Association between Haematological Parameters and Exposure to a Mixture of Organophosphate and Neonicotinoid Insecticides among Male Farmworkers in Northern Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Questionnaire Data Collection
2.2. Study Area, Study Subjects and Enrolment
2.3. Urine Collection
2.4. Blood Collection
2.5. Urinary OP Metabolite Analysis
2.6. Urinary NEO and Their Metabolite (NEO/m) Analysis
2.7. Haematological Parameter Analysis
2.8. QC and Quality Assurance
2.8.1. Urinary OP Metabolite Analysis
2.8.2. Urinary NEO/m Analysis
2.9. Statistical Analysis
Group2 = (ACE, CLO, THX, IMI, SUF, N-dm-ACE, dm-CLO and Of-IMI)] + βiXi
3. Results
3.1. Sociodemographic Characteristics of the Study Participants
3.2. Urinary DAP and NEO/m Concentrations
3.3. Haematological Parameters among Male Farmworkers
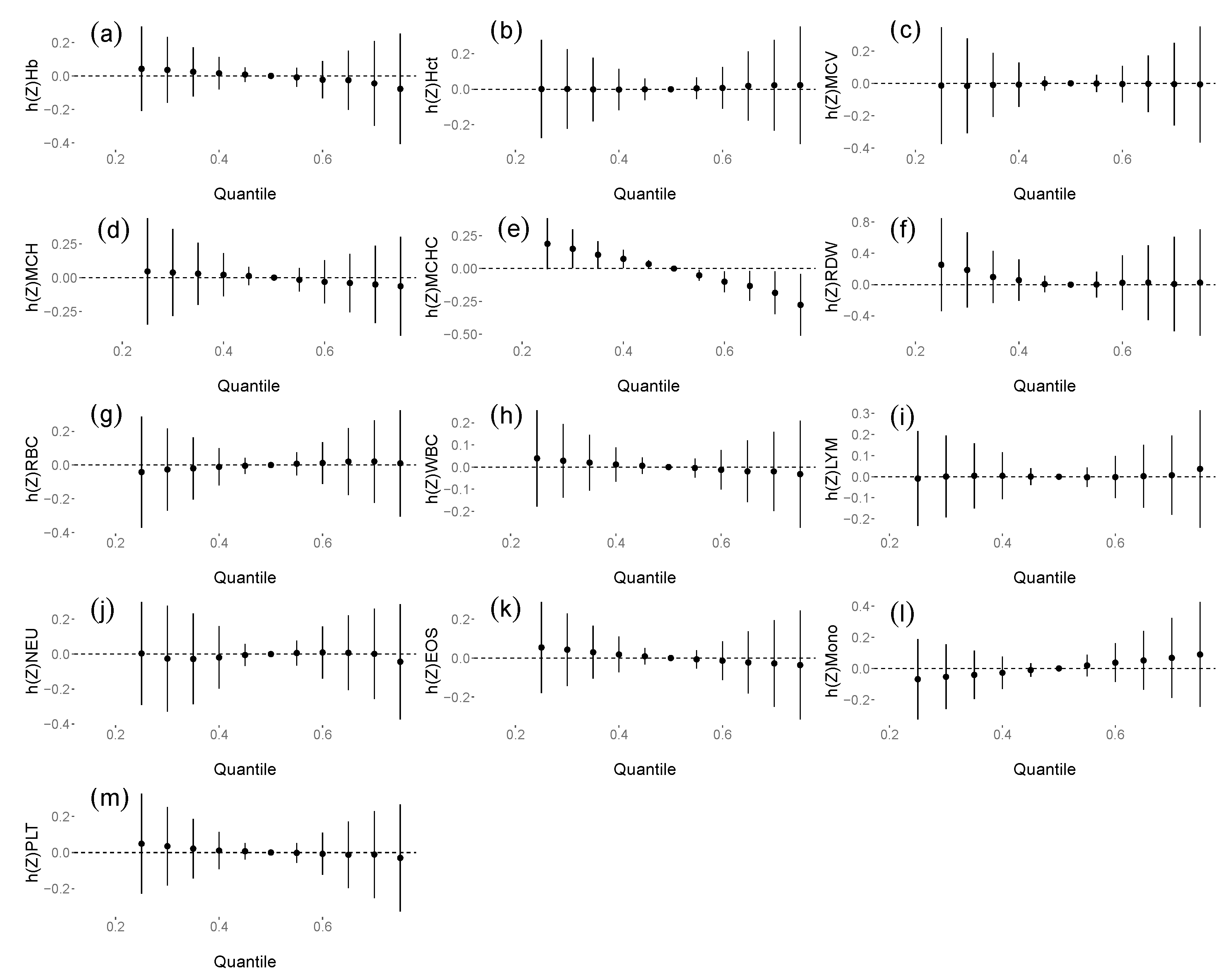
3.4. The Associations between DAP and NEO/m Concentrations and Haematological Parameters
3.5. Sensitivity Analysis
4. Discussion
4.1. Urinary DAP and NEO/m Concentrations
4.2. Associations between Insecticide Exposure and Haematological Parameters
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barr, D.B.; Bravo, R.; Weerasekera, G.; Caltabiano, L.M.; Whitehead, R.D.; Olsson, A.; Caudill, S.P.; Schober, S.E.; Pirkle, J.L.; Sampson, E.J.; et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ. Health Perspect. 2004, 112, 186–200. [Google Scholar] [CrossRef]
- Badr, A.M. Organophosphate toxicity: Updates of malathion potential toxic effects in mammals and potential treatments. Environ. Sci. Pollut. Res. 2020, 27, 26036–26057. [Google Scholar] [CrossRef]
- Ueyama, J.; Saito, I.; Kondo, T.; Taki, T.; Kimata, A.; Saito, S.; Ito, Y.; Murata, K.; Iwata, T.; Gotoh, M.; et al. Urinary concentrations of organophosphorus insecticide metabolites in Japanese workers. Chemosphere 2012, 87, 1403–1409. [Google Scholar] [CrossRef]
- Hongsibsong, S.; Prapamontol, T.; Dong, J.X.; Bever, C.S.; Xu, Z.L.; Gee, S.J.; Hammock, B.D. Exposure of consumers and farmers to organophosphate and synthetic pyrethroid insecticides in Northern Thailand. J. Verbrauch. Leb. 2019, 14, 17–23. [Google Scholar] [CrossRef]
- Gillezeau, C.; Alpert, N.; Joshi, P.; Taioli, E. Urinary dialkylphosphate metabolite levels in US adults—National health and nutrition examination survey 1999–2008. Int. J. Environ. Res. Public Health 2019, 16, 4605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, D.B.; Needham, L.L. Analytical methods for biological monitoring of exposure to pesticides: A review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 778, 5–29. [Google Scholar] [CrossRef]
- Nishihama, Y.; Nakayama, S.F.; Isobe, T.; Jung, C.; Iwai-shimada, M. Urinary Metabolites of Organophosphate Pesticides among Pregnant Women Participating in the Japan Environment and Children ’ s Study ( JECS ). Int. J. Environ. Res. Public Health 2021, 18, 5929. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Hongsibsong, S.; Kerdnoi, T. Urinary dialkylphosphate metabolites and health symptoms among farmers in Thailand. Arch. Environ. Occup. Heal. 2017, 72, 145–152. [Google Scholar] [CrossRef]
- Sun, H.; Sun, M.L.; Barr, D.B. Exposure to organophosphorus insecticides and increased risks of health and cancer in US women. Environ. Toxicol. Pharmacol. 2020, 80, 103474. [Google Scholar] [CrossRef]
