitis/chronic fatigue syndrome. Syst. Rev. 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.; Fuite, J.; Kreitz, A.; Vernon, S.D.; Klimas, N.; Fletcher, M.A. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav. Immun. 2010, 24, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- Brenu, E.W.; van Driel, M.L.; Staines, D.R.; Ashton, K.J.; Ramos, S.B.; Keane, J.; Klimas, N.G.; Marshall-Gradisnik, S.M. Immunological abnormalities as potential biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J. Transl. Med. 2011, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, D.B.; Light, A.R.; Light, K.C.; Broderick, G.; Shields, M.R.; Dougherty, R.J.; Meyer, J.; VanRiper, S.; Stegner, A.J.; Ellingson, L.D.; et al. Neural consequences of post-exertion malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Brain Behav. Immun. 2017, 62, 87–99. [Google Scholar] [CrossRef]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: A patient-centered.; cross-sectional survey. PLoS ONE 2018, 13, e0197811. [Google Scholar] [CrossRef] [Green Version]
- Nijs, J. Pain in Patients with Chronic FatigueSyndrome: Time for Specific Pain Treatment? Pain Physician 2012, 15, E677–E686. [Google Scholar] [CrossRef]
- Meeus, M.; Nijs, J.; Van Oosterwijck, J.; Van Alsenoy, V.; Truijen, S. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education: A double-blind randomized controlled trial. Arch. Phys. Med. Rehabil. 2010, 91, 1153–1159. [Google Scholar] [CrossRef]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Lefever, I.; Huybrechts, L.; Lambrecht, L.; Paul, L. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: An experimental study. J. Intern. Med. 2010, 268, 265–278. [Google Scholar] [CrossRef]
- Ball, N.; Buchwald, D.S.; Schmidt, D.; Goldberg, J.; Ashton, S.; Armitage, R. Monozygotic twins discordant for chronic fatigue syndrome: Objective measures of sleep. J. Psychosom. Res. 2004, 56, 207–212. [Google Scholar] [CrossRef]
- Benrud-Larson, L.M.; Dewar, M.S.; Sandroni, P.; Rummans, T.A.; Haythornthwaite, J.A.; Low, P.A. Quality of life in patients with postural tachycardia syndrome. Mayo Clin. Proc. 2002, 77, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnden, L.R.; Kwiatek, R.; Crouch, B.; Burnet, R.; Del Fante, P. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome. NeuroImage Clin. 2016, 11, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Staines, D.; Nilius, B.; Smith, P.; Marshall-Gradisnik, S. Novel identification and characterisation of Transient receptor potential melastatin 3 ion channels on Natural Killer cells and B lymphocytes: Effects on cell signalling in Chronic fatigue syndrome/Myalgic encephalomyelitis patients. Biol. Res. 2016, 49, 27. [Google Scholar] [CrossRef]
- Nguyen, T.; Johnston, S.; Clarke, L.; Smith, P.; Staines, D.; Marshall-Gradisnik, S. Impaired calcium mobilization in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients is associated with transient receptor potential melastatin 3 ion channels. Clin. Exp. Immunol. 2017, 187, 284–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanas, H.; Muraki, K.; Balinas, C.; Eaton-Fitch, N.; Staines, D.; Marshall-Gradisnik, S. Validation of impaired Transient Receptor Potential Melastatin 3 ion channel activity in natural killer cells from Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis patients. Mol. Med. 2019, 25, 14. [Google Scholar] [CrossRef] [Green Version]
