Point-of-Care COVID-19 Antigen Testing in Exposed German Healthcare Workers—A Cost Model
Abstract
:1. Introduction
2. Materials and Methods
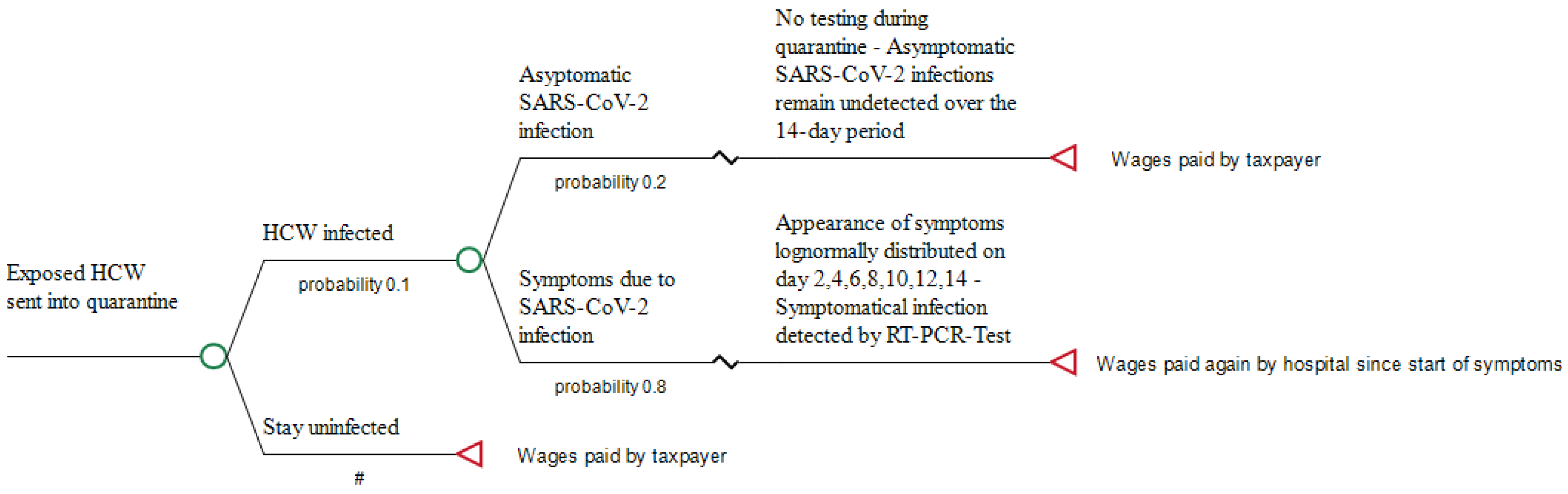
2.1. Strategy for Managing Exposed HCW
2.2. Incubation Period and Prevalence of Infections in Exposed HCW
2.3. Costs of Testing and Outcomes
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Method
| Day | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
|---|---|---|---|---|---|---|---|
| probability | p(0–2) = 0.09 | p(2–4) = 0.38 | p(4–6) = 0.30 | p(6–8) = 0.09 | p(8–10) = 0.08 | p(10–12) = 0.04 | p(12–14) = 0.01 |
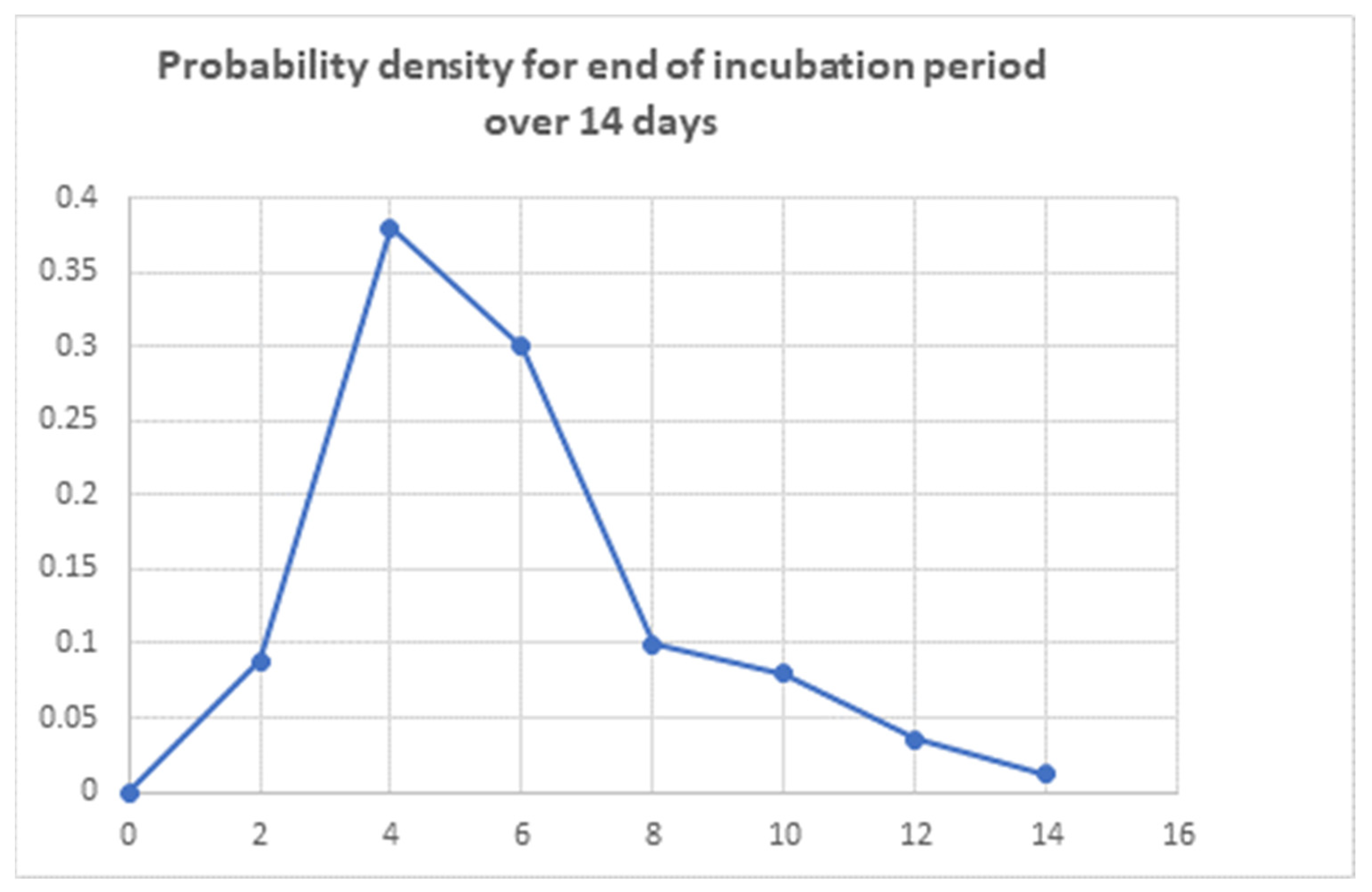
- Sensitivity in infected subjects with symptoms: 0.8 [p(S) = 0.8]
- Sensitivity in infected subjects without symptoms: 0.581 [p(NS) = 0.581]
- Specificity: 0.989 [p(NI) = 0.989]
| Persons Not Infected (No.) | d2 | d4 | d6 | d8 | d10 | d12 | d14 | Sum after 14 Days |
|---|---|---|---|---|---|---|---|---|
| TN | 890.1 | 880.31 | 870.63 | 861.05 | 851.58 | 842.21 | 832.95 | 832.95 |
