Detection of Nail Oncometabolite SAICAR in Oral Cancer Patients and Its Molecular Interactions with PKM2 Enzyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. VTGE Assisted Purification of Nail Metabolites
2.3. Molecular Docking
2.4. Molecular Dynamics (MD) Simulations
2.5. vNN-ADMET Toxicity Prediction
3. Results
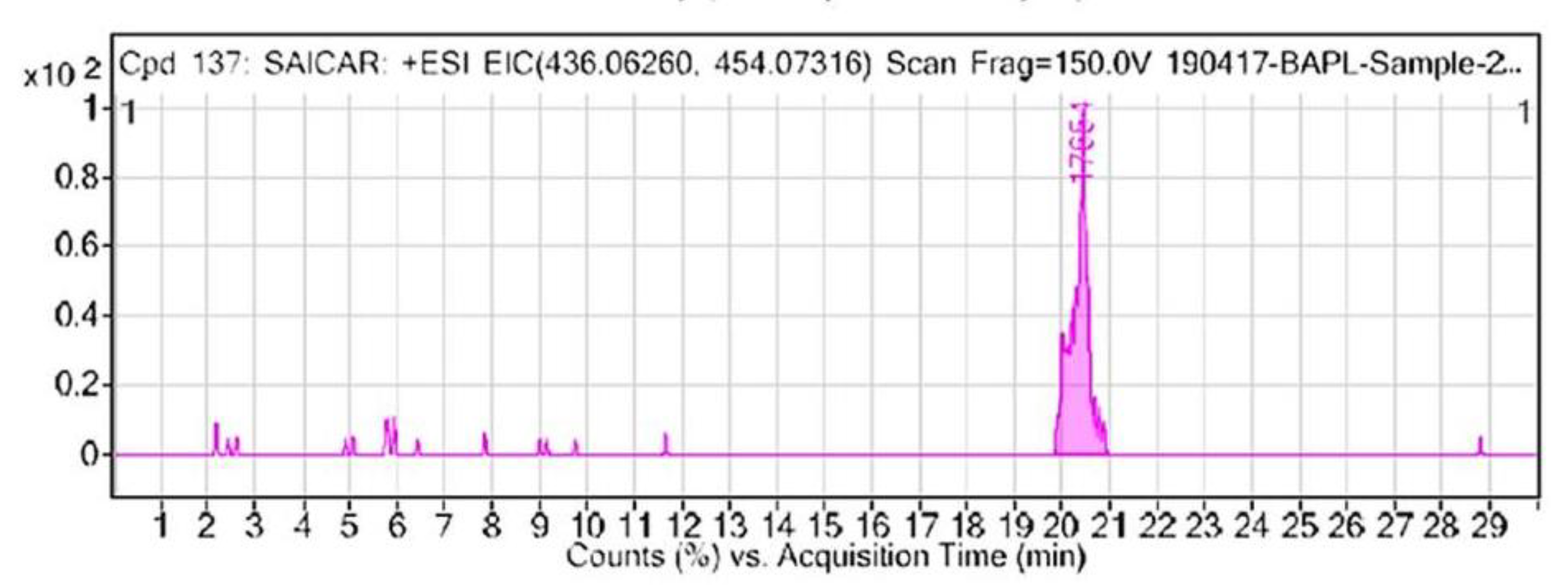
3.1. Identification of Oncometabolite from Nails of Oral Cancer Patients
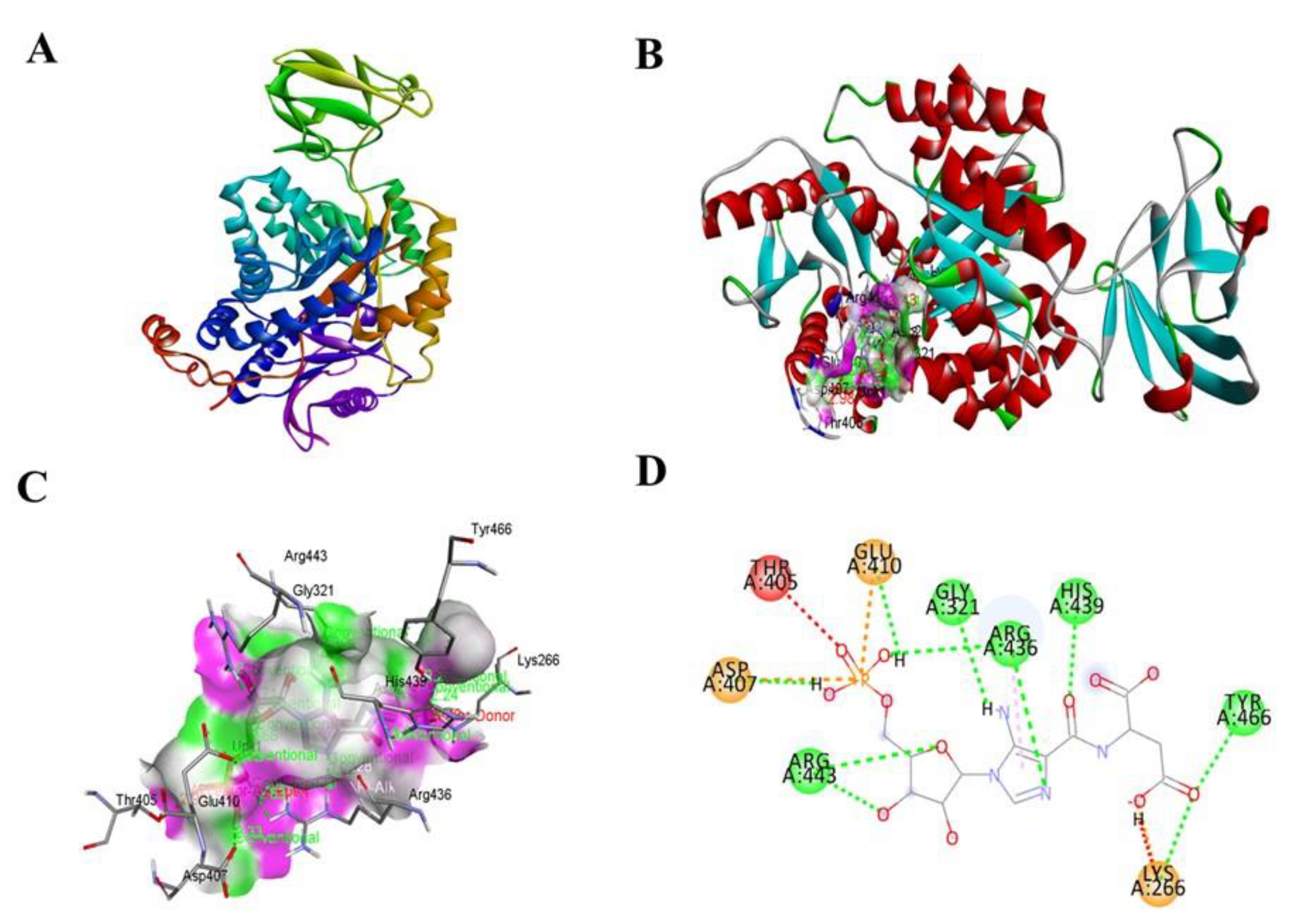
3.2. Molecular Docking
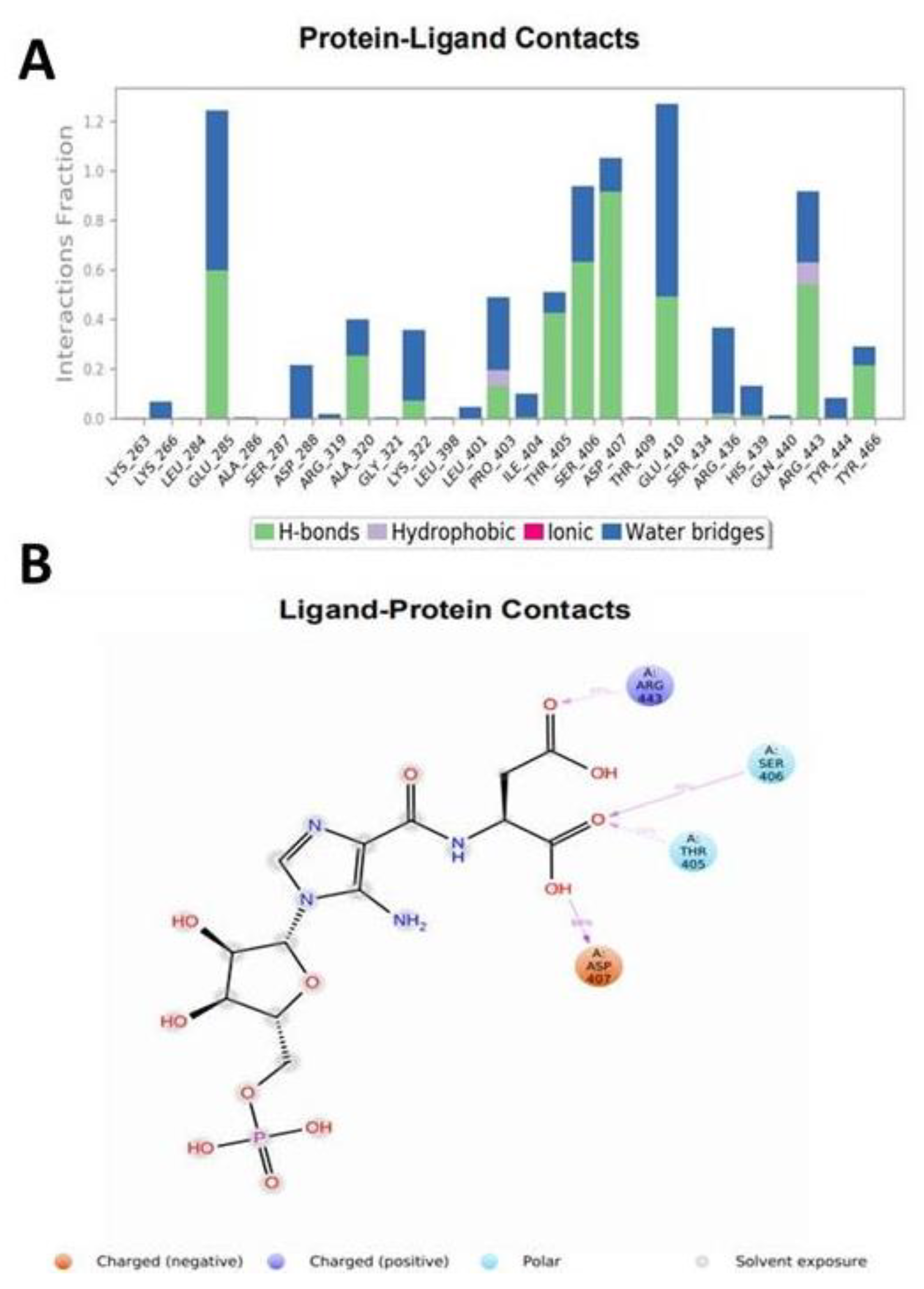
3.3. Molecular Dynamics (MD) Simulations
3.4. vNN-ADMET Toxicity Prediction
4. Discussion
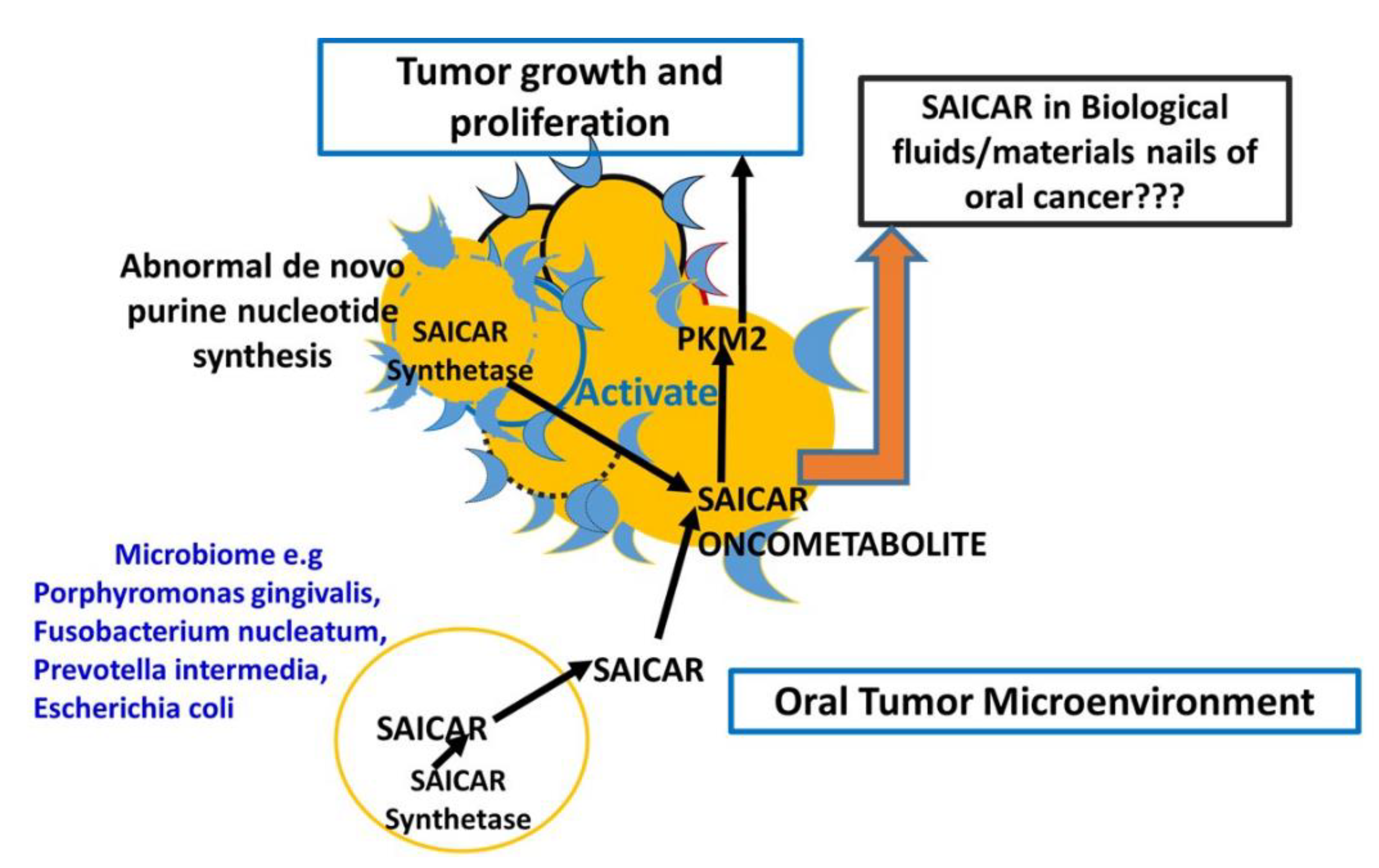
Sharing of Saicar between Oral Cancer Cell and Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. Investigation and identification of potential biomarkers in human saliva for the early diagnosis of oral squamous cell carcinoma. Clin. Chim. Acta 2014, 427, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Bhatia, N.; Lalla, Y.; Vu, A.; John, K.; Gupta, V.; Baeten, J.; Johnson, A.; Kademani, D. Advances in Early Detection and Diagnostic Adjuncts in Oral Cavity Cancer. In Contemporary Oral Oncology: Biology, Epidemiology, Etiology, and Prevention; Kuriakose, M.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 355–421. ISBN 978-3-319-14911-0. [Google Scholar]
