Air Quality in a Dental Clinic during Er:YAG Laser Usage for Cavity Preparation on Human Teeth—An Ex-Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.2. Experiment 2
2.3. Instrumentation and Experimental Set Up
3. Results
3.1. Experiment1 (Baseline Measurements)
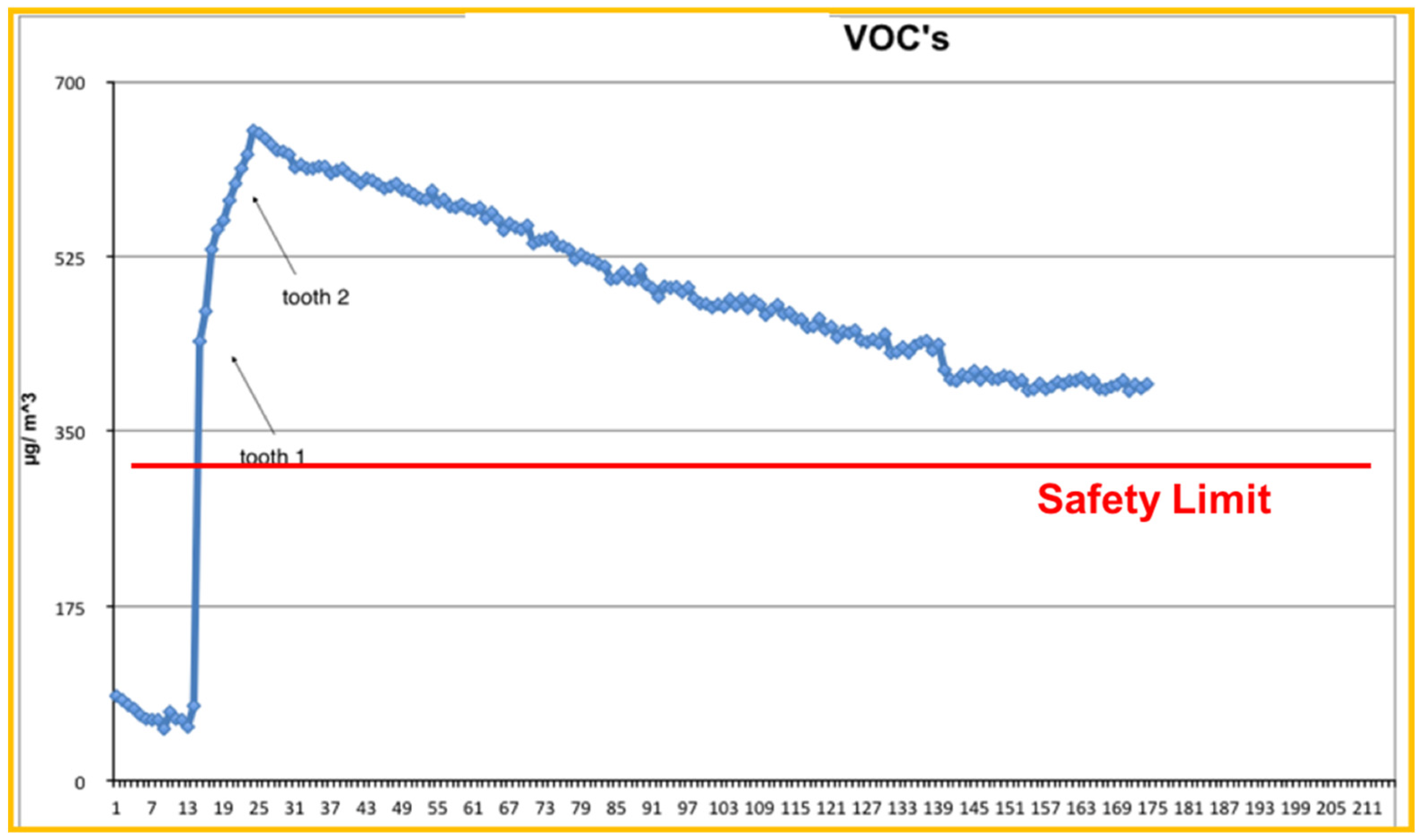
3.1.1. Particulate Matter
3.1.2. Total VOCs
3.2. Experiment 2
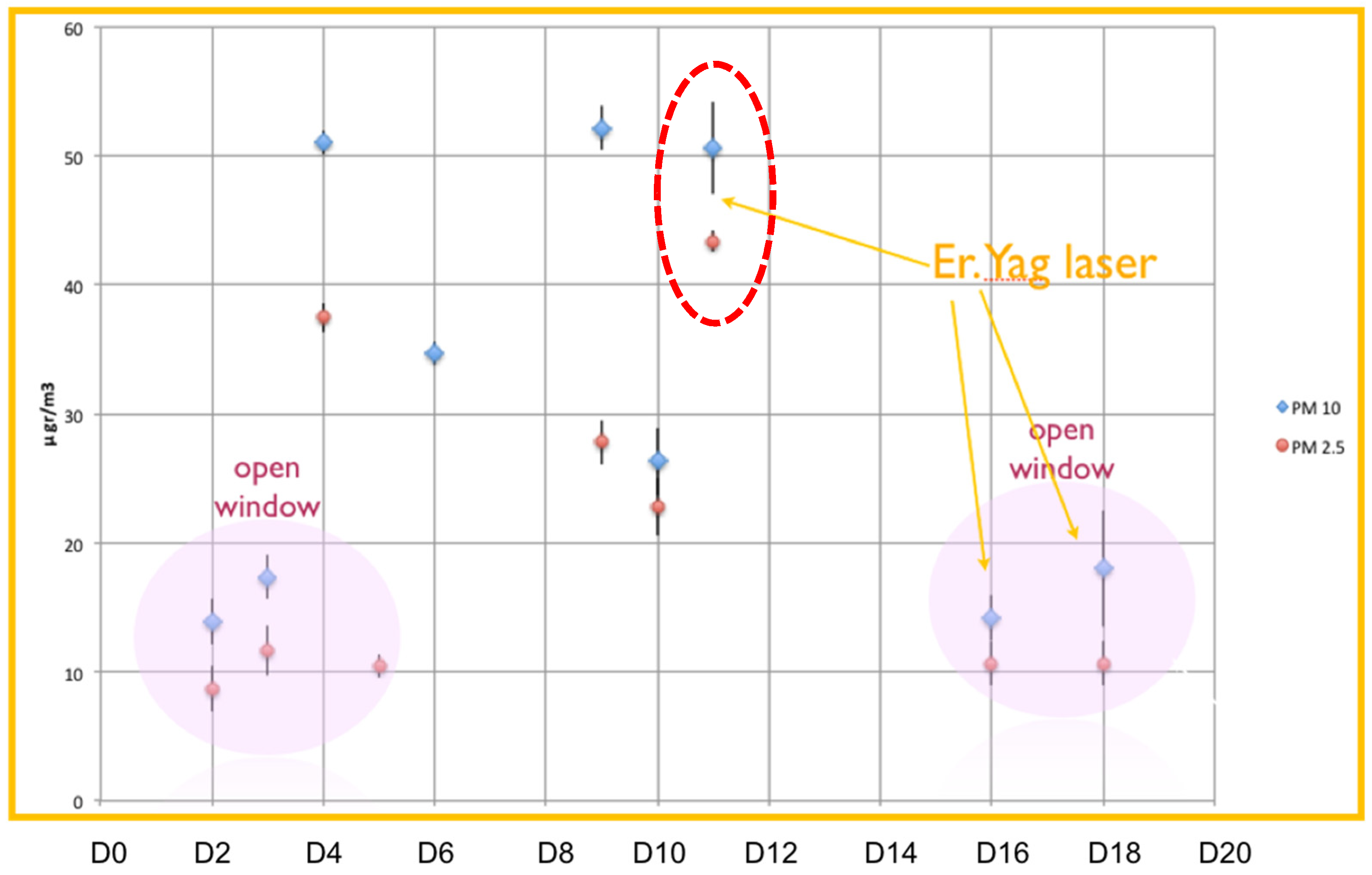
3.2.1. Particulate Matter
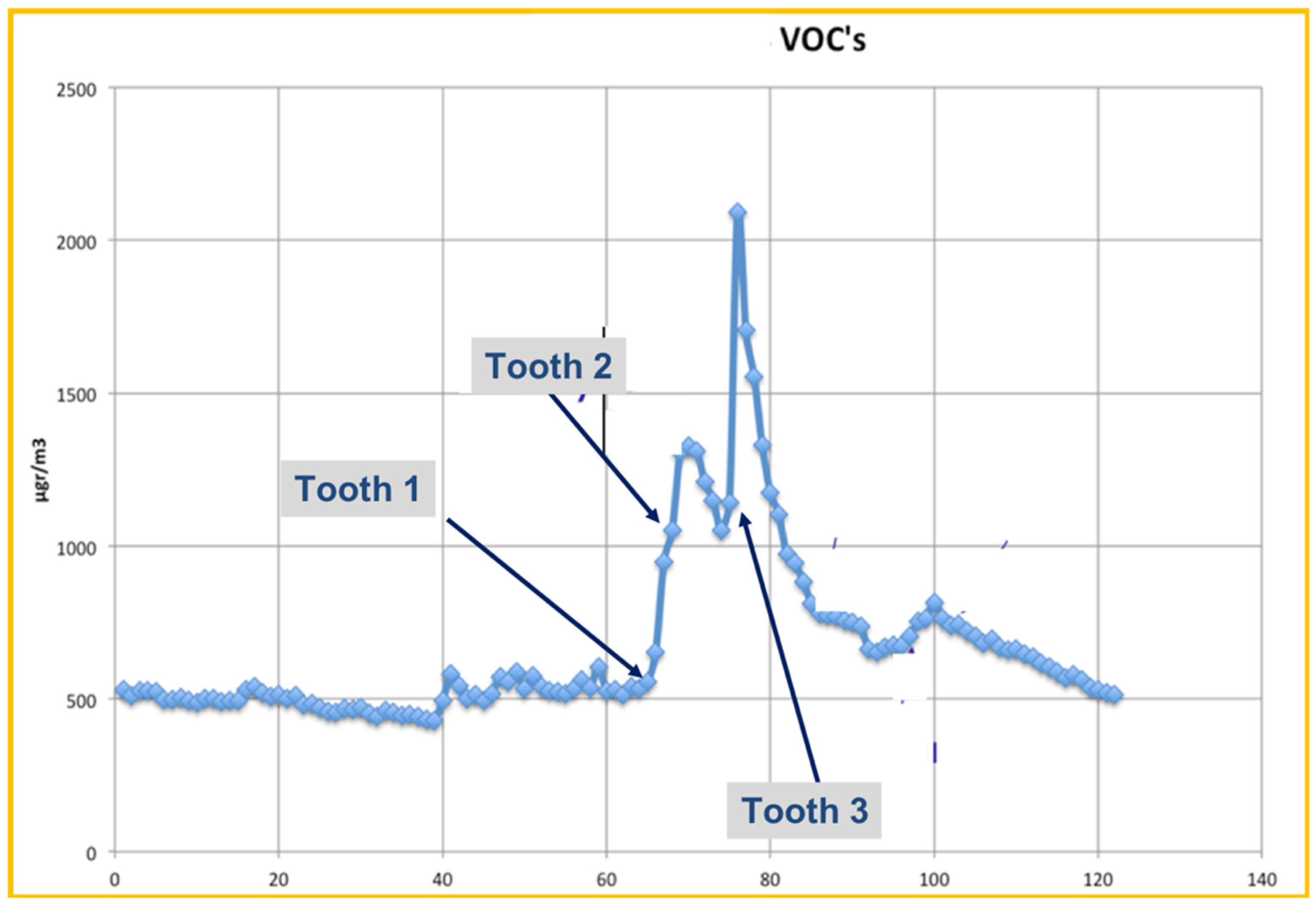
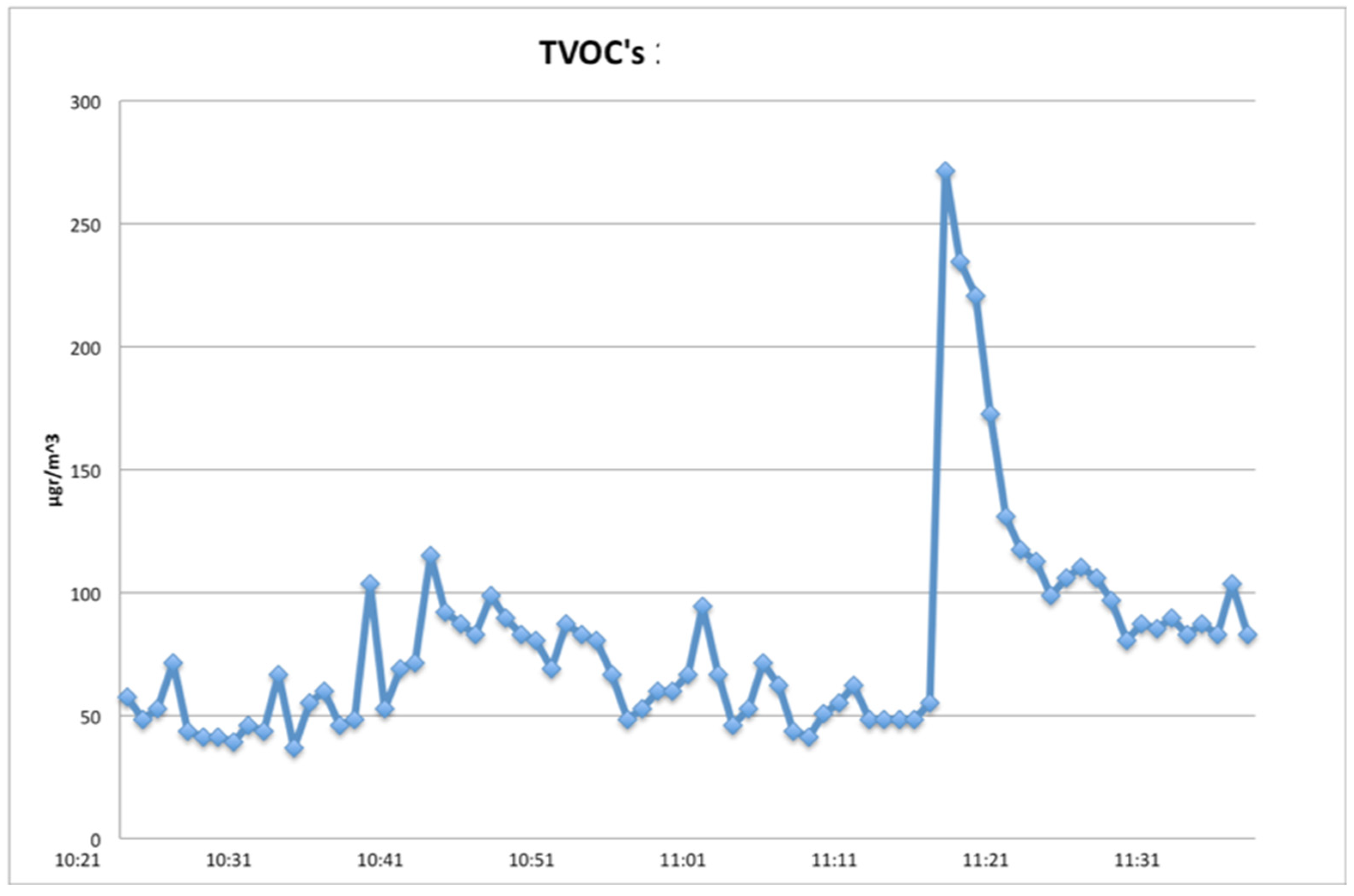
3.2.2. Total VOCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helmis, C.G.; Tzoutzas, J.; Flocas, H.A.; Halios, C.H.; Stathopoulou, O.I.; Assimakopoulos, V.D.; Panis, V.; Apostolatou, M.; Sgouros, G.; Adam, E. Indoor air quality in a dentistry clinic. Sci. Total Environ. 2007, 377, 349–365. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Evans, D.G.; Jenkinson, I.D.; Evans, P.A.; Vadgama, V.J.; Vadgama, J.; Jarad, F.; Grey, N.; Chilcott, R.P. SARS-CoV-2: Characterisation and mitigation of risks associated with aerosol generating procedures in dental practices. Br. Dent. J. 2021, 7, 1–7. [Google Scholar]
- Miller, R.L.; Micik, R.E. Air pollution and its control in the dental office. Dent. Clin. N. Am. 1978, 22, 453–476. [Google Scholar]
- Harrel, S.; Molinari, J. Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J. Am. Dent. Assoc. 2004, 135, 429–437. [Google Scholar] [CrossRef]
- Bennett, A.M.; Fulford, M.R.; Walker, J.T.; Bradshaw, D.J.; Martin, M.V.; Marsh, P.D. Microbial aerosols in general dental practice. Br. Dent. J. 2000, 189, 664–667. [Google Scholar] [CrossRef]