- Hongsibsong, S.; Sittitoon, N.; Sapbamrer, R. Association of health symptoms with low-level exposure to organophosphates, DNA damage, AChE activity, and occupational knowledge and practice among rice, corn, and double-crop farmers. J. Occup. Health 2017, 59, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Sekhotha, M.M.; Monyeki, K.D.; Sibuyi, M.E. Exposure to agrochemicals and cardiovascular disease: A review. Int. J. Environ. Res. Public Health 2016, 13, 229. [Google Scholar] [CrossRef] [Green Version]
- Yaqub, S.A.; Rahamon, S.K.; Arinola, O.G. Haematological and Immunological Indices in Nigerian Farmworkers Occupationally Exposed to Organophosphate Pesticides. Electron. J. Gen. Med. 2014, 11, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhang, C.; Hu, R.; Li, Y.; Yin, Y.; Chen, Z.; Cai, J.; Cui, F. Association between occupational exposures to pesticides with heterogeneous chemical structures and farmer health in China. Sci. Rep. 2016, 6, 25190. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Tao, Y.; Phung, D.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Liu, Q.; He, M.; Pan, X.; Li, R.; et al. Urinary monitoring of neonicotinoid imidacloprid exposure to pesticide applicators. Sci. Total Environ. 2019, 669, 721–728. [Google Scholar] [CrossRef]
- Suwannarin, N.; Prapamontol, T.; Isobe, T.; Nishihama, Y.; Nakayama, S.F. Characteristics of exposure of reproductive-age farmworkers in chiang mai province, thailand, to organophosphate and neonicotinoid insecticides: A pilot study. Int. J. Environ. Res. Public Health 2020, 17, 7871. [Google Scholar] [CrossRef]
- Ospina, M.; Wong, L.Y.; Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Calafat, A.M. Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015–2016 national health and nutrition examination survey. Environ. Res. 2019, 176, 108555. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Mahai, G.; Wan, Y.; Yang, Z.; He, Z.; Xu, S.; Xia, W. Assessment of imidacloprid related exposure using imidacloprid-olefin and desnitro-imidacloprid: Neonicotinoid insecticides in human urine in Wuhan, China. Environ. Int. 2020, 141, 105785. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Beck, M.E. Nicotinic acetylcholine receptor agonists: A milestone for modern crop protection. Angew. Chem.-Int. Ed. 2013, 52, 9464–9485. [Google Scholar] [CrossRef]
- Song, S.; Zhang, T.; Huang, Y.; Zhang, B.; Guo, Y.; He, Y.; Huang, X.; Bai, X.; Kannan, K. Urinary Metabolites of Neonicotinoid Insecticides: Levels and Recommendations for Future Biomonitoring Studies in China. Environ. Sci. Technol. 2020, 54, 8210–8220. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, D.; Fang, H.; Han, M.; Tang, C.; Wu, J.; Chen, Y.; Jiang, Q. Predictors, sources, and health risk of exposure to neonicotinoids in Chinese school children: A biomonitoring-based study. Environ. Int. 2020, 143, 105918. [Google Scholar] [CrossRef]
- Harada, K.H.; Tanaka, K.; Sakamoto, H.; Imanaka, M.; Niisoe, T.; Hitomi, T.; Kobayashi, H.; Okuda, H.; Inoue, S.; Kusakawa, K.; et al. Biological Monitoring of human exposure to neonicotinoids using urine samples, and neonicotinoid excretion kinetics. PLoS ONE 2016, 11, e0146335. [Google Scholar] [CrossRef] [Green Version]