- Sankaranarayanan, R.; Somanathan, T.; Thomas, G.; Ramadas, K. Screening for Oral Cancer. In Contemporary Oral Oncology: Biology, Epidemiology, Etiology, and Prevention; Kuriakose, M.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 423–444. ISBN 978-3-319-14911-0. [Google Scholar]
- Rai, V.; Mukherjee, R.; Ghosh, A.K.; Routray, A.; Chakraborty, C. “Omics” in oral cancer: New approaches for biomarker discovery. Arch. Oral Biol. 2018, 87, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Santosh, A.B.R.; Jones, T.; Harvey, J. A review on oral cancer biomarkers: Understanding the past and learning from the present. J. Cancer Res. Ther. 2016, 12, 486–492. [Google Scholar] [CrossRef]
- Nass, S.J.M. Cancer Biomarkers: The Promises and Challenges of Improving Detection and Treatment; Nass, S.J., Moses, H.L., Eds.; The National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-10386-2. [Google Scholar]
- Mazurek, S. Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 2011, 43, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-R.; Tian, M.-X.; Yang, L.-X.; Lin, Y.-L.; Jin, L.; Ding, Z.-B.; Shen, Y.-H.; Peng, Y.-F.; Gao, D.-M.; Zhou, J.; et al. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget 2015, 6, 846–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, W.; Wang, Y.; Chen, X.; Shi, Y.; Wang, J.; Zhang, J.; Qian, J.; Li, R.; Tao, T.; Wei, W.; et al. PKM2 promotes glucose metabolism and cell growth in gliomas through a mechanism involving a let-7a/c-Myc/hnRNPA1 feedback loop. Oncotarget 2015, 6, 13006–13018. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, H.; Yang, J.J.; Liu, X.; Liu, Z.-R. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 2012, 45, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Alquraishi, M.; Puckett, D.L.; Alani, D.S.; Humidat, A.S.; Frankel, V.D.; Donohoe, D.R.; Whelan, J.; Bettaieb, A. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic. Biol. Med. 2019, 143, 176–192. [Google Scholar] [CrossRef]
- Anastasiou, D.; Yu, Y.; Israelsen, W.J.; Jiang, J.-K.; Boxer, M.B.; Hong, B.S.; Tempel, W.; Dimov, S.; Shen, M.; Jha, A.; et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012, 8, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Zahra, K.; Dey, T.; Ashish; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.E.; Tan, I.S.; Lee, Y.