- Held, K.; Tóth, B.I. TRPM3 in Brain (Patho)Physiology. Front. Cell Dev. Biol. 2021, 9, 635659. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Lin, J.C.; Sheriff, S.; Maudsley, A.A.; Younger, J.W. Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: Assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2020, 14, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.B.; O’Connor, P.J.; Lange, G.; Steffener, J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. NeuroImage 2007, 36, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.F.; Yu, R.; Tang, T.B.; Tam, W.W.; Tran, B.; Quek, T.T.; Hwang, S.H.; Chang, C.W.; Ho, C.S.; Ho, R.C. Validating a functional near-infrared spectroscopy diagnostic paradigm for Major Depressive Disorder. Sci. Rep. 2020, 10, 9740. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean (SD) Males (n = 35) | Mean (SD) Females (n = 75) |
|---|---|---|
| Age (years) | 39.03 (8.3) | 37.41 (8.6) |
| BMI (kg/m2) | 25.23 (2.7) | 24.14 (4.1) |
| FFM (kg) | 64.50 (6.2) | 47.74 (5.7) |
| Fat (%) | 19.71 (4.4) | 28.66 (6.7) |
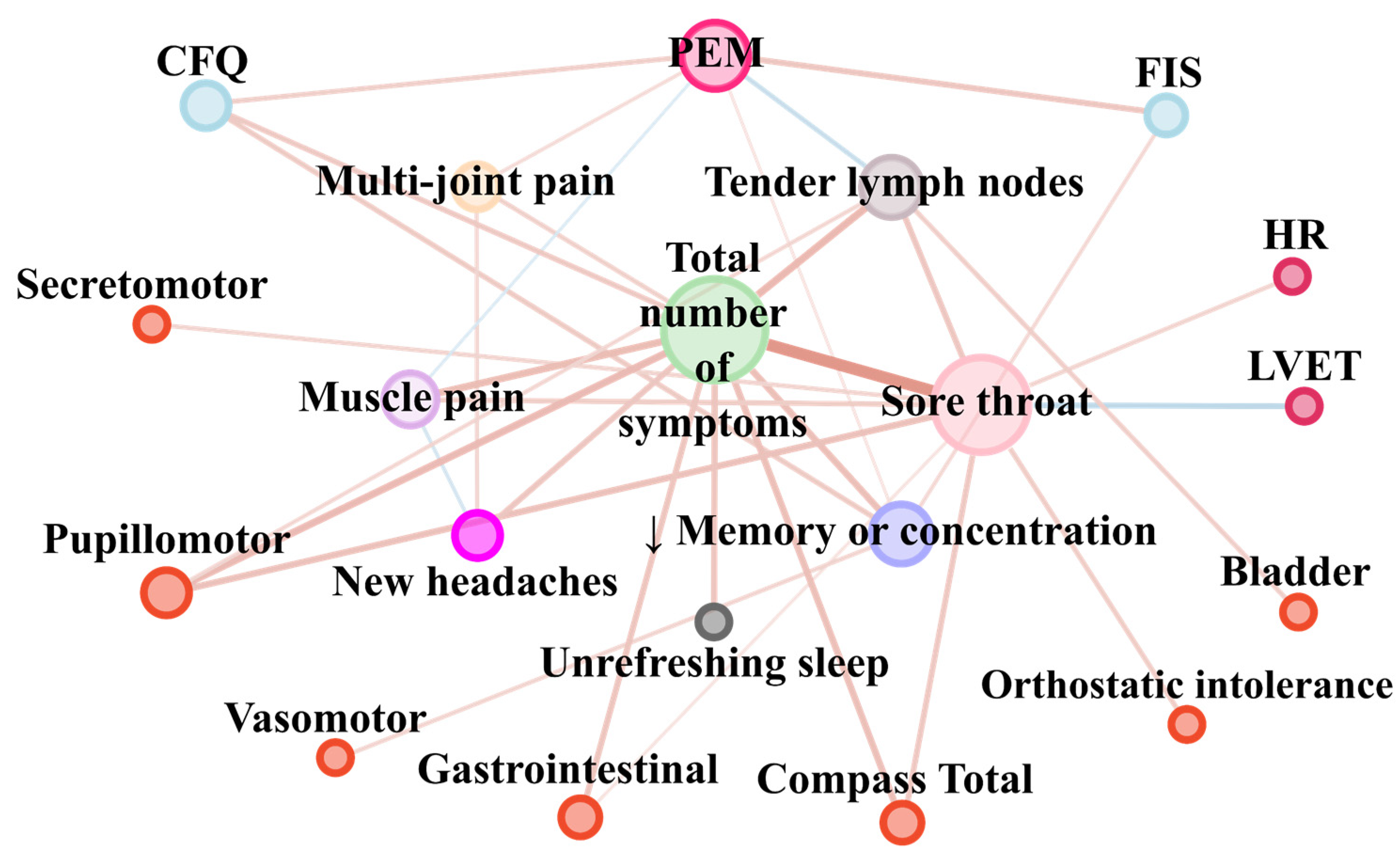
| Pair of Parameters | Correlation Coefficient (rho or tau) | FDR p-Value |
|---|---|---|
| Bladder and Tender cervical or axillary lymph nodes | 0.26 | 0.001 |
| CFQ and Total number of symptoms | 0.29 | 0.02 |
| CFQ and PEM | 0.26 | 0.001 |
| CFQ and Impaired memory or concentration | 0.28 | 0.000 |
| Compass Total and Total number of symptoms | 0.35 | 0.004 |
| Compass Total and Sore throat | 0.30 | 0.00000000006 |
| FIS and PEM | 0.31 | 0.0002 |
| FIS and Impaired memory or concentration | 0.21 | 0.01 |
| Gastrointestinal and Total number of symptoms | 0.32 | 0.009 |
| Gastrointestinal and Sore throat | 0.18 | 0.05 |
| HR and Sore throat | 0.23 | 0.005 |
| Impaired memory or concentration and CFQ | 0.28 | 0.0004 |
| Impaired memory or concentration and FIS | 0.21 | 0.01 |
| Impaired memory or concentration and Vasomotor | 0.23 | 0.005 |
| Impaired memory or concentration and PEM | 0.16 | 0.03 |
| Impaired memory or concentration and Total number of symptoms | 0.35 | 0.001 |
| LVET and Sore throat | −0.30 | 0.0002 |
| Multi-joint pain and PEM | 0.20 | 0.008 |
| Multi-joint pain and New headaches | 0.22 | 0.004 |
| Multi-joint pain and Total number of symptoms | 0.25 | 0.03 |
| Muscle pain and PEM | −0.17 | 0.03 |
| Muscle pain and New headaches | −0.20 | 0.009 |
| Muscle pain and Sore throat | 0.27 | 0.0003 |
| Muscle pain and Total number of symptoms | 0.37 | 0.0007 |
| New headaches and Muscle pain | −0.20 | 0.009 |
| New headaches and Multi-joint pain | 0.22 | 0.004 |
| New headaches and Total number of symptoms | 0.31 | 0.004 |
| Orthostatic intolerance and Sore throat | 0.27 | 0.0009 |
| PEM and CFQ | 0.26 | 0.001 |
| PEM and FIS | 0.31 | 0.0002 |
| PEM and Impaired memory or concentration | 0.16 | 0.03 |
| PEM and Muscle pain | −0.17 | 0.03 |
| PEM and Multi-joint pain | 0.20 | 0.008 |
| PEM and Tender cervical or axillary lymph nodes | −0.25 | 0.0007 |
| Pupillomotor and Total number of symptoms | 0.38 | 0.0009 |