| FP | 9.9 | 9.79 | 9.68 | 9.58 | 9.47 | 9.37 | 9.26 | 67.05 |
| Total | 900 | 890.1 | 880.31 | 870.63 | 861.05 | 851.58 | 842.21 | 900 |
| Persons Newly Infected (No.) | d2 | d4 | d6 | d8 | d10 | d12 | d14 | Sum after 14 Days |
|---|---|---|---|---|---|---|---|---|
| TP (S) | 5.652 | 24.32 | 19.216 | 6.003 | 5.476 | 2.285 | 0.928 | 63.88 |
| FN (S) | 1.413 | 6.08 | 4.804 | 1.501 | 1.369 | 0.571 | 0.242 | 15.98 |
| TP (NS) | 1.024 | 4.408 | 3.483 | 1.088 | 0.993 | 0.414 | 0.177 | 11.587 |
| FN (NS) | 0.742 | 3.192 | 2.522 | 0.788 | 0.719 | 0.3 | 0.27 | 8.553 |
| total | 8.831 | 38 | 30.025 | 9.38 | 8.557 | 3.57 | 1.637 | 100 |
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, P.; Vu, D.L.; L’Huillier, A.G.; Schibler, M.; Kaiser, L.; Jacquerioz, F. Clinical features of covid-19. BMJ 2020, 369, 1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John Hopkins University. Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 11 August 2021).