-S. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science 2012, 338, 1069–1072. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.E.; Doctor, Z.M.; Dwyer, Z.W.; Lee, Y.-S. SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol. Cell 2014, 53, 700–709. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Chakravarthy, S.; Tokuda, J.M.; Pollack, L.; Bowman, G.D.; Lee, Y.-S. Succinyl-5-aminoimidazole-4-carboxamide-1-ribose 5’-Phosphate (SAICAR) Activates Pyruvate Kinase Isoform M2 (PKM2) in Its Dimeric Form. Biochemistry 2016, 55, 4731–4736. [Google Scholar] [CrossRef] [Green Version]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrödinger Release Maestro, Schrödinger. Available online: https://www.schrodinger.com/citations (accessed on 10 October 2021).
- D. E. Shaw Research Desmond Molecular Dynamics System. Available online: https://www.deshawresearch.com/resources_desmond.html (accessed on 10 October 2021).
- Griffin, J.L.; Shockcor, J.P. Metabolic profiles of cancer cells. Nat. Rev. Cancer 2004, 4, 551–561. [Google Scholar] [CrossRef]
- Tiziani, S.; Lopes, V.; Günther, U.L. Early stage diagnosis of oral cancer using 1H NMR-based metabolomics. Neoplasia 2009, 11, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Kumar; Kothari, J.; Bhatkar, D.; Mitruka, M.; Pal, R.; Sarode, S.C.; Sharma, N.K. Detection of urinary metabolites of metabolic pathway disorders using vertical tube gel electrophoresis and liquid chromatography–high-resolution mass spectrometry techniques. J. Dent. Res. Rev. 2020, 7, 36. [Google Scholar] [CrossRef]
- Imamura, K.; Tanaka, T. Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies. J. Biochem. 1972, 71, 1043–1051. [Google Scholar] [CrossRef]
- Xie, G.X.; Chen, T.L.; Qiu, Y.P.; Shi, P.; Zheng, X.J.; Su, M.M.; Zhao, A.H.; Zhou, Z.T.; Jia, W. Urine metabolite profiling offers potential early diagnosis of oral cancer. Metabolomics 2012, 8, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kothari, J.; Bhatkar, D.; Mitruka, M.; Pal, R.; Sarode, S.; Sharma, N. Detection of Urinary Metabolites of Metabolic Pathway Disorders by Using VTGE and LC-HRMS Techniques. bioRxiv. 2019. Available online: https://www.biorxiv.org/content/10.1101/814970v1 (accessed on 10 October 2021).