- Gavel, M.J.; Richardson, S.D.; Dalton, R.L.; Soos, C.; Ashby, B.; McPhee, L.; Forbes, M.R.; Robinson, S.A. Effects of 2 Neonicotinoid Insecticides on Blood Cell Profiles and Corticosterone Concentrations of Wood Frogs (Lithobates sylvaticus). Environ. Toxicol. Chem. 2019, 38, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Américo-Pinheiro, J.H.P.; da Cruz, C.; Aguiar, M.M.; Torres, N.H.; Ferreira, L.F.R.; Machado-Neto, J.G. Sublethal Effects of Imidacloprid in Hematological Parameters of Tilapia (Oreochromis niloticus). Water Air Soil Pollut. 2019, 230, 193. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ji, L.; Hu, Y.; Zhang, J.; Wang, C.; Ding, G.; Chen, L.; Kamijima, M.; Ueyama, J.; et al. Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ. Int. 2017, 108, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kongtip, P.; Techasaensiri, B.; Nankongnab, N.; Adams, J.; Phamonphon, A.; Surach, A.; Sangprasert, S.; Thongsuksai, A.; Srikumpol, P.; Woskie, S. The impact of prenatal organophosphate pesticide exposures on thai infant neurodevelopment. Int. J. Environ. Res. Public Health 2017, 14, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedler, N.; Rohitrattana, J.; Siriwong, W.; Suttiwan, P.; Ohman Strickland, P.; Ryan, P.B.; Rohlman, D.S.; Panuwet, P.; Barr, D.B.; Robson, M.G. Neurobehavioral effects of exposure to organophosphates and pyrethroid pesticides among Thai children. Neurotoxicology 2015, 48, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Rappazzo, K.; Warren, J.; Meyer, R.; Herring, A.; Sanders, A.; Brownstein, N.C.; Luben, T.J. Maternal Residential Exposure to Agricultural Pesticides and Birth Defects in a 2003-2005 North Carolina Birth Cohort. Birth Defects Res. Part A Clin. Mol. Teratol. 2017, 98, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of neonicotinoid pesticide exposure on human health: A systematic review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, G.; Kuribayashi, R.; Ikenaka, Y.; Ichise, T.; Nakayama, S.M.M.; Ishizuka, M.; Taira, K.; Fujioka, K.; Sairenchi, T.; Kobashi, G.; et al. LC-ESI/MS/MS analysis of neonicotinoids in urine of very low birth weight infants at birth. PLoS ONE 2019, 14, e0219208. [Google Scholar] [CrossRef]
- Cremonese, C.; Piccoli, C.; Pasqualotto, F.; Clapauch, R.; Koifman, R.J.; Koifman, S.; Freire, C. Occupational exposure to pesticides, reproductive hormone levels and sperm quality in young Brazilian men. Reprod. Toxicol. 2017, 67, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Contreras, L.; Gómez-Pérez, R.; Rojas, G.; Cruz, I.; Berrueta, L.; Salmen, S.; Colmenares, M.; Barreto, S.; Balza, A.; Zavala, L.; et al. Occupational exposure to organophosphate and carbamate pesticides affects sperm chromatin integrity and reproductive hormone levels among venezuelan farm workers. J. Occup. Health 2013, 55, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio, R.; Ocampo-Gómez, G.; Morán-Martínez, J.; Borja-Aburto, V.; López-Cervantes, M.; Uribe, M.; Torres-Sánchez, L.; Cebrián, M.E. Pesticide exposure alters follicle-stimulating hormone levels in Mexican agricultural workers. Environ. Health Perspect. 2005, 113, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; Cremonese, C.; Koifman, R.; Koifman, S.; Freire, C. Occupational exposure to pesticides and hematological alterations: A survey of farm residents in the south of brazil. Cienc. Saude Coletiva 2019, 24, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.S.; Karunamoorthy, P.; Kondhalkar, S.J.; Ambikapathy, M.; Beerappa, R. Assessment of hematological, biochemical effects and genotoxicity among pesticide sprayers in grape garden. J. Occup. Med. Toxicol. 