Shock Index as a Predictor for Angiographic Hemostasis in Life-Threatening Traumatic Oronasal Bleeding
Abstract
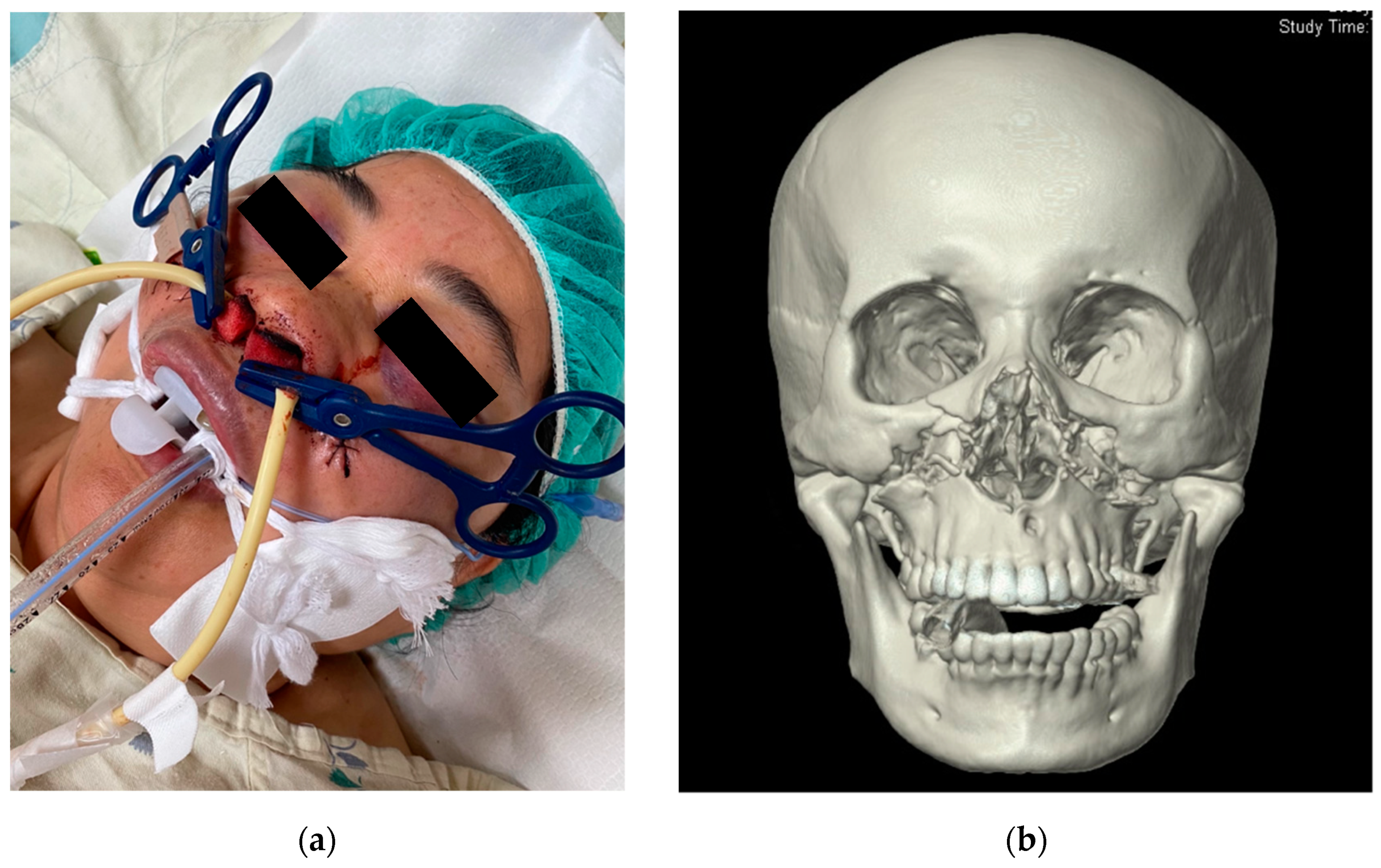
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, N.R.; Ledgard, J.P.; Katsaros, J. Massive hemorrhage in facial fracture patients: Definition, incidence, and management. Plast. Reconstr. Surg. 2009, 123, 680–690. [Google Scholar] [CrossRef]
- Liao, C.C.; Hsu, Y.P.; Chen, C.T.; Tseng, Y.Y. Transarterial embolization for intractable oronasal hemorrhage associated with craniofacial trauma: Evaluation of prognostic factors. J. Trauma Acute Care Surg. 2007, 63, 827–830. [Google Scholar] [CrossRef]
- Cogbill, T.H.; Cothren, C.C.; Ahearn, M.K.; Cullinane, D.C.; Kaups, K.L.; Scalea, T.M. Management of maxillofacial injuries with severe oronasal hemorrhage: A multicenter perspective. J. Trauma Acute Care Surg. 2008, 65, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Bynoe, R.P.; Kerwin, A.J.; Parker, H.H.; Nottingham, J.M.; Bell, R.M.; Yost, M.J. Maxillofacial injuries and life-threatening hemorrhage: Treatment with transcatheter arterial embolization. J. Trauma Acute Care Surg. 2003, 55, 74–79. [Google Scholar] [CrossRef]
- Shimoyama, T.; Kaneko, T.; Horie, N. Initial management of massive oral bleeding after midfacial fracture. J. Trauma Acute Care Surg. 2003, 54, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Dagum, A.B. A critical review of the literature and an evidence-based approach for life-threatening hemorrhage in maxillofacial surgery. Ann. Plast. Surg. 2012, 69, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Tseng, Y.Y.; Chen, C.T. Transarterial embolisation for intractable post- traumatic oronasal haemorrhage following traumatic brain injury: Evaluation of prognostic factors. Injury 2008, 39, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Singam, P.; Thanabalan, J.; Mohammed, Z. Superselective embolisation for control of intractable epistaxis from maxillary artery injury. Biomed. Imaging Interv. J. 2011, 7, e3. [Google Scholar]
- Perry, M.; Dancey, A.; Mireskandari, K.; Oakley, P.; Davies, S.; Cameron, M. Emergency care in facial trauma-a maxillofacial and ophthalmic perspective. Injury 2005, 36, 875–896. [Google Scholar] [CrossRef]
- Komiyama, M.; Nishikawa, M.; Kan, M.; Shigemoto, T.; Kaji, A. Endovascular treatment of intractable oronasal bleeding associated with severe craniofacial injury. J. Trauma Acute Care Surg. 1998, 44, 330–334. [Google Scholar] [CrossRef]
- Salsamendi, J.T.; Gortes, F.J.; Ayala, A.R.; Palacios, J.D.; Tewari, S.; Narayanan, G. Transarterial embolization of a hyperfunctioning aldosteronoma in a patient with bilateral adrenal nodules. Radiol. Case Rep. 2017, 12, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, K.O.; Harmon, J.J.; Walters, Z.; Samy, R.; de Alarcon, A.; Stevens, S.M. Facial Palsy Following Embolization of a Juvenile Nasopharyngeal Angiofibroma. Ann. Otol. Rhinol. Laryngol. 2018, 127, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.; Farb, R.; Agid, R. Endovascular treatment of epistaxis. Am. J. Neuroradiol. 2009, 30, 1637–1645. [Google Scholar] [CrossRef]
