Acute Toxicity and Ecotoxicological Risk Assessment of Three Volatile Pesticide Additives on the Earthworm—Eisenia fetida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Tested Biota
2.3. Acute Lethal Toxicity and Growth Inhibition Test
2.4. Avoidance Test
2.5. Statistical Analysis
3. Results and Discussion
3.1. Acute Lethal Toxicity Test
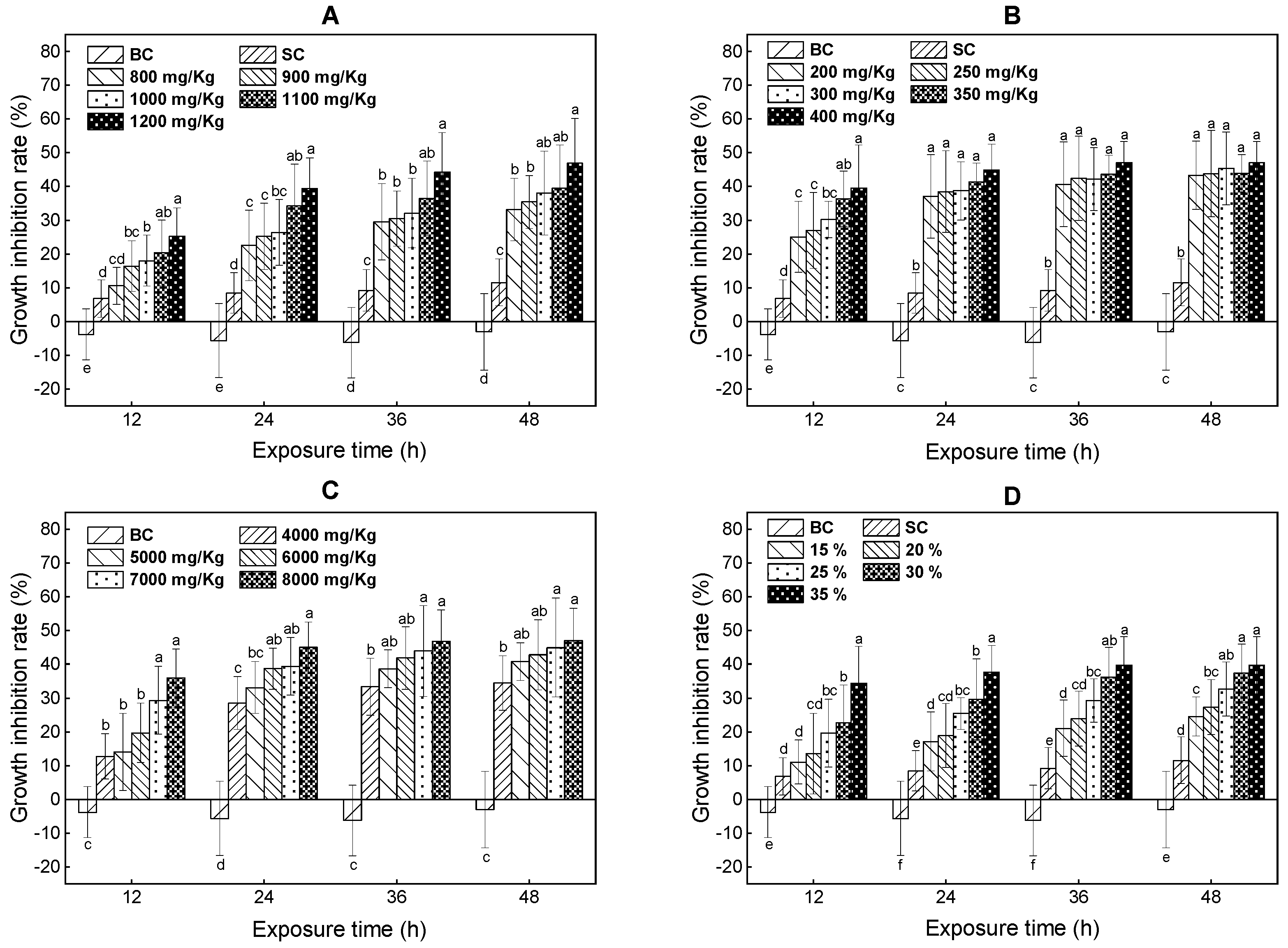
3.2. Growth Inhibition Effects
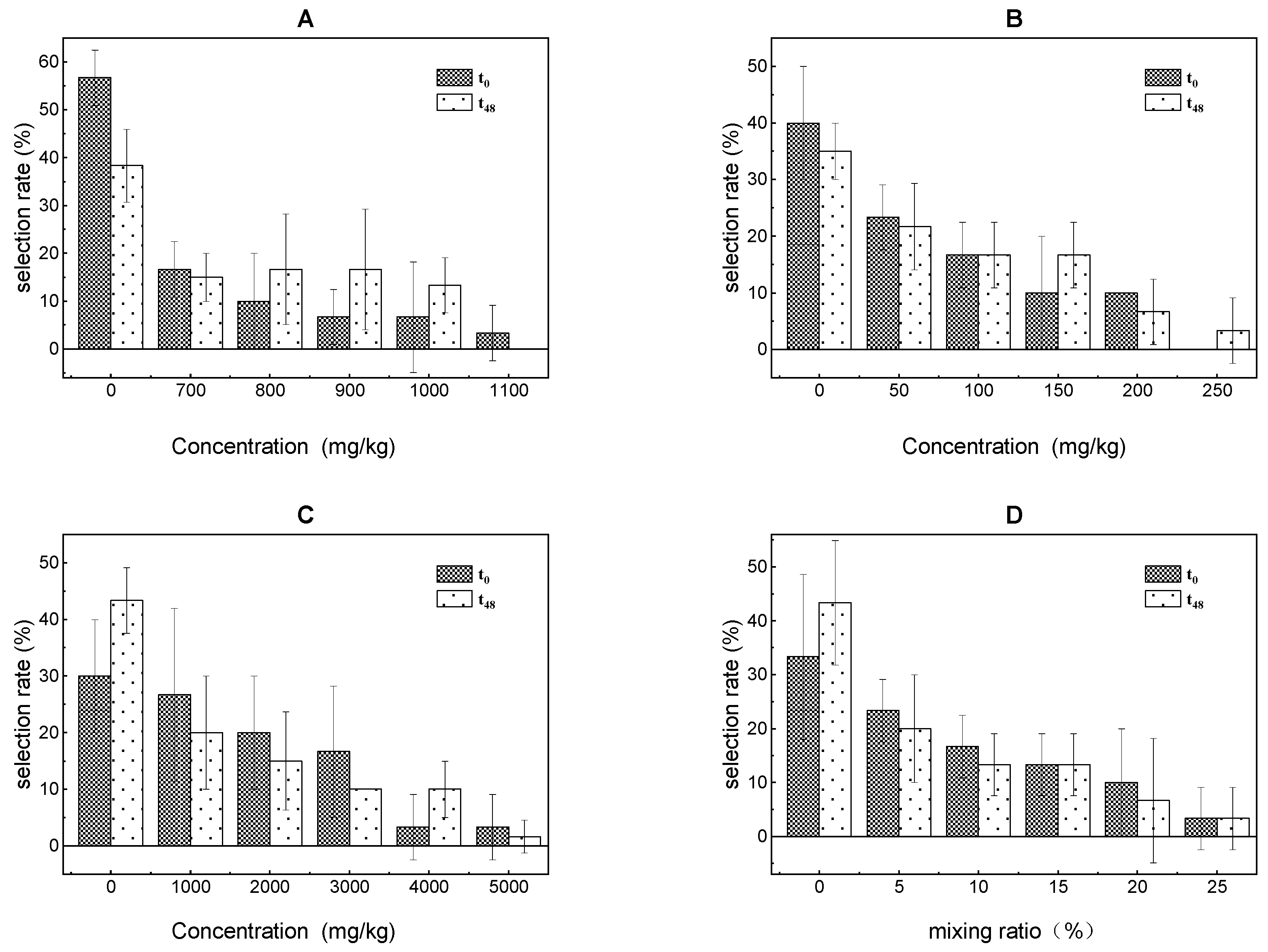
3.3. Avoidance Response Effect
3.4. Ecotoxicological Risk Assessment of Three PAs
4. Conclusions
- (1)
- The 24 h-LC50 of toluene, xylene and trichloroethylene on E. fetida were 300.23, 1190.45, and 5332.36 mg/kg, respectively, and the 48 h-LC50 of toluene, xylene, and trichloroethylene were 221.62, 962.89, and 4522.41 mg/kg, respectively. The decreasing order of toxicity at the 24 h and 48 h exposure were both: xylene > toluene > trichloroethylene. The ternary mixture exhibited a significant synergistic effect response on E. fetida.