- Kedjarune, U.; Kukiattrakoon, B.; Yapong, B.; Chowanadisai, S.; Leggat, P. Bacterial aerosols in the dental clinic: Effect of time, position and type of treatment. Int. Dent. J. 2000, 50, 103–107. [Google Scholar] [CrossRef]
- Matys, J.; Grzech-Leśniak, K. Dental Aerosol as a Hazard Risk for Dental Workers. Materials (Basel) 2020, 13, 5109. [Google Scholar] [CrossRef] [PubMed]
- Girdler, N.M.; Sterling, P.A. Investigation of nitrous oxide pollution arising from inhalational sedation for the extraction of teeth in child patients. Int. J. Paediatr. Dent. 1998, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Helmis, C.G.; Tzoutzas, J.; Flocas, H.A.; Halios, C.H.; Assimakopoulos, V.D.; Stathopoulou, O.I.; Panis, V.; Apostolatou, M. Emissions of total volatile organic compounds and indoor environment assessment in dental clinics in Athens, Greece. Int. Dent. J. 2008, 58, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Godwin, C.C.; Batterman, S.A.; Sahni, S.P.; Peng, C.-Y. Indoor environment quality in dental clinics: Potential concerns from particulate matter. Am. J. Dent. 2003, 16, 260–266. [Google Scholar]
- Barrett, W.L.; Garber, S.M. Surgical smoke: A review of the literature. Is this just a lot of hot air? Surg. Endosc. 2003, 17, 979–987. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Alexis, N.; Bacchus, H.; Bernstein, I.L.; Fritz, P.; Horner, E.; Li, N.; Mason, S.; Nel, A.; Oullette, J.; et al. The health effects of non-industrial indoor air pollution. J. Allergy ClinImmunol. 2008, 121, 585–591. [Google Scholar] [CrossRef]
- Nimmo, A.; Werley, M.S.; Martin, J.S.; Tansy, M.F. Particulate inhalation during the removal of amalgam restorations. J. Prosthet. Dent. 1990, 63, 228–233. [Google Scholar] [CrossRef]