- Tung, T.C.; Tseng, W.S.; Chen, C.T.; Lai, J.P.; Chen, Y.R. Acute life-threatening injuries in facial fracture patients: A review of 1025 patients. J. Trauma Acute Care Surg. 2000, 49, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, N.; Walsh, R.; Brennan, P.; Walsh, M. Treatment of intractable epistaxis using arterial embolization. Clin. Otolaryngol. Allied Sci. 2001, 26, 307–309. [Google Scholar] [CrossRef]
- Ho, K.; Hutter, J.J.; Eskridge, J.; Khan, U.; Boorer, C.J.; Hopper, R.A. The management of life-threatening haemorrhage following blunt facial trauma. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 1257–1262. [Google Scholar] [CrossRef]
- Bouloux, G.F.; Perciaccante, V.J. Massive hemorrhage during oral and maxillofacial surgery: Ligation of the external carotid artery or embolization? J. Oral Maxillofac. Surg. 2009, 67, 1547–1551. [Google Scholar] [CrossRef]
- Bachar, G.; Esmat, N.; Stern, S.; Litvin, S.; Knizhnik, M.; Perlow, E. Transarterial embolization for acute head and neck bleeding: Eight-Year experience with emphasis on rebleeding risk in cancer patients. Laryngoscope 2013, 123, 1220–1226. [Google Scholar] [CrossRef]
- Wiratama, B.Y.; Chen, P.L.; Chao, C.J.; Wang, M.H.; Saleh, W.; Lin, H.A.; Pai, C.W. Effect of Distance to Trauma Centre, Trauma Centre level, and Trauma Centre Region on Fatal Injuries among Motorcyclists in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 2998. [Google Scholar] [CrossRef]
- Malone, D.L.; Dunne, J.; Tracy, J.K.; Putnam, A.T.; Scalea, T.M.; Napolitano, L.M. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J. Trauma Acute Care Surg. 2003, 54, 898–907. [Google Scholar] [CrossRef]
- Birkhahn, R.H.; Gaeta, T.J.; Terry, D.; Bove, J.J.; Tloczkowski, J. Shock index in diagnosing early acute hypovolemia. Am. J. Emerg. Med. 2005, 23, 323–326. [Google Scholar] [CrossRef]
- DeMuro, J.P.; Simmons, S.; Jax, J.; Gianelli, S.M. Application of the Shock Index to the prediction of need for hemostasis intervention. Am. J. Emerg. Med. 2013, 31, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.H.; Rau, C.S.; Hsu, S.Y.; Wu, S.C.; Kuo, P.J.; Hsieh, H.Y.; Chen, Y.C.; Hsieh, C.H. Using the Reverse Shock Index at the Injury Scene and in the Emergency Department to Identify High-Risk Patients: A Cross-Sectional Retrospective Study. Int. J. Environ. Res. Public Health 2016, 13, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.C.; Rau, C.Y.; Kuo, S.C.H.; Chien, P.C.; Hiseh, H.Y.; Hsieh, C.H. The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data. Int. J. Environ. Res. Public Health 2018, 15, 2346. [Google Scholar] [CrossRef] [Green Version]