| Pupillomotor and Sore throat | 0.34 | 0.00002 |
| Pupillomotor and Tender cervical or axillary lymph nodes | 0.22 | 0.009 |
| Secretomotor and Sore throat | 0.23 | 0.005 |
| Sore throat and Orthostatic intolerance | 0.27 | 0.0009 |
| Sore throat and Secretomotor | 0.23 | 0.005 |
| Sore throat and Gastrointestinal | 0.18 | 0.0497 |
| Sore throat and Pupillomotor | 0.34 | 0.00002 |
| Sore throat and Compass Total | 0.30 | 0.0002 |
| Sore throat and HR | 0.23 | 0.005 |
| Sore throat and LVET | −0.30 | 0.0002 |
| Sore throat and Muscle pain | 0.27 | 0.0003 |
| Sore throat and Tender cervical or axillary lymph nodes | 0.31 | 0.00004 |
| Sore throat and Total number of symptoms | 0.61 | 0.00000000006 |
| Tender cervical or axillary lymph nodes and Bladder | 0.26 | 0.001 |
| Tender cervical or axillary lymph nodes and Pupillomotor | 0.22 | 0.009 |
| Tender cervical or axillary lymph nodes and Sore throat | 0.31 | 0.00004 |
| Tender cervical or axillary lymph nodes and PEM | −0.25 | 0.0007 |
| Tender cervical or axillary lymph nodes and Total number of symptoms | 0.39 | 0.0003 |
| Total number of symptoms and CFQ | 0.29 | 0.02 |
| Total number of symptoms and Gastrointestinal | 0.32 | 0.009 |
| Total number of symptoms and Pupillomotor | 0.38 | 0.0009 |
| Total number of symptoms and Compass Total | 0.35 | 0.004 |
| Total number of symptoms and unrefreshing sleep | 0.32 | 0.004 |
| Total number of symptoms and Impaired memory or concentration | 0.35 | 0.001 |
| Total number of symptoms and Muscle pain | 0.37 | 0.0007 |
| Total number of symptoms and Multi-joint pain | 0.25 | 0.03 |
| Total number of symptoms and New headaches | 0.31 | 0.004 |
| Total number of symptoms and Sore throat | 0.61 | 0.00000000006 |
| Total number of symptoms and Tender cervical or axillary lymph nodes | 0.39 | 0.0003 |
| unrefreshing sleep and Total number of symptoms | 0.32 | 0.004 |
| Vasomotor and Impaired memory or concentration | 0.23 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawski, S.; Słomko, J.; Newton, J.L.; Eaton-Fitch, N.; Staines, D.R.; Marshall-Gradisnik, S.; Zalewski, P. Network Analysis of Symptoms Co-Occurrence in Chronic Fatigue Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 10736. https://doi.org/10.3390/ijerph182010736
Kujawski S, Słomko J, Newton JL, Eaton-Fitch N, Staines DR, Marshall-Gradisnik S, Zalewski P. Network Analysis of Symptoms Co-Occurrence in Chronic Fatigue Syndrome. International Journal of Environmental Research and Public Health. 2021; 18(20):10736. https://doi.org/10.3390/ijerph182010736
Chicago/Turabian StyleKujawski, Sławomir, Joanna Słomko, Julia L. Newton, Natalie Eaton-Fitch, Donald R. Staines, Sonya Marshall-Gradisnik, and Paweł Zalewski. 2021. "Network Analysis of Symptoms Co-Occurrence in Chronic Fatigue Syndrome" International Journal of Environmental Research and Public Health 18, no. 20: 10736. https://doi.org/10.3390/ijerph182010736
APA StyleKujawski, S., Słomko, J., Newton, J. L., Eaton-Fitch, N., Staines, D. R., Marshall-Gradisnik, S., & Zalewski, P. (2021). Network Analysis of Symptoms Co-Occurrence in Chronic Fatigue Syndrome. International Journal of Environmental Research and Public Health, 18(20), 10736. https://doi.org/10.3390/ijerph182010736