- Mirabelli, M.C.; Vaidyanathan, A.; Flanders, W.D.; Qin, X.; Garbe, P. Outdoor PM2.5, Ambient Air Temperature, and Asthma Symptoms in the Past 14 Days among Adults with Active Asthma. Environ. Health Perspect. 2016, 124, 1882–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rexhepi, I.; Mangifesta, R.; Santilli, M.; Guri, S.; Di Carlo, P.; D’Addazio, G.; Caputi, S.; Sinjari, B. Effects of Natural Ventilation and Saliva Standard Ejectors during the COVID-19 Pandemic: A Quantitative Analysis of Aerosol Produced during Dental Procedures. Int. J. Environ. Res. Public Health 2021, 18, 7472. [Google Scholar] [CrossRef] [PubMed]
- Micik, R.E.; Miller, R.L.; Mazzarella, M.A.; Ryge, G. Studies on dental aerobiology. I. Bacterial aerosols generated during dental procedures. J. Dent. Res. 1969, 48, 49–56. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Air Quality Guidelines for Europe, 2nd ed.; Regional Publications, European Series, No. 91; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- McKinley, I.B., Jr.; Ludlow, M.O. Hazards of laser smoke during endodontic therapy. J. Endod. 1994, 20, 558–559. [Google Scholar] [CrossRef]
- Correa-Afonso, A.M.; Pécora, J.D.; Palma-Dibb, R.G. Influence of pulse repetition rate on temperature rise and working time during composite filling removal with the Er:YAG laser. Photomed. Laser Surg. 2008, 26, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, E.C.; Skoulas, E.; Pepelassi, E.; Koidis, P.; Tzoutzas, I.G. The Use of Lasers in Dental Materials: A Review. Materials (Basel) 2021, 18, 3370. [Google Scholar] [CrossRef]