- Vandromme, M.J.; Griffin, R.L.; McGwin, G.; Weinberg, J.A.; Rue, L.W.; Kerby, J.D. Prospective identification of patients at risk for massive transfusion: An imprecise endeavor. Am. Surg. 2011, 77, 155–161. [Google Scholar] [CrossRef]
- Mutschler, M.; Nienaber, U.; Münzberg, M.; Wölfl, C.; Schoechl, H.; Paffrath, T. The Shock Index revisited—A fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU®. Crit. Care 2013, 17, R172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, C.S.; Wu, S.C.; Kuo, S.C.H.; Kuo, P.J.; Hsu, S.Y.; Chen, Y.C.; Hsieh, H.Y.; Hsieh, C.H.; Liu, H.T. Prediction of Massive Transfusion in Trauma Patients with Shock Index, Modified Shock Index, and Age Shock Index. Int. J. Environ. Res. Public Health 2016, 13, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.C.; Rau, C.S.; Kuo, P.J.; Liu, H.T.; Hsu, S.Y.; Hsieh, C.H. Significance of Blood Transfusion Units in Determining the Probability of Mortality among Elderly Trauma Patients Based on the Geriatric Trauma Outcome Scoring System: A Cross-Sectional Analysis Based on Trauma Registered Data. Int. J. Environ. Res. Public Health 2018, 15, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakasone, Y.; Ikeda, O.; Yamashita, Y.; Kudoh, K.; Shigematsu, Y.; Harada, K. Shock index correlates with extravasation on angiographs of gastrointestinal hemorrhage: A logistics regression analysis. Cardiovasc. Interv. Radiol. 2007, 30, 861–865. [Google Scholar] [CrossRef]
- Kuo, L.W.; Yang, S.J.; Fu, C.Y.; Liao, C.H.; Wang, S.Y.; Wu, S.C. Relative hypotension increases the probability of the need for angioembolisation in pelvic fracture patients without contrast extravasation on computed tomography scan. Injury 2016, 47, 37–42. [Google Scholar] [CrossRef]
- Cole, E.; Weaver, A.; Gall, L.; West, A.; Nevin, D.; Tallach, R.; O’Neill, B.; Lahiri, S.; Allard, S.; Tai, N.; et al. A Decade of Damage Control Resuscitation: New Transfusion Practice, New Survivors, New Directions. Ann. Surg. 2021, 273, 1215–1220. [Google Scholar] [CrossRef]
- MacLeod, J.B.A.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early coagulopathy predicts mortality in trauma. J. Trauma 2003, 55, 39–44. [Google Scholar] [CrossRef]
- Kornblith, L.Z.; Moore, H.B.; Cohen, M.J. Trauma-induced coagulopathy: The past, present, and future. J. Thromb. Haemost. 2019, 17, 852–862. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs. a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef]