- (2)
- The three volatile PAs and their ternary mixture significantly inhibited the growth of E. fetida. The significant inhibition concentration and mixing rates were 900 mg/kg, 200 mg/kg, 4000 mg/kg and 25% at 12 h. The AT values of E. fetida for the toluene, xylene, and trichloroethylene and their ternary mixture treatments were 1100 mg/kg, 250 mg/kg, 5000 mg/kg and 25%.
- (3)
- By comparing the PNEC values of E. fetida for the three volatile PAs and the investigated concentrations in the environment, toluene exhibited a medium to high ecotoxicological risk, and xylene exhibited a high ecotoxicological risk to the ecosystem.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Jiang, Z.; Cao, X.; Su, H.; Shao, H.; Jin, F.; Zheng, L.; EI-Aty, A.M.A.; Wang, J. SPE/GC–MS Determination of 2-Pyrrolidone, N -Methyl-2-pyrrolidone, and N -Ethyl-2-pyrrolidone in Liquid Pesticide Formulations. Chromatographia 2018, 81, 359–364. [Google Scholar] [CrossRef]
- Mesnage, R.; Antoniou, M.N. Ignoring Adjuvant Toxicity Falsifies the Safety Profile of Commercial Pesticides. Front. Public Health 2017, 5, 361. [Google Scholar] [CrossRef] [Green Version]
- Cl, A.; Jie, Z.A.; Ning, Y.A.; Yw, B.; Jing, W.A.; Fj, A. Dissipation and dietary risk assessment of tristyrylphenol ethoxylate homologues in cucumber after field application. Food Chem. 2020, 338, 127–988. [Google Scholar]
- Jiang, D.; Cheng, Z.; Chen, X.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Pan, X.; An, X.; Zheng, Y. Occurrences of eight common-used pesticide adjuvants in ten vegetable species and implications for dietary intake in North China. Food Chem. 2021, 347, 128–984. [Google Scholar] [CrossRef]
- Cserhati, T. Alkyl Ethoxylated and Alkylphenol Ethoxylated Nonionic Surfactants: Interaction with Bioactive Compounds and Biological Effects. Environ. Health Perspect. 1995, 103, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Revilla, A.S.; Pestana, C.R.; Pardo-Andreu, G.L.; Santos, A.C.; Uyemura, S.A.; Gonzales, M.E.; Curti, C. Potential toxicity of toluene and xylene evoked by mitochondrial uncoupling. Toxicol. Vitr. 2007, 21, 782–788. [Google Scholar] [CrossRef]
- Johnson, E.M.; Gabel, B.; Christian, M.S.; Sica, E. The developmental toxicity of xylene and xylene isomers in the Hydra assay. Toxicol. Appl. Pharmacol. 1986, 82, 323–328. [Google Scholar] [CrossRef]
- Salama, M.M.; El-Naggar, D.A.; Abdel-Rahman, R.H.; Elhak, S.A.G. Toxic Effects of Trichloroethylene on Rat Neuroprogenitor Cells. Front. Pharmacol. 2018, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Piola, L.; Fuchs, J.; Oneto, M.L.; Basack, S.; Kesten, E.; Casabe, N. Comparative toxicity of two glyphosate-based formulations to Eisenia andrei under laboratory conditions. Chemosphere 2013, 91, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, Q.; Zhang, J.; Yuan, S.; Liu, X. Joint Toxicity of Acetamiprid and Co-Applied Pesticide Adjuvants on Honeybees under Semifield and Laboratory Conditions. Environ. Toxicol. Chem. 2019, 38, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Yuan-Qing, B.U.; Wang, Z.C.; Zhi, Y.; Wang, J.Y.; Shan, Z.J. Investigation and Risk Analysis of Pesticide Inert Ingredients in Pesticide Products. Agrochemicals 2014, 53, 932–936. [Google Scholar]