- Frentzen, M.; Koort, H.J. Lasers in Dentistry: New possibilities with advancing laser technology? Int. Dent. J. 1990, 40, 323–332. [Google Scholar]
- Widgor, H.A.; Walsh, J.T., Jr.; Featherstone, J.D.B.; Visuri, S.R.; Fried, D.; Waldvogel, J.L. Lasers in Dentistry. Lasers Surg. Med. 1995, 16, 103–133. [Google Scholar]
- Carrigan, P.J.; Morse, D.R.; Furst, M.L.; Sinai, I.H. A scanning electron microscopic evaluation of human dentinal tubules according to age and location. J. Endod. 1984, 10, 359–363. [Google Scholar] [CrossRef]
- Alevantis, L.E.; Xenaki-Petreas, M. Indoor air quality in practice. In Series: Energy Conservation in Buildings; Appendix A; Santamouris, M., Asimakopoulos, D.N., Eds.; CIENE, EC; University of Athens: Athens, Greece, 1996. [Google Scholar]
- Moritz, A.; Gutknecht, N.; Goharkhay, K.; Schoop, U.; Wernisch, J.; Sperr, W. In vitro irradiation of infected root canals with a diode laser: Results of microbiologic, infrared spectrometric, and stain penetration examinations. Quintessence Int. 1997, 28, 205–209. [Google Scholar]
- Kurzmann, C.; Meire, M.A.; Lettner, S.; Farmakis, E.T.R.; Moritz, A.; De Moor, R.J.G. The efficacy of ultrasonic and PIPS (photon-induced acoustic streaming) irrigation to remove artificially placed dentine debris plugs out of an artificial and natural root model. Lasers Med. Sci. 2020, 35, 719–728. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Konstantinidis, I.; Karoussis, I.K.; Ma, X.; Chu, H. Systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J. Periodontol. 2014, 85, 1203–1213. [Google Scholar] [CrossRef]