- Christie, S.A.; Kornblith, L.Z.; Howard, B.M.; Conroy, A.S.; Kunitake, R.C.; Nelson, M.F.; Hendrickson, C.M.; Calfee, C.S.; Callcut, R.A.; Cohen, M.J. Characterization of distinct coagulopathic phenotypes in injury: Pathway-specific drivers and implications for individualized treatment. J. Trauma Acute Care Surg. 2017, 82, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Kerby, J.D.; Kalkwarf, K.J.; Van Belle, G.; Fox, E.E.; Cotton, B.A.; Cohen, M.J.; Schreiber, M.A.; Brasel, K.J.; Bulger, E.M.; et al. Earlier time to hemostasis is associated with decreased mortality and rate of complications: Results from the Pragmatic Randomized Optimal Platelet and Plasma Ratio trial. J. Trauma Acute Care Surg. 2019, 87, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Maegele, M.; Schöchl, H.; Menovsky, T.; Maréchal, H.; Miklas, M.; Buki, A.; Stanworth, S. Coagulopathy and haemorrhagic progression in traumatic brain injury: Advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017, 16, 630–647. [Google Scholar] [CrossRef]
- Böhm, J.K.; Gütin, H.; Thorn, S.; Schäfer, N.; Rambach, V.; Schöchl, H.; Grottke, O.; Rossaint, R.; Stanworth, S.; Nicola, C.; et al. Global Characterisation of Coagulopathy in Isolated Traumatic Brain Injury (iTBI): A CENTER-TBI Analysis. Neurocrit. Care 2021, 35, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Li, L.; Schäfer, N.; Huang, Q.; Maegele, M.; Gu, Z. Endothelial glycocalyx in traumatic brain injury associated coagulopathy: Potential mechanisms and impact. J. NeuroInflamm. 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Nikolaou, G.; Rufibach, K.; Holzmann, D. On the effectiveness of treatment options in epistaxis: An analysis of 678 interventions. Rhinology 2011, 49, 474–478. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wu, C.; Wang, L.J.; Wong, Y.C.; Chen, H.W.; Wang, C.J. Predictive factors for early failure of transarterial embolization in blunt hepatic injury patients. Clin. Radiol. 2014, 69, e505–e511. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Yu, C.Y.; Chen, R.C.; Huang, G.S.; Liu, C.H.; Hsu, H.H. Transarterial treatment of acute gastrointestinal bleeding: Prediction of treatment failure by clinical and angiographic parameters. J. Chin. Med. Assoc. 2012, 75, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, S.; Tozer, K.R.; Chen, J. An overview of embolic agents. Semin. Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyon, J.J.; Littlehales, T.; Rangarajan, B.; Hoey, E.T.; Ganeshan, A. Endovascular embolization: Review of currently available embolization agents. Curr. Probl. Diagn. Radiol. 2014, 43, 35–53. [Google Scholar] [CrossRef]
- Dubel, G.J.; Ahn, S.H.; Soares, G.M. Transcatheter embolization in the management of epistaxis. Semin. Interv. Radiol. 2013, 30, 249–262. [Google Scholar]




| Variables | TAE (n = 39) | Non-TAE (n = 11) | p Value |
|---|---|---|---|
| Age | 34.48 ± 19.41 | 38.54 ± 10.86 | 0.12 |
| Mechanism | |||
| MVA | 18 (46.2%) | 8 (72.7%) | 0.17 |
| Pedestrian | 8 (20.5%) | 0 | - |
| Fall | 7 (17.9%) | 2 (18.2%) | - |
| Miscellaneous | 6 (15.4%) | 1 (9.1%) | - |