- Duan, W.; Meng, F.; Wang, F.; Liu, Q. Environmental behavior and eco-toxicity of xylene in aquatic environments: A review. Ecotoxicol. Environ. Saf. 2017, 145, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Portales, A.M.; Batcho, K.G.; Freeman, J.L. Developmental toxicity of trichloroethylene in zebrafish (Danio rerio). Environ. Sci. Process Impacts 2020, 22, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.; Yin, L.; Cancan, Z.; Shulan, Z. Ecotoxicological responses of the earthworm Eisenia fetida to EDTA addition under turfgrass growing conditions. Chemosphere 2018, 220, 56–60. [Google Scholar]
- Noah, F. Earthworms’ place on Earth. Science 2019, 366, 425–426. [Google Scholar]
- Yao, X.; Zhang, F.; Qiao, Z.; Yu, H.; Sun, S.; Li, X.; Zhang, J.; Jiang, X. Toxicity of thifluzamide in earthworm (Eisenia fetida). Ecotoxicol. Environ. Saf. 2020, 188, 109880. [Google Scholar] [CrossRef] [PubMed]
- Yuanbo, L.; Xing, W.; Zhenjun, S. Ecotoxicological effects of petroleum-contaminated soil on the earthworm Eisenia fetida. J. Hazard. Mater. 2020, 393, 122384. [Google Scholar]
- OECD. Earthworm Acute Toxicity Tests. Guidelines for the Testing of Chemicals; Test no. 207; Organization for Economic Cooperation and Development: Paris, France, 1984. [Google Scholar]
- Standardization Administration of the People’ s Republic of China. Part 15: Earthworm acute toxicity test GB/T 3127015-2014. In Test Guidelines on Environmental Safety Assessment for Chemical Pesticides; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2014. [Google Scholar]
- An, Y.J. Assessing soil ecotoxicity of methyl tert-butyl ether using earthworm bioassay; closed soil microcosm test for volatile organic compounds. Environ. Pollut. 2005, 134, 181–186. [Google Scholar]
- ISO. Part 1: Tests with earthworms (Eisenia fetida and Eisenia andrei) ISO/DIS 17512-1. In Soil Quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour; ISO (International Organization for Standardization): Geneva, Switzerland, 2008. [Google Scholar]
- Chao, G.; Ji, L.; Zhengtao, L.; Jingbo, X. Avoidance Behavior and Acute Toxicity of Eisenia fetida under Exposure to Lead, Cadmium and Chromium in Soil. Res. Environ. Sci. 2015, 28, 1596–1601. [Google Scholar]
- Marking. Toxicity of chemical mixtures. In Fundamentals of Aquatic Toxicology; Rand, G., Petroceli, S., Eds.; Hemisphere Publishing Corporation: Washington, DC, USA, 1985; pp. 164–176. [Google Scholar]
- Tang, R.; Lan, P.; Ding, C.; Wang, J.; Zhang, T.; Wang, X. A new perspective on the toxicity of arsenic-contaminated soil: Tandem mass tag proteomics and metabolomics in earthworms. J. Hazard Mater. 2020, 398, 122825. [Google Scholar] [CrossRef]
- Shao, Y.; Du, Z.; Zhang, C.; Zhu, L.; Wang, J.; Wang, J. Acute Toxicity of Imidazole Nitrate Ionic Liquids with Varying Chain Lengths to Earthworms (Eisenia foetida). Bull. Environ. Contam. Toxicol. 2017, 99, 213–217. [Google Scholar] [CrossRef]
- Verhaar, H.; Leeuwen, C.; Hermens, J. Classifying environmental pollutants. Chemosphere 1992, 25, 471–491. [Google Scholar] [CrossRef]
- Marzio, W.D.; Saenz, M.E. QSARs for aromatic hydrocarbons at several trophic levels. Environ. Toxicol. 2010, 21, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Qian, Y.; Zhao, X.; Wang, Q. Ternary toxicological interactions of insecticides, herbicides, and a heavy metal on the earthworm Eisenia fetida. J. Hazard. Mater. 2015, 284, 233–240. [Google Scholar] [CrossRef]