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19 (accessed on 11 March 2020).
- Osterloh, F. Krankenhäuser: Folgen des Personalmangels. Dtsch. Arztebl. 2019, 116, A-613/B-503/C-495. [Google Scholar]
- Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden)–DESTATIS. Gesundheit. Grunddaten der Krankenhäuser; Fachserie 12, Reihe 6.1.1. Krankenhäuser 2017, 2.8; Bettenführende Fachabteilungen: Wiesbaden, Germany, 2019. [Google Scholar]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [PubMed]
- Bundesministerium für Gesundheit. Verordnung zum Anspruch auf Testung in Bezug auf einen Direkten Erregernachweis des Coronavirus SARS-CoV-2 (Coronavirus-Testverordnung–TestV). Available online: https://www.gesetze-im-internet.de/coronatestv_2021-07/TestV.pdf (accessed on 11 August 2021).
- Centers for Disease Control. Interim Guidance for Antigen Testing for SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed on 11 August 2021).
- Paltiel, A.D.; Zheng, A.; Walensky, R.P. Assessment of SARS-CoV-2 Screening Strategies to 365 Permit the Safe Reopening of College Campuses in the United States. JAMA Netw. Open 2020, 3, e2016818. [Google Scholar] [CrossRef] [PubMed]
- Larremore, D.B.; Wilder, B.; Lester, E.; Shehata, S.; Burke, J.M.; Hay, J.A.; Tambe, M.; Mina, M.J.; Parker, R. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021, 7, eabd5393. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. COVID-19: Entlassungskriterien aus der Isolierung. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Entlassmanagement.html (accessed on 31 March 2021).
- Robert Koch Institut. Optionen zum Management von Kontaktpersonen unter Personal der Kritischen Infrastruktur bei Personalmangel-Options for Managing Contact Persons among Staff in the Critical Infrastructure in the Event of a Staff Shortage. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Personal_KritIs.html (accessed on 30 April 2021).
- Diel, R.; Nienhaus, A. Point-of-care COVID-19 antigen testing in German emergency rooms-a cost-benefit analysis. Pulmonology 2021. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Epidemiologischer Steckbrief zu SARS-CoV-2 und COVID-19. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html (accessed on 14 July 2021).
- McAloon, C.; Collins, Á.; Hunt, K.; Barber, A.; Byrne, A.W.; Butler, F.; Casey, M.; Griffin, J.; Lane, E.; McEvoy, D.; et al. Incubation period of COVID-19: A rapid systematic review and meta-analysis of observational research. BMJ Open 2020, 10, e039652. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Kontaktpersonen und Quarantäne. Available online: https://www.rki.de/SharedDocs/FAQ/NCOV2019/FAQ_Liste_Kontaktpersonemanagement.html (accessed on 9 July 2021).