- Olivi, G.; Genovese, M.D. Laser restorative dentistry in children and adolescents. Eur. Arch. Paediatr. Dent. 2011, 12, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Skondra, F.G.; Koletsi, D.; Eliades, T.; Farmakis, E.T.R. The Effect of Low-Level Laser Therapy on Bone Healing After Rapid Maxillary Expansion: A Systematic Review. Photomed. Laser Surg. 2018, 36, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzanakakis, E.C.; Beketova, A.; Papadopoulou, L.; Kontonasaki, E.; Tzoutzas, I.G. Novel Femto Laser Patterning of High Translucent Zirconia as an Alternative to Conventional Particle Abrasion. Dent. J. (Basel) 2021, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Belting, C.M.; Haberfelde, G.C.; Juhl, L.K. Spread of organisms from Dental air rotor. J. Am. Dent. Assoc. 1964, 68, 648–651. [Google Scholar] [CrossRef]
- Larato, D.C. Sterilization of the high speed dental handpiece. Dent. Dig. 1966, 72, 107–109. [Google Scholar]
- Toroğlu, M.S.; Haytaç, M.C.; Köksal, F. Evaluation of aerosol contamination during debonding procedures. Angle Orthod. 2001, 71, 299–306. [Google Scholar]
- Samaranayake, L.P.; Fakhruddin, K.S.; Buranawat, B.; Panduwawala, C. The efficacy of bio-aerosol reducing procedures used in dentistry: A systematic review. Acta Odontol. Scand. 2021, 79, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, J. Dental bioaerosol as an occupational hazard in a dentist’s workplace. Ann. Agric. Environ. Med. 2007, 14, 203–207. [Google Scholar] [PubMed]
- Travaglini, E.A.; Larato, D.C.; Martin, A. Dissemination of organism-bearing droplets by high-speed dental drills. J. Prosth. Dent. 1966, 16, 132–139. [Google Scholar] [CrossRef]