- Bluemlein, K.; Grüning, N.-M.; Feichtinger, R.G.; Lehrach, H.; Kofler, B.; Ralser, M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget 2011, 2, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesen, R.H.; Castellani, R.J.; Lee, J.C.; Braun, W. Allostery in rabbit pyruvate kinase: Development of a strategy to elucidate the mechanism. Biochemistry 1998, 37, 15266–15276. [Google Scholar] [CrossRef]
- Mazurek, S. Pyruvate kinase type M2: A key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Scher. Found. Symp. Proc. 2007, 99–124. [Google Scholar] [CrossRef]
- Luo, W.; Semenza, G.L. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metab. 2012, 23, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Mancuso, A.; Tong, X.; Ward, P.S.; Fan, J.; Rabinowitz, J.D.; Thompson, C.B. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl. Acad. Sci. USA 2012, 109, 6904–6909. [Google Scholar] [CrossRef] [Green Version]
- Chaneton, B.; Hillmann, P.; Zheng, L.; Martin, A.C.L.; Maddocks, O.D.K.; Chokkathukalam, A.; Coyle, J.E.; Jankevics, A.; Holding, F.P.; Vousden, K.H.; et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 2012, 491, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Lu, Z. Pyruvate kinase M2 at a glance. J. Cell Sci. 2015, 128, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Hu, H.; Chang, R.; Zhong, J.; Knabel, M.; O’Meally, R.; Cole, R.N.; Pandey, A.; Semenza, G.L. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011, 145, 732–744. [Google Scholar] [CrossRef] [Green Version]
- Kurihara-Shimomura, M.; Sasahira, T.; Nakashima, C.; Kuniyasu, H.; Shimomura, H.; Kirita, T. The Multifarious Functions of Pyruvate Kinase M2 in Oral Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2907. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-J.; Kim, J.Y.; Lee, D.Y.; Zhang, X.; Bazarsad, S.; Chung, W.-Y.; Kim, J. PKM2 enhances cancer invasion via ETS-1-dependent induction of matrix metalloproteinase in oral squamous cell carcinoma cells. PLoS ONE 2019, 14, e0216661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, H.P.; O’Reilly, F.J.; Wear, M.A.; O’Neill, J.R.; Fothergill-Gilmore, L.A.; Hupp, T.; Walkinshaw, M.D. M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc. Natl. Acad. Sci. USA 2013, 110, 5881–5886. [Google Scholar] [CrossRef] [Green Version]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.W.; et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Shirai, T.; Nazarewicz, R.R.; Wallis, B.B.; Yanes, R.E.; Watanabe, R.; Hilhorst, M.; Tian, L.; Harrison, D.G.; Giacomini, J.C.; Assimes, T.L.; et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016, 213, 337–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.; Raj, A.K.; Lokhande, K.B.; Almasri, M.A.; Alzahrani, K.J.; Almeslet, A.S.; Swamy, K.V.; Sarode, G.S.; Sarode, S.C.; Patil, S.; et al. Detection of Nail Oncometabolite SAICAR in Oral Cancer Patients and Its Molecular Interactions with PKM2 Enzyme. Int. J. Environ. Res. Public Health 2021, 18, 11225. https://doi.org/10.3390/ijerph182111225
Patel R, Raj AK, Lokhande KB, Almasri MA, Alzahrani KJ, Almeslet AS, Swamy KV, Sarode GS, Sarode SC, Patil S, et al. Detection of Nail Oncometabolite SAICAR in Oral Cancer Patients and Its Molecular Interactions with PKM2 Enzyme. International Journal of Environmental Research and Public Health. 2021; 18(21):11225. https://doi.org/10.3390/ijerph182111225
Chicago/Turabian StylePatel, Rushikesh, Ajay Kumar Raj, Kiran Bharat Lokhande, Mazen A. Almasri, Khalid J. Alzahrani, Asma Saleh Almeslet, K. Venkateswara Swamy, Gargi S. Sarode, Sachin C. Sarode, Shankargouda Patil, and et al. 2021. "Detection of Nail Oncometabolite SAICAR in Oral Cancer Patients and Its Molecular Interactions with PKM2 Enzyme" International Journal of Environmental Research and Public Health 18, no. 21: 11225. https://doi.org/10.3390/ijerph182111225
APA StylePatel, R., Raj, A. K., Lokhande, K. B., Almasri, M. A., Alzahrani, K. J., Almeslet, A. S., Swamy, K. V., Sarode, G. S., Sarode, S. C., Patil, S., & Sharma, N. K. (2021). Detection of Nail Oncometabolite SAICAR in Oral Cancer Patients and Its Molecular Interactions with PKM2 Enzyme. International Journal of Environmental Research and Public Health, 18(21), 11225. https://doi.org/10.3390/ijerph182111225