- Rivett, L.; Sridhar, S.; Sparkes, D.; Routledge, M.; Jones, N.K.; Forrest, S.; Young, J.; Pereira-Dias, J.; Hamilton, W.L.; Ferris, M.; et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife 2020, 9, e58728. [Google Scholar] [CrossRef] [PubMed]
- Reusken, C.B.; Buiting, A.; Bleeker-Rovers, C.; Diederen, B.; Hooiveld, M.; Friesema, I.; Koopmans, M.; Kortbeek, T.; Lutgens, S.P.; Meijer, A.; et al. Rapid assessment of regional SARS-CoV-2 community transmission through a convenience sample of healthcare workers, The Netherlands, March 2020. Eurosurveillance 2020, 25, 2000334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Stefanizzi, P.; Gesualdo, L.; Migliore, G.; Fucilli, F.I.M.; Cavone, D.; Delfino, M.C.; et al. COVID-19 hospital outbreaks: Protecting healthcare workers to protect frail patients. An Italian observational cohort study. Int. J. Infect. Dis. 2021, 102, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Quidel: Sofia SARS Antigen FIA. Available online: https://www.quidel.com/immunoassays/rapid-sars-tests/sofia-sars-antigen-fia (accessed on 9 August 2021).
- Robert Koch Institute. Hinweise zur Testung von Patienten auf Infektion mit dem Neuartigen Coronavirus SARS-CoV-2-Zur Bewertung der Ergebnisse aus AG-Testen. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Vorl_Testung_nCoV.html;jsessionid=0DABCE15E5CF14EE43D11785FCF2BCA7.internet062?nn=13490888#doc13490982bodyText7 (accessed on 8 July 2021).
- Gesetz zur Wirtschaftlichen Sicherung der Krankenhäuser und zur Regelung der Krankenhauspflegesätze (Krankenhausfinanzierungsgesetz-KHG). § 26 Zusatzentgelt für Testungen auf das Coronavirus SARS-CoV-2 im Krankenhaus [Law on the Economic Security of Hospitals and on the Regulation of Hospital Care Rates (Hospital Financing Act-KHG). Section 26 Additional Fee for Tests for the SARS-CoV-2 Coronavirus in Hospitals]. Available online: https://www.gesetze-im-internet.de/khg/BJNR010090972.html (accessed on 11 August 2021).
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef] [PubMed]
- Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden)-DESTATIS. Fachserie 16, Reihe 2.3: Verdienste und Arbeitskosten –Arbeitnehmerverdienste. 4.5.1 Durchschnittliche Bruttojahresverdienste und Sonderzahlungen nach Wirtschaftszweigen Arbeitnehmer im Jahr 2019 im Krankenhaus (Q861); Statistisches Bundesamt: Wiesbaden, Germany, 2020. [Google Scholar]
- Working Days in Germany 2021. Available online: https://www.arbeitstage.de/arbeitsstunden_jahr_2021.htm (accessed on 9 August 2021).
- California Department of Public Health. Updated Testing Guidance. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/Updated-COVID-19-Testing-Guidance.aspx (accessed on 16 June 2021).
- Robert Koch Institut. Nationale Teststrategie–Anwendung von Antigentesten zum Screenen von Personal Empfohlen. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Teststrategie/Nat-Teststrat.html (accessed on 14 July 2021).
- NHS. Asymptomatic Staff Testing for COVID-19. Available online: https://www.england.nhs.uk/coronavirus/publication/asymptomatic-staff-testing/ (accessed on 9 August 2021).
- Gabler, J.; Raabe, T.; Röhrl, K.; von Gaudecker, H.M. The Effectiveness of Strategies to Contain SARS-CoV-2: Testing, Vaccinations, and NPIs. arXiv 2021, arXiv:2106.11129. [Google Scholar]
- Centers for Disease Control and Prevention. Science Brief: Options to Reduce Quarantine for Contacts of Persons with SARS-CoV-2 Infection Using Symptom Monitoring and Diagnostic Testing. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-options-to-reduce-quarantine.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fmore%2Fscientific-brief-options-to-reduce-quarantine.html (accessed on 9 August 2021).
- Robert Koch Institut. SARS-CoV-2 Kontaktpersonennachverfolgung für Medizinisches Personal in Arztpraxis und Krankenhausbei Relevantem Personalmangel. Available online: https://edoc.rki.de/bitstream/handle/176904/6586.4/Kontaktpers-Medizin_Nachverfolg_A3_20-04-22_V8.pdf?sequence=9&isAllowed=y (accessed on 22 April 2021).