- Belden, J.B.; Lydy, M.J. Joint toxicity of chlorpyrifos and esfenvalerate to fathead minnows and midge larvae. Environ. Toxicol. Chem. 2010, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ya, Z.; Jianying, Z.; Yaoxuan, L.; Gangping, S.; Lizhong, Z.; Daohui, L. Environmentally Relevant Concentrations of the Flame Retardant Tris(1,3-dichloro-2-propyl) Phosphate Inhibit the Growth and Reproduction of Earthworms in Soil. Environ. Sci. Technol. Lett. 2019, 212, 358–364. [Google Scholar]
- Gintarė, S.; Jūratė, Č. Assessment of Toxicity to Earthworm Eisenia fetida of Lead Contaminated Shooting Range Soils with Different Properties. Bull. Environ. Contam. Toxicol. 2019, 103, 559–564. [Google Scholar]
- Yan, X.; Wang, J.; Zhu, L.; Wang, J.; Li, S.; Kim, Y.M. Oxidative stress, growth inhibition, and DNA damage in earthworms induced by the combined pollution of typical neonicotinoid insecticides and heavy metals. Sci. Total Environ. 2020, 754, 141–873. [Google Scholar]
- Xian, C.; Wang, X.; Gu, X.; Yang, J.; Rong, J. Oxidative stress responses and insights into the sensitivity of the earthworms Metaphire guillelmi and Eisenia fetida to soil cadmium. Sci. Total Environ. 2017, 574, 300–306. [Google Scholar]
- Liu, T.; Wang, X.; Chen, D.; Li, Y.; Wang, F. Growth, reproduction and biochemical toxicity of chlorantraniliprole in soil on earthworms (Eisenia fetida). Ecotoxicol. Environ. Saf. 2017, 150, 18–25. [Google Scholar] [CrossRef]
- Liang, J.; Xia, X.; Zaman, W.Q.; Zhang, W.; Lin, K.; Hu, S.; Lin, Z. Bioaccumulation and toxic effects of decabromodiphenyl ether in the presence of nanoscale zero-valent iron in an earthworm-soil system. Chemosphere 2017, 169, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, Z.; Zhu, T.; Shu, W. Toxicity of soil antimony to earthworm Eisenia fetida (Savingy) before and after the aging process. Ecotoxicol. Environ. Saf. 2020, 207, 111–278. [Google Scholar]
- Liu, Y.; Xu, K.; Cheng, J. Different Nanomaterials for Soil Remediation Affect Avoidance Response and Toxicity Response in Earthworm (Eisenia fetida). Bull. Environ. Contam. Toxicol. 2020, 104, 477–483. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Y.-X.; Wang, X.-F.; Cheng, J.-M. Effect of Nano-Carbon Black Surface Modification on Toxicity to Earthworm (Eisenia fetida) Using Filter Paper Contact and Avoidance Test. Bull. Environ. Contam. Toxicol. 2019, 103, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Rmbke, J.; de Brito, M.T.; Scheffczyk, A. Effects of three pesticides on the avoidance behavior of earthworms in laboratory tests performed under temperate and tropical conditions. Environ. Pollut. 2008, 153, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Brighton, M.M.; Willis, G.; Munyaradzi, G.M. Ecotoxicological effects of citrus processing waste on earthworms, Lumbricus terrestris L. Ind. Crop. Prod. 2017, 110, 123–129. [Google Scholar]
- Syed, Z.; Alexander, D.; Ali, J.; Unrine, J.; Shoults-Wilson, W.A. Chemosensory cues alter earthworm (Eisenia fetida) avoidance of lead-contaminated soil. Environ. Toxicol. Chem. 2016, 36, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, J.; Zhao, L.; Li, J.; Liu, H. Effects of perfluorooctanoic acid and perfluorooctane sulfonate on acute toxicity, superoxide dismutase, and cellulase activity in the earthworm Eisenia fetida. Environ. Sci. Pollut. Res. 2017, 24, 18188–18194. [Google Scholar]
