Metabarcoding Analysis of Harmful Algal Bloom Species in the Western Pacific Seamount Regions
Abstract
:1. Introduction
2. Materials and Methods
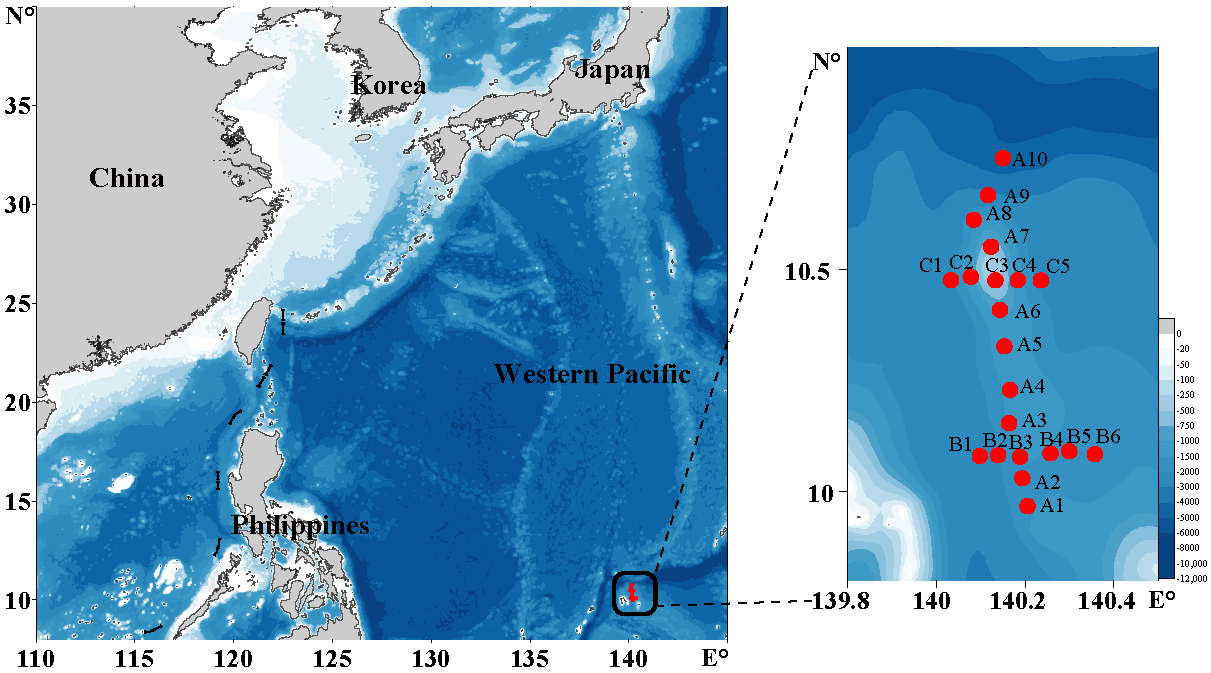
2.1. Sampling Sites and Sample Preparation
2.2. DNA Extraction and Sequencing
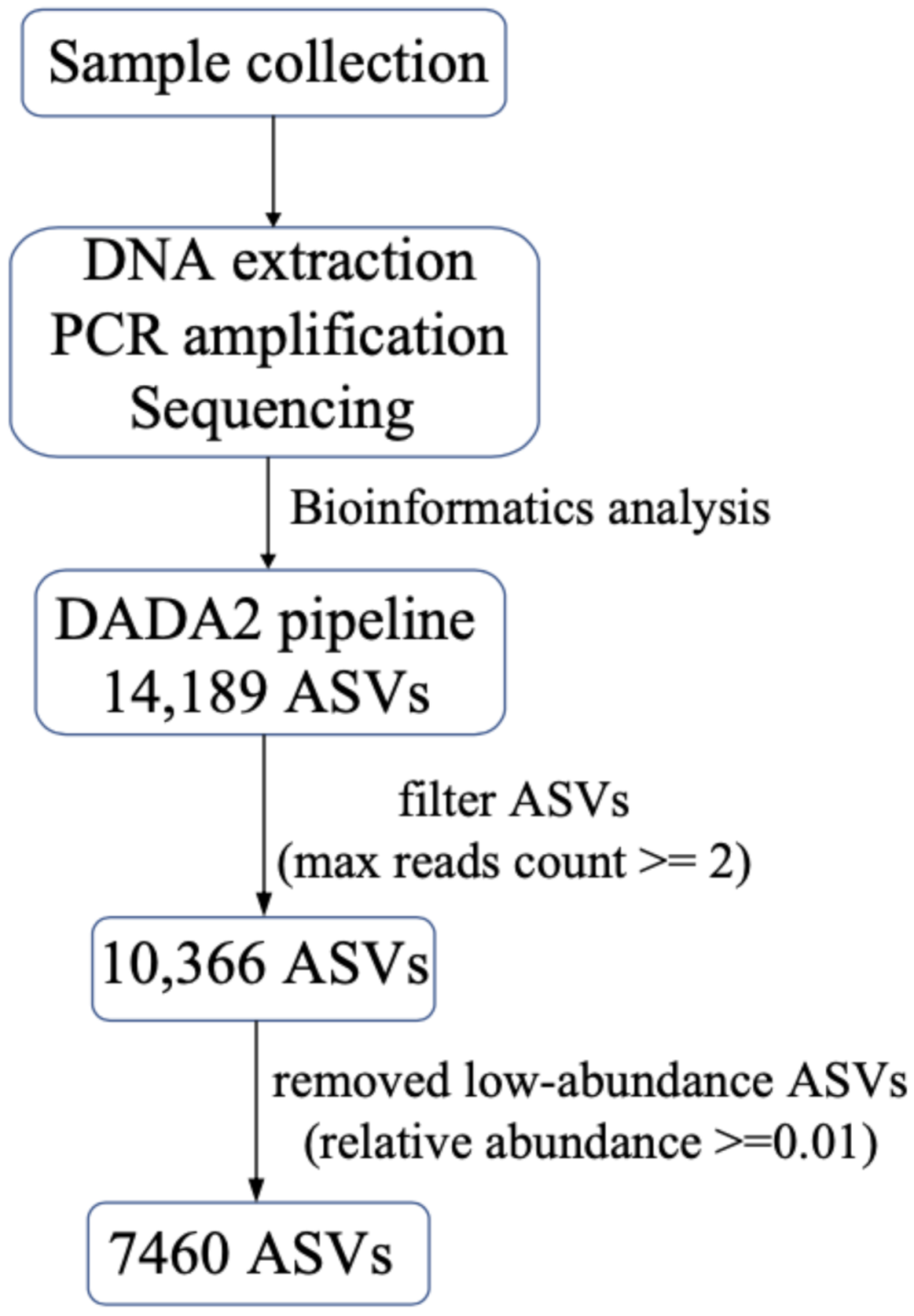
2.3. Bioinformatics Analysis
3. Results
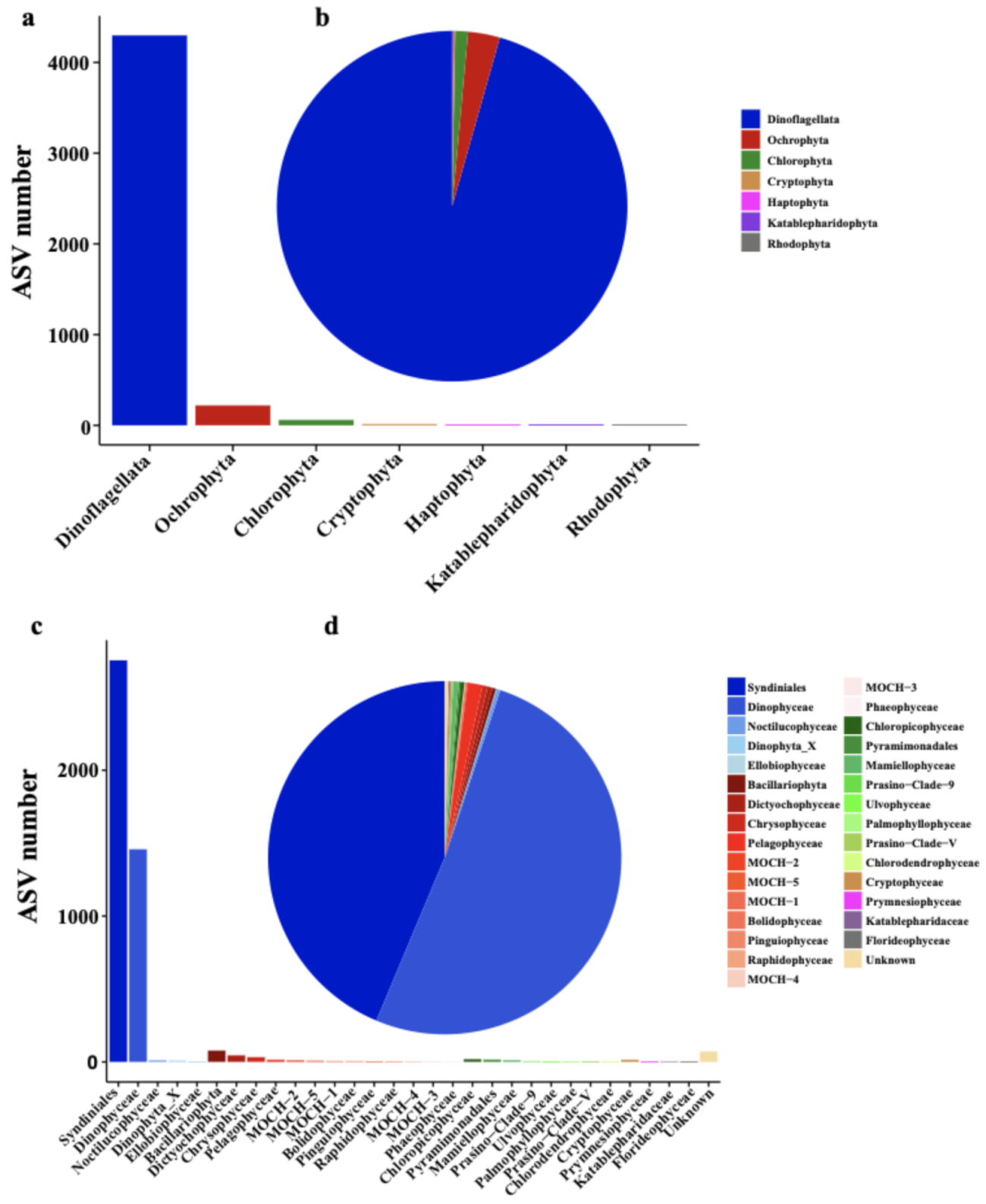
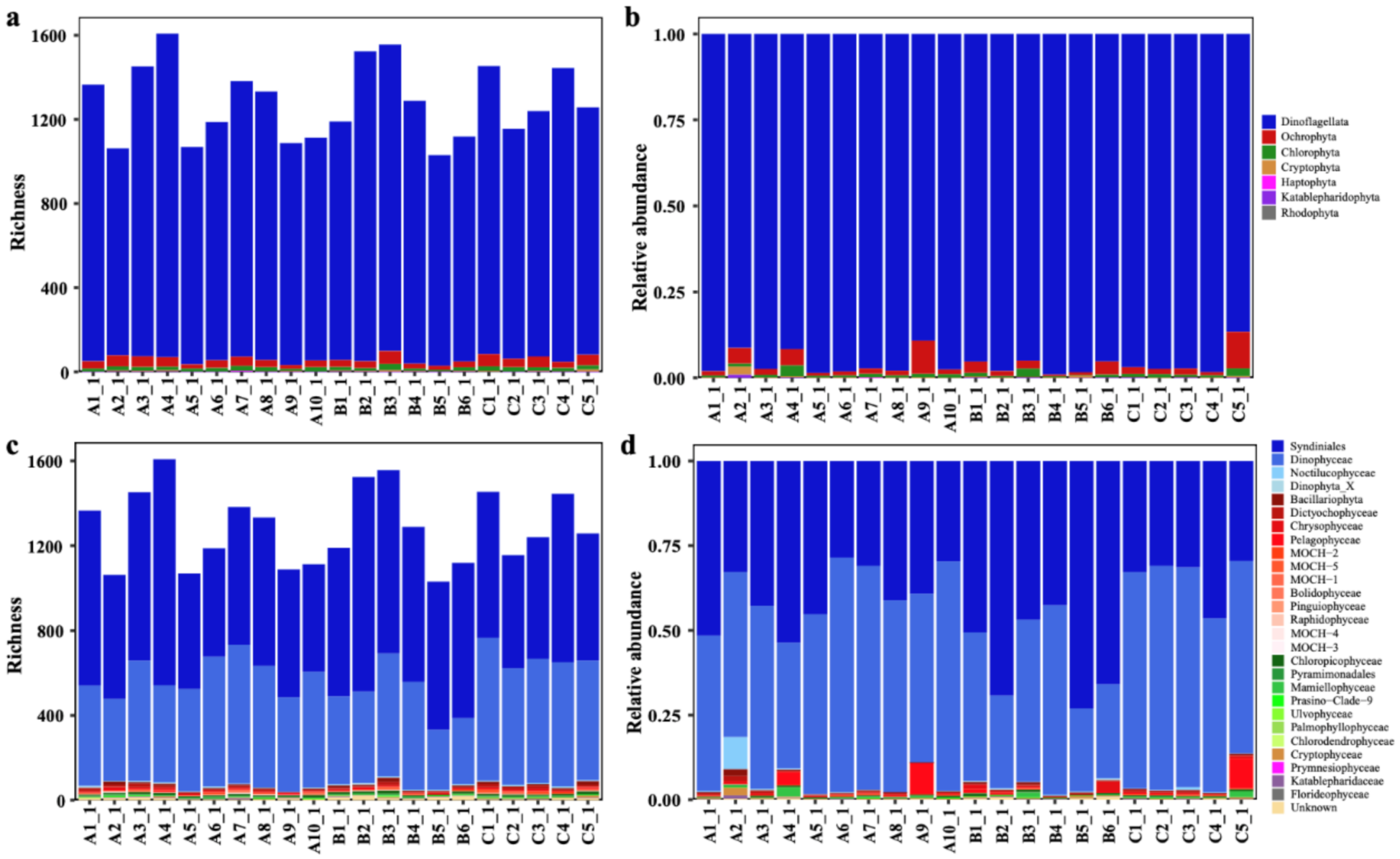
3.1. Phytoplankton Composition and Relative Abundance in the Western Pacific Seamount Regions
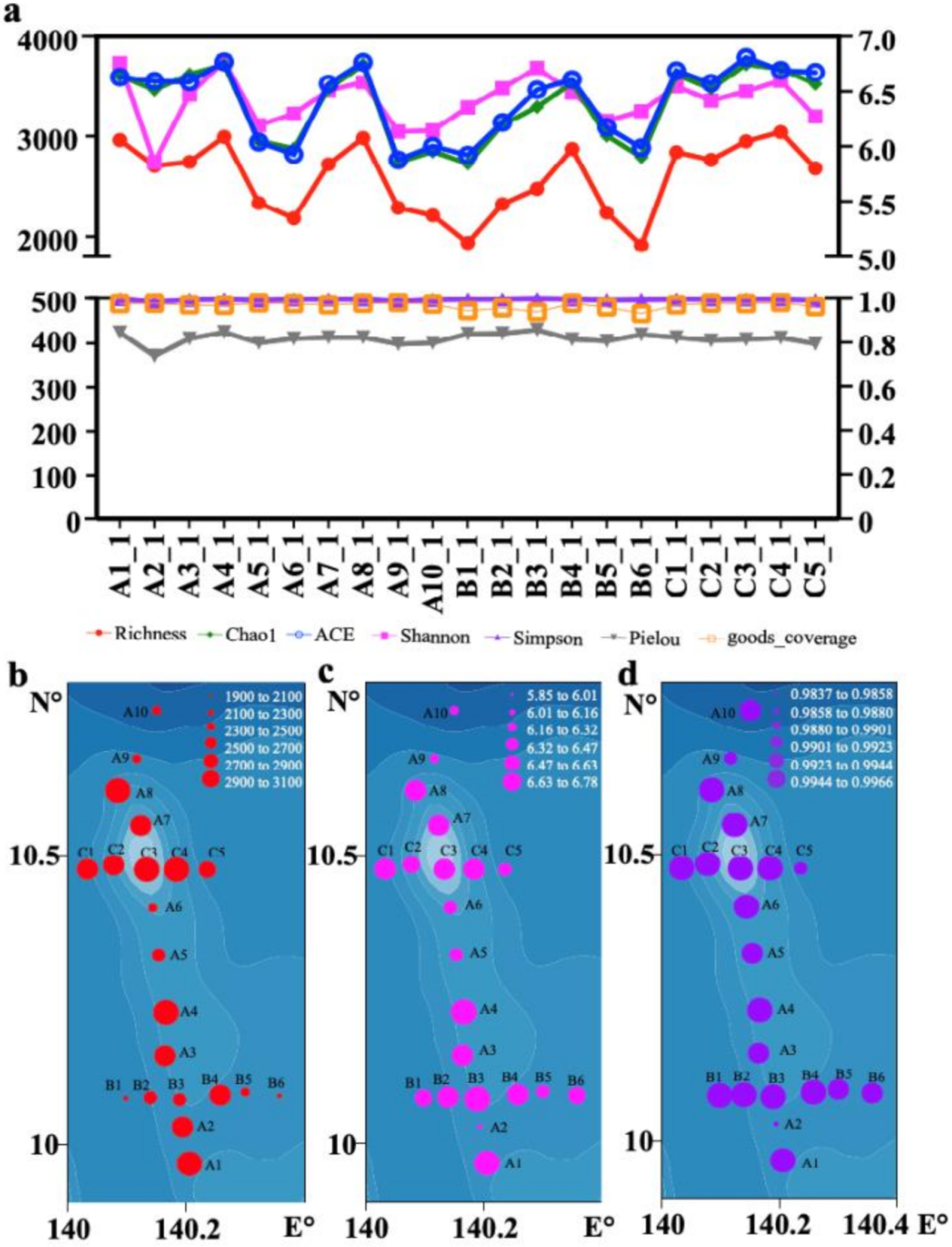
3.2. Phytoplankton Composition, Diversity, and Distribution Patterns in the Western Pacific Seamount Regions
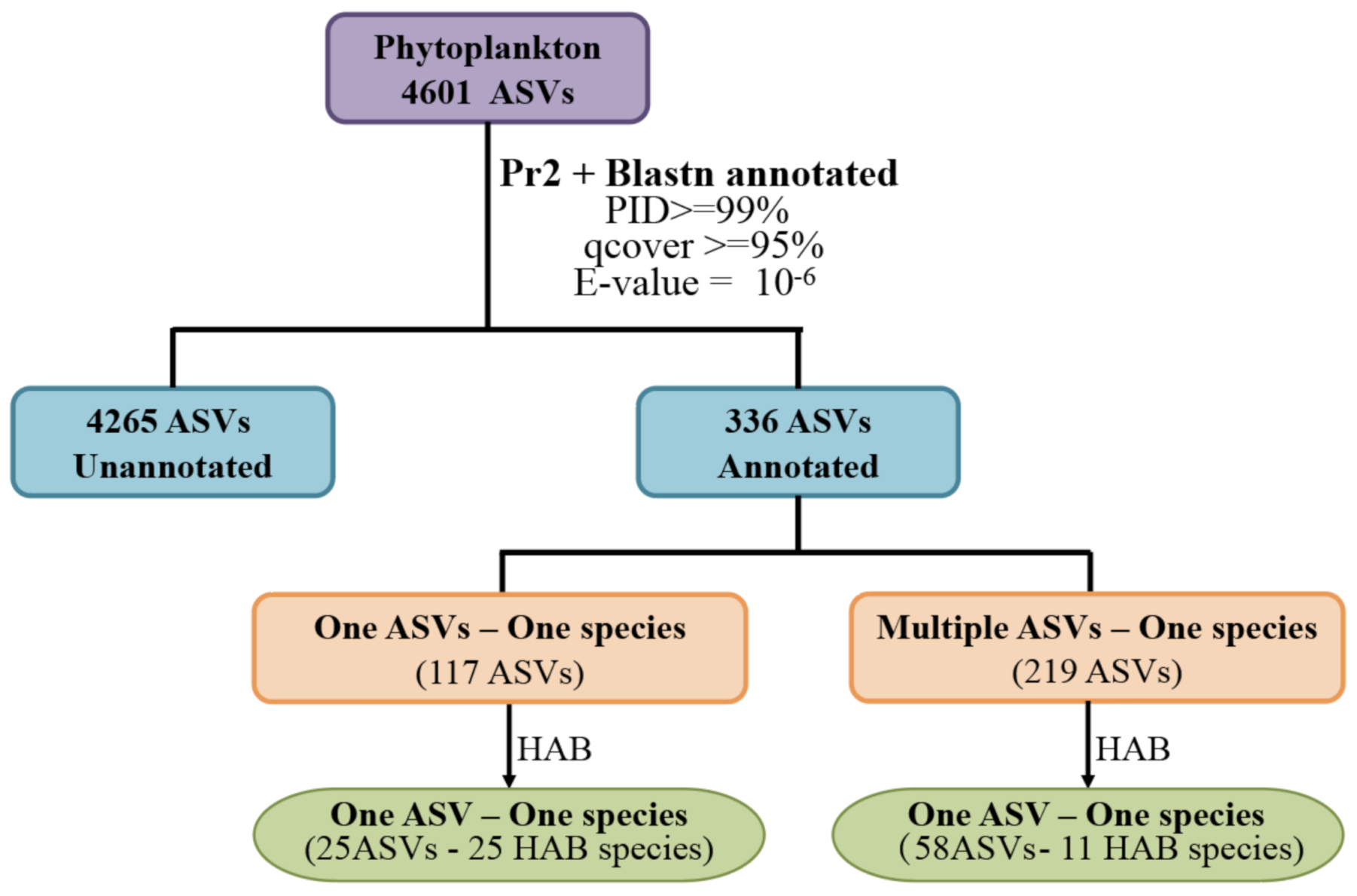
3.3. HAB Species Composition and Distribution in the Western Pacific Seamount Regions
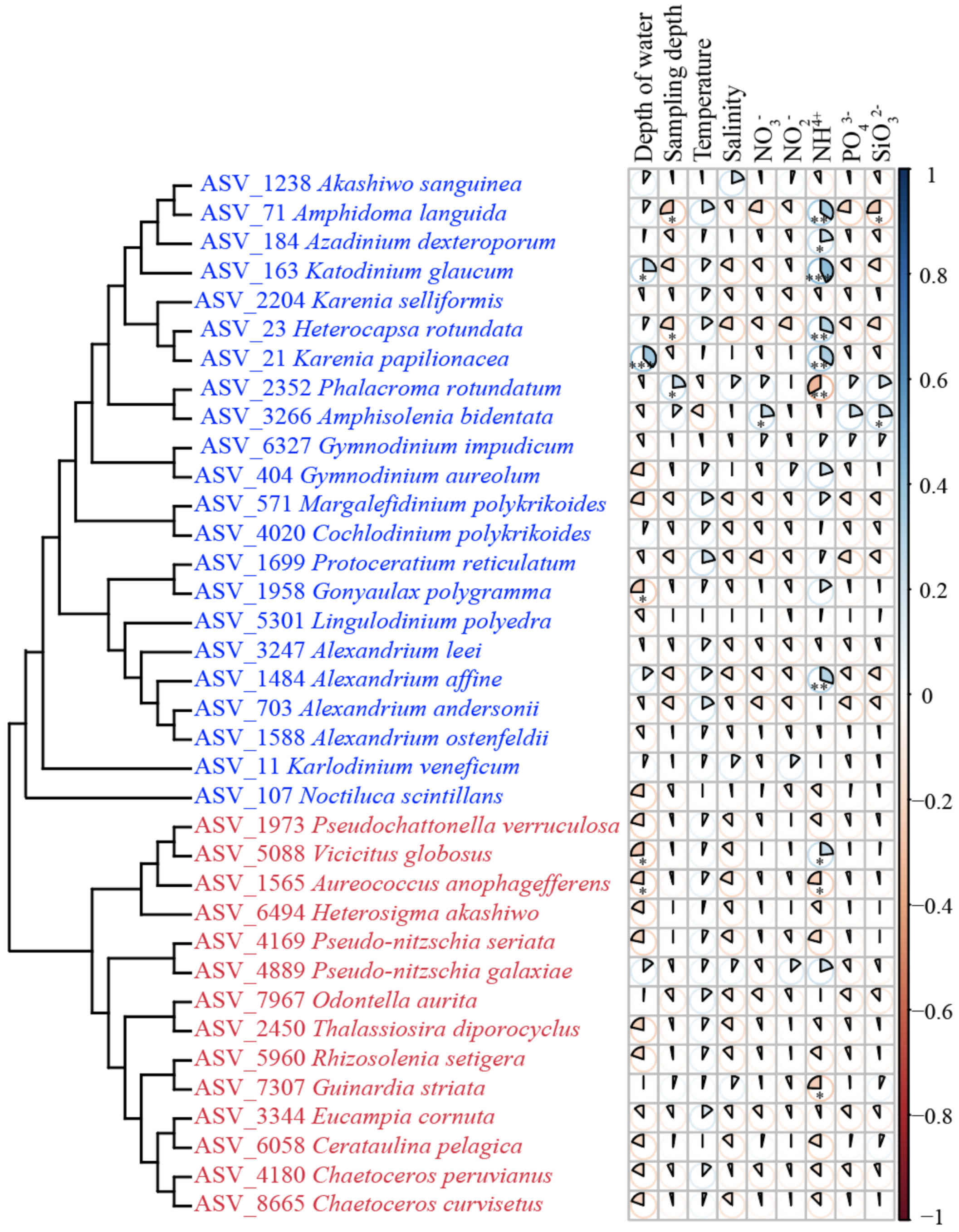