- Smith, R.L.; Gibson, L.L.; Martinez, P.P.; Ke, R.; Mirza, A.; Conte, M.; Gallagher, N.; Conte, A.; Wang, L.; Fredrickson, R.; et al. Longitudinal assessment of diagnostic test performance over the course of acute SARS-CoV-2 infection. medRxiv 2021. preprint. [Google Scholar]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]

| Variables Category | Value (Mean Costs in EUR) | Reference |
|---|---|---|
| Costs of POCT antigen test (taking the Sofia SARS Antigen FIA® as example) | 12 | [14] |
| Preanalytics of the POC antigen test and waiting for its result t (30 min) | 14.88 | Calculated from [26] |
| Wage costs per HCW and day | 167.58 | Calculated from [25] |
| Persons Newly Infected (No.) | d2 | d4 | d6 | d8 | d10 | d12 | d14 | Sum after 14 Days |
|---|---|---|---|---|---|---|---|---|
| TP (S) | 5.652 | 24.32 | 19.216 | 6.003 | 5.476 | 2.285 | 0.928 | 63.88 |
| FN (S) | 1.413 | 6.08 | 4.804 | 1.501 | 1.369 | 0.571 | 0.242 | 15.98 |
| TP (NS) | 1.024 | 4.408 | 3.483 | 1.088 | 0.993 | 0.414 | 0.177 | 11.587 |
| FN (NS) | 0.742 | 3.192 | 2.522 | 0.788 | 0.719 | 0.3 | 0.27 | 8.553 |
| total | 8.831 | 38 | 30.025 | 9.38 | 8.557 | 3.57 | 1.637 | 100 |
| (a) Costs at the expense of the employer (hospital) | |
| Costs due to continuation of wage for all symptomatically diseased HCW, starting from the respective day of symptoms until the end of quarantine: * | |
| 7.065 infected HCW on day 2 × EUR 167.58 × 12 remaining days | EUR 14,207.43 |
| 30.4 infected HCW on day 4 × EUR 167.58 × 10 remaining days | EUR 50,944.32 |
| 24.02 infected HCW on day 6 × EUR 167.58 × 8 remaining days | EUR 32,202.17 |
| 7.504 infected HCW on day 8 × EUR 167.58 × 6 remaining days | EUR 7545.12 |
| 6.845 infected HCW on day 10 × EUR 167.58 × 4 remaining days | EUR 4588.34 |
| 2.856 infected HCW on day 12 × EUR 167.58 × 2 remaining days | EUR 957.22 |
| 0.956 infected HCW on day 14 × EUR 167.58 | EUR 196.07 |
| Total cost for 1000 HCW sent into quarantine: EUR 110,640.67 (±20%: EUR 88,512.54 to EUR 132,768.80) | |
| Cost per exposed HCW sent into quarantine from the hospital´s perspective: EUR 110.64 (±20%: EUR 88.51 to EUR 132.77) | |
| (b) Costs at the expense of the taxpayer | |
| Reimbursement of wages to the employer (hospital) for all HCW sent into quarantine as long as no symptoms appear: 1000 HCW × EUR 167.58 × 14 days (EUR 2346,120; ±20%: 1876,896 to EUR 2815,344) minus EUR 110,604.67 (±20%: EUR 88,512.54 to EUR 132,768.80; see Table 3a) | |
| Total cost for 1000 HCW sent into quarantine: EUR 2235,479.33 (±20%: EUR 1744,127.20 to EUR 2726,831.46) | |
| Cost per exposed HCW sent into quarantine from the taxpayer´s perspective: EUR 2235.48 (±20%: EUR 1744.13 to EUR 2726.83) | |
| (a) Costs at the expense of the employer (hospital) | |
| |
| 1000 (on day 2) × EUR 26.88 testing costs | EUR 26,880 |
| 991.911 [1000–8.089] (on day 4) × EUR 26.88 testing costs | EUR 26,662.57 |
| 957.103 [991.911–34.808] (on day 6) × EUR 26.88 testing costs | EUR 25,726.93 |
| 929.6 [957.103–27.503] (on day 8) × EUR 26.88 testing costs | EUR 24,987.65 |