2015, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Manyilizu, W.B.; Mdegela, R.H.; Kazwala, R.; Nonga, H.; Muller, M.; Lie, E.; Skjerve, E.; Lyche, J.L. Association of long-term pesticide exposure and biologic parameters in female farm workers in Tanzania: A cross sectional study. Toxics 2016, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Shearer, J.J.; Beane Freeman, L.E.; Liu, D.; Andreotti, G.; Hamilton, J.; Happel, J.; Lynch, C.F.; Alavanja, M.C.; Hofmann, J.N. Longitudinal investigation of haematological alterations among permethrin-exposed pesticide applicators in the Biomarkers of Exposure and Effect in Agriculture study. Occup. Environ. Med. 2019, 76, 467–470. [Google Scholar] [CrossRef]
- Kwanhian, W.; Yimthiang, S.; Jawjit, S.; Mahaboon, J.; Thirarattanasunthon, P.; Vattanasit, U. Hematological indices of pesticide exposure on rice farmers in Southern Thailand. Kesmas 2019, 14, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, A.N.; Prapamontol, T.; Narksen, W.; Srinual, N.; Barr, D.B.; Riederer, A.M. Pilot study of pesticide knowledge, attitudes, and practices among pregnant women in northern Thailand. Int. J. Environ. Res. Public Health 2012, 9, 3365–3383. [Google Scholar] [CrossRef] [Green Version]
- Taneepanichskul, N.; Norkaew, S.; Siriwong, W.; Robson, M.G. Pesticide application and safety behaviour among male and female chili-growings farmers in Hua Rua Sub-District. J. Health Res. 2012, 26, 193–197. [Google Scholar]
- Panuwet, P.; Prapamontol, T.; Chantara, S.; Thavornyuthikarn, P.; Montesano, M.A.; Whitehead, R.D.; Barr, D.B. Concentrations of urinary pesticide metabolites in small-scale farmers in Chiang Mai Province, Thailand. Sci. Total Environ. 2008, 407, 655–668. [Google Scholar] [CrossRef]
- Sapbamrer, R. Pesticide Use, Poisoning, and Knowledge and Unsafe Occupational Practices in Thailand. New Solut. 2018, 28, 283–302. [Google Scholar] [CrossRef]
- Suwannarin, N.; Prapamontol, T.; Isobe, T.; Nishihama, Y.; Hashimoto, Y.; Mangklabruks, A.; Pantasri, T.; Chantara, S.; Naksen, W.; Nakayama, S.F. Exposure to Organophosphate and Neonicotinoid Insecticides and Its Association with Steroid Hormones among Male Reproductive-Age Farmworkers in Northern Thailand. Int. J. Environ. Res. Public Health 2021, 18, 5599. [Google Scholar] [CrossRef] [PubMed]
- Aroonvilairat, S.; Kespichayawattana, W.; Sornprachum, T.; Chaisuriya, P.; Siwadune, T.; Ratanabanangkoon, K. Effect of pesticide exposure on immunological, hematological and biochemical parameters in thai orchid farmers—A cross-sectional study. Int. J. Environ. Res. Public Health 2015, 12, 5846–5861. [Google Scholar] [CrossRef] [Green Version]
- Prapamontol, T.; Sutan, K.; Laoyang, S.; Hongsibsong, S.; Lee, G.; Yano, Y.; Hunter, R.E.; Ryan, P.B.; Barr, D.B.; Panuwet, P. Cross validation of gas chromatography-flame photometric detection and gas chromatography-mass spectrometry methods for measuring dialkylphosphate metabolites of organophosphate pesticides in human urine. Int. J. Hyg. Environ. Health 2014, 217, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Just, A.C.; Adibi, J.J.; Rundle, A.G.; Calafat, A.M.; Camann, D.E.; Hauser, R.; Silva, M.J.; Whyatt, R.M. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2014, 16, 493–508. [Google Scholar] [CrossRef]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health A Glob. Access Sci. Source 2018, 17, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Osaka, A.; Ueyama, J.; Kondo, T.; Nomura, H.; Sugiura, Y.; Saito, I.; Nakane, K.; Takaishi, A.; Ogi, H.; Wakusawa, S.; et al. Exposure characterization of three major insecticide lines in urine of young children in Japan-neonicotinoids, organophosphates, and pyrethroids. Environ. Res. 2016, 147, 89–96. [Google Scholar] [CrossRef]