3.4. Environmental Factors Correlated with HAB Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granéli, E.; Weberg, M.; Salomon, P.S. Harmful algal blooms of allelopathic microalgal species: The role of eutrophication. Harmful Algae 2008, 8, 94–102. [Google Scholar] [CrossRef]
- Lin, S.; Ji, N.; Luo, H. Recent progress in marine harmful algal bloom research. Oceanol. Et Limnol. Sinica 2019, 50, 496–508. [Google Scholar]
- Smayda, T.J. Reflections on the ballast water dispersal—harmful algal bloom paradigm. Harmful Algae 2007, 6, 601–622. [Google Scholar] [CrossRef]
- Doblin, M.A.; Popels, L.C.; Coyne, K.J.; Hutchins, D.A.; Cary, S.C.; Dobbs, F.C. Transport of the harmful bloom alga Aureococcus anophagefferens by oceangoing ships and coastal boats. Appl. Environ. Microbiol. 2004, 70, 6495–6500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Probyn, T.; Pitcher, G.; Pienaar, R.; Nuzzi, R. Brown Tides and Mariculture in Saldanha Bay, South Africa. Mar. Pollut. Bull. 2001, 42, 405–408. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Qiu, L.M.; Yu, R.C.; Kong, F.Z.; Wang, Y.F.; Yan, T.; Gobler, C.J.; Zhou, M.-J. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae 2012, 19, 117–124. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Ma, Z.; Hu, Z.; Deng, Y.; Yang, A.; Lin, S.; Yi, L.; Chai, Z.; Gobler, C.J. 3000 km and 1500-year presence of Aureococcus anophagefferens reveals indigenous origin of brown tides in China. Mol. Ecol. 2019, 28, 4065–4076. [Google Scholar] [CrossRef]
- Pitcher, T.J.; Bulman, C. Raiding the larder: A quantitative evaluation framework and strophic signature for seamount food webs. In Seamounts: Ecology, Fisheries, and Conservation; Pitcher, T.J., Morato, T., Hart, P.J.B., Clark, M.R., Haggan, N., Santos, R.S., Eds.; Blackwell: Oxford, UK, 2007; pp. 282–295. [Google Scholar]