| 921.008 [929.6–8.592] (on day 10) × EUR 26.88 testing costs | EUR 24,756.70 |
| 913.17 [921.008–7.838] (on day 12) × EUR 26.88 testing costs | EUR 24,546.01 |
| 909.9 [913.17–3.27](on day 14) × EUR 26.88 testing costs | EUR 24,458.11 |
| Subtotal costs: EUR 178,017.97 (±20%: EUR 142,414.38 to EUR 213,621.56) | |
| |
| 7.065 infected HCW on day 2 × EUR 167.58 × 12 remaining days | EUR 14,207.43 |
| 30.4 infected HCW on day 4 × EUR 167.58 × 10 remaining days | EUR 50,944.32 |
| 24.02 infected HCW on day 6 × EUR 167.58 × 8 remaining days | EUR 32,202.17 |
| 7.504 infected HCW on day 8 × EUR 167.58 × 6 remaining days | EUR 7545.12 |
| 6.845 infected HCW on day 10 × EUR 167.58 × 4 remaining days | EUR 4588.34 |
| 2.856 infected HCW on day 12 × EUR 167.58 × 2 remaining days | EUR 957.22 |
| 0.956 infected HCW on day 14 × EUR 167.58 | EUR 196.07 |
| Subtotal costs: EUR 110,640.67 (±20%: EUR 88,512.54 to EUR 132,768.80) | |
| Total cost for 1000 HCW sent to work: EUR 288,658.64 (±20%: EUR 230,926.92 to EUR 346,390.36) | |
| Cost per exposed HCW sent to work from the hospital´s perspective: EUR 288.66 (±20%: EUR 230.93 to EUR 346.39) | |
| (b) Costs at the expense of the taxpayer | |
| Costs of sending asymptomatically infected HCW into isolation [TP (NS)] | |
| 1.024 × EUR 167.58 (on day 2) × 12 remaining days | EUR 2059.22 |
| 4.408 × EUR 167.58 (on day 4) × 10 remaining days | EUR 7386.93 |
| 3.483 × EUR 167.58 (on day 6) × 8 remaining days | EUR 4669.45 |
| 1.088 × EUR 167.58 (on day 8) × 6 remaining days | EUR 1093.96 |
| 0.993 × EUR 167.58 (on day 10) × 4 remaining days | EUR 665.63 |
| 0.414 × EUR 167.58 (on day 12) × 2 remaining days | EUR 138.76 |
| 0.177 × EUR 167.58 (on day 14) | EUR 29.66 |
| Total cost for 1000 HCW sent to work: EUR 16,043.61 (±20%: EUR 12,834.89 to EUR 19,252.33) | |
| Cost per exposed HCW sent to work from the taxpayer´s perspective: EUR 16.04 (±20%: 12.83 to EUR 19.25) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diel, R.; Hittel, N.; Nienhaus, A. Point-of-Care COVID-19 Antigen Testing in Exposed German Healthcare Workers—A Cost Model. Int. J. Environ. Res. Public Health 2021, 18, 10767. https://doi.org/10.3390/ijerph182010767
Diel R, Hittel N, Nienhaus A. Point-of-Care COVID-19 Antigen Testing in Exposed German Healthcare Workers—A Cost Model. International Journal of Environmental Research and Public Health. 2021; 18(20):10767. https://doi.org/10.3390/ijerph182010767
Chicago/Turabian StyleDiel, Roland, Norbert Hittel, and Albert Nienhaus. 2021. "Point-of-Care COVID-19 Antigen Testing in Exposed German Healthcare Workers—A Cost Model" International Journal of Environmental Research and Public Health 18, no. 20: 10767. https://doi.org/10.3390/ijerph182010767
APA StyleDiel, R., Hittel, N., & Nienhaus, A. (2021). Point-of-Care COVID-19 Antigen Testing in Exposed German Healthcare Workers—A Cost Model. International Journal of Environmental Research and Public Health, 18(20), 10767. https://doi.org/10.3390/ijerph182010767