- Oya, N.; Ito, Y.; Ebara, T.; Kato, S.; Hioki, K.; Aoi, A.; Ueyama, J.; Oguri, T.; Shoji, N.; Sugiura-Ogasawara, M.; et al. Exposure levels of organophosphate pesticides in Japanese diapered children: Contributions of exposure-related behaviors and mothers’ considerations of food selection and preparation. Environ. Int. 2020, 134, 105294. [Google Scholar] [CrossRef]
- van den Dries, M.A.; Pronk, A.; Guxens, M.; Spaan, S.; Voortman, T.; Jaddoe, V.W.; Jusko, T.A.; Longnecker, M.P.; Tiemeier, H. Determinants of organophosphate pesticide exposure in pregnant women: A population-based cohort study in the Netherlands. Int. J. Hyg. Environ. Health 2018, 221, 489–501. [Google Scholar] [CrossRef]
- Ueyama, J.; Nomura, H.; Kondo, T.; Saito, I.; Ito, Y.; Osaka, A.; Kamijima, M. Biological monitoring method for urinary neonicotinoid insecticides using LC-MS/MS and its application to Japanese adults. J. Occup. Health 2014, 56, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Marfo, J.T.; Fujioka, K.; Ikenaka, Y.; Nakayama, S.M.M.; Mizukawa, H.; Aoyama, Y.; Ishizuka, M.; Taira, K. Relationship between urinary n-desmethyl-acetamiprid and typical symptoms including neurological findings: A prevalence case-control study. PLoS ONE 2015, 10, e0142172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueyama, J.; Harada, K.H.; Koizumi, A.; Sugiura, Y.; Kondo, T.; Saito, I.; Kamijima, M. Temporal Levels of Urinary Neonicotinoid and Dialkylphosphate Concentrations in Japanese Women between 1994 and 2011. Environ. Sci. Technol. 2015, 49, 14522–14528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, T.; Liu, F.; Zhang, J.; Wu, Y.; Sun, H. Occurrence and Profile Characteristics of the Pesticide Imidacloprid, Preservative Parabens, and Their Metabolites in Human Urine from Rural and Urban China. Environ. Sci. Technol. 2015, 49, 14633–14640. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Robinson, M.; Kannan, K. A simple method for the analysis of neonicotinoids and their metabolites in human urine. Environ. Chem. 2019, 16, 171–178. [Google Scholar] [CrossRef]
- Li, A.J.; Martinez-Moral, M.P.; Kannan, K. Variability in urinary neonicotinoid concentrations in single-spot and first-morning void and its association with oxidative stress markers. Environ. Int. 2020, 135, 105415. [Google Scholar] [CrossRef]
- Zhang, T.; Song, S.; Bai, X.; He, Y.; Zhang, B.; Gui, M.; Kannan, K.; Lu, S.; Huang, Y.; Sun, H. A nationwide survey of urinary concentrations of neonicotinoid insecticides in China. Environ. Int. 2019, 132, 105114. [Google Scholar] [CrossRef]
- Del Prado-Lu, J.L. Pesticide exposure, risk factors and health problems among cutflower farmers: A cross sectional study. J. Occup. Med. Toxicol. 2007, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Iza, S.C.; Rodríguez, A.I.; Prieto-Suarez, E. Assessment of hematological parameters in workers exposed to organophosphorus pesticides, carbamates and pyrethroids in Cundinamarca 2016-2017. Rev. Salud Publica 2017, 19, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Fareed, M.; Pathak, M.K.; Bihari, V.; Kamal, R.; Srivastava, A.K.; Kesavachandran, C.N. Adverse Respiratory Health and Hematological Alterations among Agricultural Workers Occupationally Exposed to Organophosphate Pesticides: A Cross-Sectional Study in North India. PLoS ONE 2013, 8, e69755. [Google Scholar] [CrossRef]