- Alothmani, O. Air Quality in the Endodontist’s Dental Surgery. N. Z. Endod. J. 2009, 39, 7–16. [Google Scholar]
- Ionescu, A.C.; Brambilla, E.; Manzoli, L.; Orsini, G.; Gentili, V.; Rizzo, R. Aerosols modification with H2O2 reduces airborne contamination by dental handpieces. J. Oral Microbiol. 2021, 29, 1881361. [Google Scholar] [CrossRef] [PubMed]
- DIRECTIVE 2008/50/EC. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:152:0001:0044:EN:PDF (accessed on 1 September 2021).
- WHO. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. Global Update 2005. Summary of Risk Assessment. Available online: http://apps.who.int/iris/bitstream/handle/10665/69477/WHO_SDE_PHE_OEH_06.02_eng.pdf?sequence=1 (accessed on 1 September 2021).
- Environmental Protection Agency. National Ambient Air Quality Standards for Particulate Matter, Part II; Environmental Protection Agency: Washington, DC, USA, 2013.
- Mølhave, L.; Dueholm, S.; Jensen, L.K. Assessment of exposures and health risks related to formaldehyde emissions from furniture: A case study. Indoor Air 1995, 5, 104–119. [Google Scholar] [CrossRef]
- Meire, M.A.; Poelman, D.; De Moor, R.J. Optical properties of root canal irrigants in the 300–3000-nm wavelength region. Lasers Med. Sci. 2014, 29, 1557–1562. [Google Scholar] [CrossRef]
- Miserendino, L.; Pick, R. Lasers in Dentistry Quintessence Books; Quintessence Publishing: Batavia, IL, USA, 1995. [Google Scholar]
- Freitag, L.; Chapman, G.A.; Sielczak, M.; Ahmed, A.; Russin, D. Laser smoke effect on the bronchial system. Lasers Surg. Med. 1987, 7, 283–288. [Google Scholar] [CrossRef]