- Lowe, C.N.; Butt, K.R.; Cheynier, Y.M. Assessment of avoidance behaviour by earthworms (Lumbricus rubellus and Octolasion cyaneum) in laboratory-based, linear pollution gradients. Ecotoxicol. Environ. Saf. 2016, 124, 324–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brami, C.; Glover, A.R.; Butt, K.R.; Lowe, C.N. Avoidance, biomass and survival response of soil dwelling (endogeic) earthworms to OECD artificial soil: Potential implications for earthworm ecotoxicology. Ecotoxicology 2017, 26, 576–579. [Google Scholar] [CrossRef] [Green Version]
- Zhi, Z.L. Investigation of Volatile Organic Compounds Contamination in Soil of a Pesticide Production Site in Changzhou. Environ. Monit. China 2012, 28, 67–71. [Google Scholar]
- Tan, B.; Wang, T.-Y.; Li, Q.-F.; Zhang, H.-Y.; Pang, B.; Zhu, Z.-Y.; Wang, D.-H.; Lv, Y.-L. Risk Assessment and Countermeasures of BTEX Contamination in Soils of Typical Pesticide Factory. Environ. Sci. 2014, 35, 2272–2280. [Google Scholar]
| Chemical | CAS Number | Purity (%) | Water Solubility (mg/L, 25 °C) | Log Kow a |
|---|---|---|---|---|
| Toluene | 108-88-3 | 99.5 | 515 | 2.69 |
| Xylene | 1330-20-7 | 99.0 | 160 | 3.12~3.20 |
| Trichloroethylene | 79-01-6 | 99.0 | 1100 | 2.42 |
| Chemical | Exposure Time (h) | LC50 (95% CI a)/(mg/kg) | Regression Equation | R2 |
|---|---|---|---|---|
| Toluene | 24 | 1190.45 (1134.75~1283.74) | Y = −37.81 + 12.3X | 0.992 |
| 48 | 962.89 (933.45~991.14) | Y = −46.45 + 15.56X | 0.989 | |
| Xylene | 24 | 300.23 (284.59~317.15) | Y = −20.45 + 8.26X | 0.960 |
| 48 | 221.62 (202.35~236.66) | Y = −19.47 + 8.30X | 0.960 | |
| Trichloroethylene | 24 | 5332.36 (4948.86~5679.19) | Y = −26.13 + 7.01X | 0.936 |
| 48 | 4522.41 (4124.26~4833.91) | Y = −29.92 + 8.18X | 0.978 |
| Exposure Time (h) | LC50 (95% CI d)/(mg/kg) | Mixing Rate (95% CI)/(%) | AI (95% CI) | Interaction Type | ||
|---|---|---|---|---|---|---|
| Am a | Bm b | Cm c | ||||
| 24 | 270.52 (254.35~290.79) | 62.26 (58.54~66.93) | 1270.57 (1194.60~1365.77) | 28.10 (26.42~30.20) | 0.49 (0.38~0.58) | Synergism |
| 48 | 199.41 (166.53~226.66) | 45.90 (38.33~52.17) | 936.60 (782.15~1064.58) | 20.71 (17.30~23.54) | 0.61 (0.42~0.93) | Synergism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, J.; Wu, J.; Yu, R.; Zhang, Y.; Sun, L.; Gao, Y. Acute Toxicity and Ecotoxicological Risk Assessment of Three Volatile Pesticide Additives on the Earthworm—Eisenia fetida. Int. J. Environ. Res. Public Health 2021, 18, 11232. https://doi.org/10.3390/ijerph182111232
Wang W, Zhang J, Wu J, Yu R, Zhang Y, Sun L, Gao Y. Acute Toxicity and Ecotoxicological Risk Assessment of Three Volatile Pesticide Additives on the Earthworm—Eisenia fetida. International Journal of Environmental Research and Public Health. 2021; 18(21):11232. https://doi.org/10.3390/ijerph182111232
Chicago/Turabian StyleWang, Wenqiang, Jing Zhang, Jingya Wu, Ran Yu, Yimin Zhang, Liwei Sun, and Yuexiang Gao. 2021. "Acute Toxicity and Ecotoxicological Risk Assessment of Three Volatile Pesticide Additives on the Earthworm—Eisenia fetida" International Journal of Environmental Research and Public Health 18, no. 21: 11232. https://doi.org/10.3390/ijerph182111232