- Nassar, A.M.K.; Salim, Y.M.; Malhat, F.M. Assessment of pesticide residues in human blood and effects of occupational exposure on hematological and hormonal qualities. Pak. J. Biol. Sci. 2016, 19, 95–105. [Google Scholar] [CrossRef]
- Suwannahong, K.; Sridon, A.; Pongstaporn, W.; Thongmuang, P.; Sudjaroen, Y. Biochemical and Hematological Status in Rice farmers with Chronic Pesticide Exposure, Suphan Buri, Thailand. Indian J. Public Health Res. Dev. 2020, 11, 1285–1290. [Google Scholar]
- Emam, S.J.; Salehcheh, M.; Haghighizadeh, M.H.; Jazayeri, S.M.H.M. Occupational exposure to pesticides among farmers. Pak. J. Med. Sci. 2012, 28, 120–123. [Google Scholar] [CrossRef]
- Dalbó, J.; Filgueiras, L.A.; Mendes, A.N. Effects of pesticides on rural workers: Haematological parameters and symptomalogical reports. Cien. Saude Colet. 2019, 24, 2569–2582. [Google Scholar] [CrossRef] [Green Version]
- Socolovsky, M.; Koulnis, M.; Porpiglia, E.; Hidalgo, D. Erythropoiesis: From molecular pathways to system properties. Adv. Exp. Med. Biol. 2014, 844, 37–58. [Google Scholar] [CrossRef]
- Sato, K.; Inoue, S.; Ishibashi, Y.; Ota, T.; Murano, H.; Furuyama, K.; Yang, S.; Machida, H.; Nakano, H.; Sato, M.; et al. Association between low mean corpuscular hemoglobin and prognosis in patients with exacerbation of chronic obstructive pulmonary disease. Respir. Investig. 2021, 59, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Kumar M, S.; Sanjeevi, G.; Narayanan, B.; Kurien, A.A. Thiamethoxam, a Neonicotinoid Poisoning Causing Acute Kidney Injury via a Novel Mechanism. Kidney Int. Rep. 2020, 5, 1111–1113. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.R.; Je, J.; Jeong, K.; Kim, S.; Kim, H.J.; Chang, K.C.; Park, S.W. The proximal tubular α7 nicotinic acetylcholine receptor attenuates ischemic acute kidney injury through Akt/PKC signaling-mediated HO-1 induction. Exp. Mol. Med. 2018, 50, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. WORLDWIDE INTEGRATED ASSESSMENT OF THE IMPACT OF SYSTEMIC PESTICIDES ON BIODIVERSITY AND ECOSYSTEMS Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Hussain, R.; Noreen, S.; Abbas, G.; Chodhary, I.R.; Khan, A.; Ahmed, Z.; Khan, M.K.; Akram, K.; Ulhaq, M.; et al. Dose and time-related pathological and genotoxic studies on thiamethoxam in fresh water fish (Labeo rohita) in Pakistan. Pak. Vet. J. 2020, 40, 151–156. [Google Scholar] [CrossRef]
- Kataria, S.K.; Chhillar, A.K.; Kumar, A.; Tomar, M.; Malik, V. Cytogenetic and hematological alterations induced by acute oral exposure of imidacloprid in female mice. Drug Chem. Toxicol. 2016, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, S.; Ezzi, L.; Grissa, I.; Kerkeni, E.; Neffati, F.; Bhouri, R.; sallem, A.; Najjar, M.F.; Hassine, M.; Mehdi, M.; et al. Hematological, biochemical, and toxicopathic effects of subchronic acetamiprid toxicity in Wistar rats. Environ. Sci. Pollut. Res. 2016, 23, 25191–25199. [Google Scholar] [CrossRef] [PubMed]