- Garden, J.M.; O’Banion, M.K.; Shelnitz, L.S.; Pinski, K.S.; Bakus, A.D.; Reichmann, M.E.; Sundberg, J.P. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA 1988, 26, 1199–1202. [Google Scholar] [CrossRef]
- Baggish, M.S.; Poiesz, B.J.; Joret, D.; Williamson, P.; Refai, A. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg. Med. 1991, 11, 197–203. [Google Scholar] [CrossRef]
- Hallmo, P.; Naess, O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur. Arch. Otorhinolaryngol. 1991, 248, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Bahn, S.L. Dental lasers: Safe or sorry? Compendium 1994, 15, 812–814, 816 passim; quiz 825. [Google Scholar]
- Nezhat, C.; Winer, W.K.; Nezhat, F.; Nezhat, C.; Forrest, D.; Reeves, W.G. Smoke from laser surgery: Is there a health hazard? Lasers Surg. Med. 1987, 7, 376–382. [Google Scholar] [CrossRef]
- Cochran, M.A.; Miller, C.H.; Sheldrake, M.A. The efficacy of the rubber dam as a barrier to the spread of microorganisms during dental treatment. J. Am. Dent. Assoc. 1989, 119, 141–144. [Google Scholar] [CrossRef]
- Kumbargere Nagraj, S.; Eachempati, P.; Paisi, M.; Nasser, M.; Sivaramakrishnan, G.; Verbeek, J.H. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database Syst. Rev. 2020, 10, CD013686. [Google Scholar] [PubMed]
- Nazarenko, Y. Air filtration and SARS-CoV-2. Epidemiol. Health 2020, 42, e2020049. [Google Scholar] [CrossRef]
- Cheek, E.; Guercio, V.; Shrubsole, C.; Dimitroulopoulou, S. Portable air purification: Review of impacts on indoor air quality and health. Sci. Total Environ. 2021, 766, 142585. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; Rexhepi, I.; Santilli, M.; DAddazio, G.; Chiacchiaretta, P.; Di Carlo, P.; Caputi, S. The Impact of COVID-19 Related Lockdown on Dental Practice in Central Italy-Outcomes of A Survey. Int. J. Environ. Res. Public Health 2020, 10, 5780. [Google Scholar] [CrossRef]