- Comeau, L.A.; Vezina, A.F.; Bourgeois, M.; Juniper, S.K. Relationship between phytoplankton production and the physical structure of the water column near Cobb Seamount, northeast Pacific. Deep-Sea Res. 1995, 42, 993–1005. [Google Scholar] [CrossRef]
- Mourino, B.; Fernandez, E.; Serret, P.; Harbour, D.; Sinha, B.; Pingree, R. Variability and seasonality of physical and biological fields at the Great Meteor Tablemount (subtropical NE Atlantic). Oceanol. Acta. 2001, 24, 167–185. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.P.; Coutinho, T.P.; Cabeçadas, G.; Brogueira, M.J.; Coca, J.; Ramos, M.; Calado, G.; Duarte, P. Primary production enhancement in a shallow seamount (Gorringe—Northeast Atlantic). J. Mar. Syst. 2016, 164, 13–29. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Wang, C.; Zhang, W.; Sun, X.; Li, X.; Zhao, Y.; Xiao, T. Comparison in the distribution of microbial food web components in the Y3 and M2 seamounts in the tropical western pacific. Oceanol. Limnol. Sinica 2017, 48, 1446–1455. [Google Scholar]
- Chen, Z.; Sun, J.; Zhang, G. Netz-phytoplankton community structure of the tropical Western Pacific Ocean in summer 2016. Mar. Sci. 2018, 42, 114–130. [Google Scholar]
- Guo, S.; Sun, X.; Zheng, S.; Luo, X.; Zhu, M.; Liang, J. Net phytoplankton community structure in the Y3 and M2 seamount zone in the Western Tropical Pacific. Mar. Sci. 2018, 42, 31–40. [Google Scholar]
- Bik, H.M.; Porazinska, D.L.; Creer, S.; Caporaso, J.G.; Knight, R.; Thomas, W.K. Sequencing our way towards understanding global eukaryotic biodiversity. Trends Ecol. Evol. 2012, 27, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.F.; Li, D.X.; Kong, L.F.; Li, Y.Y.; Zhang, H.; Xie, Z.X.; Lin, L.; Wang, D.Z. The diversity and biogeography of microeukaryotes in the euphotic zone of the northwestern Pacific Ocean. Sci. Total Environ. 