- Apidechkul, T.; Yeemard, F.; Chomchoei, C.; Upala, P.; Tamornpark, R. Epidemiology of thalassemia among the hill tribe population in Thailand. PLoS ONE 2021, 16, e0246736. [Google Scholar] [CrossRef] [PubMed]
- Fucharoen, S.; Winichagoon, P. Thalassemia in Southeast Asia: Problems and strategy for prevention and control. Southeast Asian J. Trop. Med. Public Health 1992, 23, 647–655. [Google Scholar] [PubMed]
- Spaan, S.; Pronk, A.; Koch, H.M.; Jusko, T.A.; Jaddoe, V.W.V.; Shaw, P.A.; Tiemeier, H.M.; Hofman, A.; Pierik, F.H.; Longnecker, M.P. Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R Study. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 286–294. [Google Scholar] [CrossRef] [Green Version]
- González-alzaga, B.; Romero-molina, D.; López-flores, I.; Giménez-asensio, M.J. Urinary levels of organophosphate pesticides and predictors of exposure in pre-school and school children living in agricultural and urban communities from south Spain. Environ. Res. 2020, 186, 109459. [Google Scholar] [CrossRef]
- Binter, A.C.; Bannier, E.; Saint-Amour, D.; Simon, G.; Barillot, C.; Monfort, C.; Cordier, S.; Pelé, F.; Chevrier, C. Exposure of pregnant women to organophosphate insecticides and child motor inhibition at the age of 10–12 years evaluated by fMRI. Environ. Res. 2020, 188, 109859. [Google Scholar] [CrossRef]
- Hu, P.; Vinturache, A.; Li, H.; Tian, Y.; Yuan, L.; Cai, C.; Lu, M.; Zhao, J.; Zhang, Q.; Gao, Y.; et al. Urinary organophosphate metabolite concentrations and pregnancy outcomes among women conceiving through in vitro fertilization in Shanghai, China. Environ. Health Perspect. 2020, 128, 097007. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Chang, C.H.; Lou, J.L.; Zhao, M.R.; Lu, C. Potential human exposures to neonicotinoid insecticides: A review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wafa, T.; Nadia, K.; Amel, N.; Ikbal, C.; Insaf, T.; Asma, K.; Hedi, M.A.; Mohamed, H. Oxidative stress, hematological and biochemical alterations in farmers exposed to pesticides. J. Environ. Sci. Health -Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Cestonaro, L.V.; Garcia, S.C.; Nascimento, S.; Gauer, B.; Sauer, E.; Göethel, G.; Peruzzi, C.; Nardi, J.; Fão, N.; Piton, Y.; et al. Biochemical, hematological and immunological parameters and relationship with occupational exposure to pesticides and metals. Environ. Sci. Pollut. Res. 2020, 27, 29291–29302. [Google Scholar] [CrossRef] [PubMed]
| Compound (ng/mL) | MDL | >MDL (%) | GM (GSD) | Range |
|---|---|---|---|---|
| DAPs: | ||||
| DMP | 5.0 | 28.7 | − | <MDL–73.4 |
| DMTP | 1.0 | 44.8 | − | <MDL–134 |
| DMDTP | 0.5 | 25.2 | − | <MDL–398 |
| DEP | 1.0 | 100.0 | 20.7 (4.5) | <MDL–5678 |
| DETP | 0.125 | 99.3 | 23.9 (4.2) | <MDL–445 |
| DEDTP | 0.25 | 79.0 | 9.3 (6.1) | <MDL–386 |
| NEO/m: | ||||
| ACE | 0.0011 | 46.9 | − | <MDL–1.5 |
| CLO | 0.0009 | 96.5 | 7.4 (3.6) | 0.01–14.6 |
| DIN | 0.003 | 16.1 | − | <MDL–1.0 |
| FLN | 0.003 | 16.8 | − | 0.004–0.02 |
| IMI | 0.007 | 99.3 | 17.4 (5.5) | 0.02–24.6 |
| NIT | 0.009 | 3.5 | − | <MDL–0.3 |
| THI | 0.007 | 4.9 | − | <MDL–0.009 |
| THX | 0.002 | 97.2 | 9.1 (4.9) | 0.004–75.8 |
| SUF | 0.002 | 35.7 | − | <MDL–0.3 |
| dm-CLO | 0.01 | 29.4 | − | <MDL–4.1 |
| N-dm-ACE | 0.007 | 99.3 | 15.8 (3.7) | <MDL–18.1 |
| Of-IMI | 0.03 | 64.3 | 5.1 (5.3) | 0.12–253 |
| THI-AM | 0.2 | 0.7 | − | <MDL–0.04 |
| Haematological Parameter | Mean ± SD | Min–Max | n (%) Normal | n (%) below the Range | n (%) above the Range |