| PM10 | PM2.5 | |
|---|---|---|
| Background (Days 1 and 2) | 28 μg/m3 | 12 μg/m3 |
| Laser Activity (Day 3) | 310 μg/m3 | 275 μg/m3 |
| Background Daily Values Open Windows | Background Daily Values Closed Windows | Daily Values Laser Usage Open Windows | Daily Values Laser Usage Closed Window | |
|---|---|---|---|---|
| PM10 (μg/m3) | 13–17 | 26–50 | 18–22 | 55 |
| PM2.5 (μg/m3) | 8–11 | 27–37 | 14 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karveli, A.; Tzoutzas, I.G.; Raptis, P.I.; Tzanakakis, E.-G.C.; Farmakis, E.T.R.; Helmis, C.G. Air Quality in a Dental Clinic during Er:YAG Laser Usage for Cavity Preparation on Human Teeth—An Ex-Vivo Study. Int. J. Environ. Res. Public Health 2021, 18, 10920. https://doi.org/10.3390/ijerph182010920
Karveli A, Tzoutzas IG, Raptis PI, Tzanakakis E-GC, Farmakis ETR, Helmis CG. Air Quality in a Dental Clinic during Er:YAG Laser Usage for Cavity Preparation on Human Teeth—An Ex-Vivo Study. International Journal of Environmental Research and Public Health. 2021; 18(20):10920. https://doi.org/10.3390/ijerph182010920
Chicago/Turabian StyleKarveli, Angeliki, Ioannis G. Tzoutzas, Panagiotis Ioannis Raptis, Emmanouil-George C. Tzanakakis, Eleftherios Terry R. Farmakis, and Constantinos G. Helmis. 2021. "Air Quality in a Dental Clinic during Er:YAG Laser Usage for Cavity Preparation on Human Teeth—An Ex-Vivo Study" International Journal of Environmental Research and Public Health 18, no. 20: 10920. https://doi.org/10.3390/ijerph182010920