| Other Injury | |||
| CNS | 23 (58.9%) | 9 (81.82%) | 0.28 |
| Chest | 15 (38.4%) | 5 (45.45%) | 0.73 |
| Abdomen | 6 (15.3%) | 0 | - |
| Pelvis | 5 (12.8%) | 0 | - |
| Extremities | 7 (17.9%) | 2 (18.18%) | 0.31 |
| Fracture | |||
| LeFort II + III | 16 (41.0%) | 5 (45.4%) | 1 |
| NOE | 2 (5.1%) | 2 (18.2%) | 0.30 |
| ZMC | 4 (10.2%) | 4 (36.4%) | 0.05 |
| Nasal | 2 (5.1%) | 0 | - |
| Mandible | 1 (2.5%) | 1 (9.1%) | 0.39 |
| Vitals | |||
| SBP | 107.12 ± 35.74 | 136.81 ± 27.53 | <0.05 * |
| HR | 126.84 ± 25.36 | 98.18 ± 24.28 | <0.05 * |
| Initial SI | 1.31 ± 0.49 | 0.73 ± 0.17 | <0.05 * |
| SI (after nasal packing) | 1.14 ± 0.37 | 0.74 ± 0.23 | <0.05 * |
| SI (after TAE) | 0.9 ± 0.31 | - | - |
| GCS | 8.17 ± 4.62 | 7.63 ± 3.72 | 0.99 |
| Laboratory Studies | |||
| Hematocrit (%) | 32.6 ± 8.6 | 34.8 ± 9 | 0.23 |
| INR | 1.84 ± 0.95 | 1.17 ± 0.14 | 0.042 * |
| Platelet (×103/μL) | 162.4 ± 66.9 | 213 ± 53.9 | 0.037 * |
| Blood Transfusion | |||
| pRBC | 6.6 ± 5 | 2.9 ± 3.2 | <0.05 * |
| WB | 1.4 ± 2.9 | 0.9 ± 2.1 | 0.77 |
| FFP | 3.1 ± 4.3 | 1.5 ± 2.6 | 0.2 |
| ISS | 28.72 ± 10.71 | 24.54 ± 11.30 | 0.32 |
| LOS | 6.61 ± 5.00 | 2.54 ± 3.35 | 1 |
| Variables | Rebleeding (n = 7) | No Rebleeding (n = 32) | p Value |
|---|---|---|---|
| Fracture | |||
| LeFort II + III | 3 | 13 | 0.08 |
| NOE | 0 | 2 | - |
| ZMC | 1 | 3 | 0.56 |
| Nasal | 0 | 2 | - |
| Mandible | 0 | 1 | - |
| Material | |||
| Coils | 1 | 4 | 0.96 |
| Gelfoam | 3 | 26 | 0.05 |
| NBCA | 2 | 3 | 0.21 |
| PVA | 0 | 2 | - |
| Bleeder | |||
| IMA | 7 | 24 | 0.07 |
| Facial | 0 | 5 | - |
| ECA | 0 | 3 | - |
| Lingual | 0 | 2 | - |
| Sphenopalatine | 0 | 1 | - |
| Ascending pharyngeal | 0 | 1 | - |
| TAE | |||
| Extravasation | 6 | 23 | 0.56 |
| Pseudoaneurysm | 5 | 11 | 0.1 |
| SI | 1.38 ± 0.72 | 1.30 ± 0.43 | 0.98 |
| ISS | 26.28 ± 11.28 | 29.38 ± 10.69 | 0.52 |
| LOS | 30.2 ± 12.37 | 21.96 ± 15.72 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, F.-Y.; Mao, S.-H.; Chuang, A.D.-C.; Wong, Y.-C.; Chen, C.-H. Shock Index as a Predictor for Angiographic Hemostasis in Life-Threatening Traumatic Oronasal Bleeding. Int. J. Environ. Res. Public Health 2021, 18, 11051. https://doi.org/10.3390/ijerph182111051
Hsu F-Y, Mao S-H, Chuang AD-C, Wong Y-C, Chen C-H. Shock Index as a Predictor for Angiographic Hemostasis in Life-Threatening Traumatic Oronasal Bleeding. International Journal of Environmental Research and Public Health. 2021; 18(21):11051. https://doi.org/10.3390/ijerph182111051
Chicago/Turabian StyleHsu, Fang-Yu, Shih-Hsuan Mao, Andy Deng-Chi Chuang, Yon-Cheong Wong, and Chih-Hao Chen. 2021. "Shock Index as a Predictor for Angiographic Hemostasis in Life-Threatening Traumatic Oronasal Bleeding" International Journal of Environmental Research and Public Health 18, no. 21: 11051. https://doi.org/10.3390/ijerph182111051