|---|---|---|---|---|---|
| Hb (g/dL) | 9.8–17.8 | 119 (83.2) | 24 (16.8) | 0 (0) | |
| Hct (%) | 46.2 ± 3.7 | 32.1–53.6 | 122 (85.3) | 2 (1.4) | 19 (13.2) |
| MCV (fL) | 87.4 ± 8.0 | 53.5–107.0 | 119 (83.2) | 20 (13.4) | 4 (2.8) |
| MCH (pg) | 28.6 ± 3.0 | 16.5–35.2 | 95 (66.4) | 48 (33.6) | 0 (0) |
| MCHC (g/dL) | 32.7 ± 1.0 | 29.8–34.8 | 136 (95.1) | 7 (4.9) | 0 (0) |
| RDW (%) | 12.0 ± 1.2 | 1.9–17.2 | 141 (98.6) | 1 (0.7) | 1 (0.7) |
| RBC count (×106/µL) | 5.3 ± 0.5 | 3.9–7.3 | 124 (86.7) | 6 (4.2) | 13 (9.1) |
| WBC count (cells/µL) | 6300 ± 1710 | 2500–12,700 | 107 (74.8) | 32 (22.3) | 4 (2.8) |
| NEU (%) | 60.4 ± 5.0 | 48.0–69.0 | 143 (100) | 0 (0) | 0 (0) |
| LYM (%) | 37.2 ± 5.5 | 12.0–51.0 | 135 (94.4) | 1 (0.7) | 7 (4.9) |
| EOS (%) | 1.0 ± 0.9 | 0–6.0 | 143 (100) | 0 (0) | 0 (0) |
| Mono (%) | 1.1 ± 0.8 | 0–4.0 | 143 (100) | 0 (0) | 0 (0) |
| PLT count (cells/µL) | 229,000 ± 67,300 | 47,000–399,000 | 130 (90.9) | 13 (9.1) | 0 (0) |
| Variable | Group | GroupPIP | CondPIP |
|---|---|---|---|
| DAPs: | |||
| DMP | 1 | 0.241 | 0.256 |
| DMTP | 1 | 0.241 | 0.043 |
| DMDTP | 1 | 0.241 | 0.122 |
| DEP | 1 | 0.241 | 0.081 |
| DETP | 1 | 0.241 | 0.258 |
| DEDTP | 1 | 0.241 | 0.239 |
| NEO/m: | |||
| ACE | 2 | 0.996 | 0.000 |
| THX | 2 | 0.996 | 0.024 |
| CLO | 2 | 0.996 | 0.936 |
| IMI | 2 | 0.996 | 0.000 |
| SUF | 2 | 0.996 | 0.016 |
| N-dm-ACE | 2 | 0.996 | 0.000 |
| dm-CLO | 2 | 0.996 | 0.023 |
| Of-IMI | 2 | 0.996 | 0.000 |
| Dependent Variable | Independent Variable | Coefficient B (95% CI) | Conditional R2 |
|---|---|---|---|
| MCHC | CLO | −0.43 (−0.7, −0.16) | 0.463 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannarin, N.; Prapamontol, T.; Isobe, T.; Nishihama, Y.; Mangklabruks, A.; Pantasri, T.; Chantara, S.; Naksen, W.; Nakayama, S.F. Association between Haematological Parameters and Exposure to a Mixture of Organophosphate and Neonicotinoid Insecticides among Male Farmworkers in Northern Thailand. Int. J. Environ. Res. Public Health 2021, 18, 10849. https://doi.org/10.3390/ijerph182010849
Suwannarin N, Prapamontol T, Isobe T, Nishihama Y, Mangklabruks A, Pantasri T, Chantara S, Naksen W, Nakayama SF. Association between Haematological Parameters and Exposure to a Mixture of Organophosphate and Neonicotinoid Insecticides among Male Farmworkers in Northern Thailand. International Journal of Environmental Research and Public Health. 2021; 18(20):10849. https://doi.org/10.3390/ijerph182010849
Chicago/Turabian StyleSuwannarin, Neeranuch, Tippawan Prapamontol, Tomohiko Isobe, Yukiko Nishihama, Ampica Mangklabruks, Tawiwan Pantasri, Somporn Chantara, Warangkana Naksen, and Shoji F. Nakayama. 2021. "Association between Haematological Parameters and Exposure to a Mixture of Organophosphate and Neonicotinoid Insecticides among Male Farmworkers in Northern Thailand" International Journal of Environmental Research and Public Health 18, no. 20: 10849. https://doi.org/10.3390/ijerph182010849
APA StyleSuwannarin, N., Prapamontol, T., Isobe, T., Nishihama, Y., Mangklabruks, A., Pantasri, T., Chantara, S., Naksen, W., & Nakayama, S. F. (2021). Association between Haematological Parameters and Exposure to a Mixture of Organophosphate and Neonicotinoid Insecticides among Male Farmworkers in Northern Thailand. International Journal of Environmental Research and Public Health, 18(20), 10849. https://doi.org/10.3390/ijerph182010849