2020, 698, 134289. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Method 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Gibson, K.; Cui, Z.; Chen, Y.; Sun, X.; Chen, N. Metabarcoding analysis of harmful algal species in Jiaozhou Bay. Harmful Algae 2020, 92, 101772. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.; Breiner, H.W.; Richards, T.A. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 2010, 19 (Suppl. S1), 21–31. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013, 41, D597–D604. [Google Scholar] [CrossRef] [Green Version]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Chao, A.; Yang, M. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika 1993, 80, 193–201. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. A Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- Good, I.J. The population frequencies of species and the estimation of population parameters. Biometrika 1953, 40, 237–264. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research; Illinois R Package Version 2.0.8; Northwestern University: Evanston, IL, USA, 2020. [Google Scholar]
- Pering, T.D.; Tamburello, G.; McGonigle, A.J.S.; Hanna, E.; Aiuppa, A. Correlation of oscillatory behaviour in Matlab using wavelets. Comput. Geosci. 2014, 70, 206–212. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [Green Version]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D.; Nakagawa, S. popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Chen, N.S.; Chen, Y. Advances in the study of biodiversity of phytoplankton and red tide species in China (II): The East China Sea. Oceanol. Et Limnol. Sinica 2021, 52, 363–384. [Google Scholar]
- Chen, N.S.; Cui, Z.M.; Xu, Q. Advances in the study of biodiversity of phytoplankton and red tide species in China (IV): The Changjiang Estuary. Oceanol. Et Limnol. Sinica 2021, 52, 402–417. [Google Scholar]
- Wang, J. HAB alga nearby Changjiang Estuary. Mar. Environ. Sci. 2002, 21, 37–41. [Google Scholar]
- Qin, Q.; Shen, J.; Reece, K.S.; Mulholland, M.R. Developing a 3D mechanistic model for examining factors contributing to harmful blooms of Margalefidinium polykrikoides in a temperate estuary. Harmful Algae 2021, 105, 102055. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, Ø.; Akselmann-Cardella, R.; Churro, C.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Lundholm, N.; Zingone, A. IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae; 2009; unpublished. [Google Scholar]
- MNR. Bulletin of China Marine Disaster; Ministry of Natural Resources: Beijing, China, 1989–2019.
- Guo, H. Illustrations of Planktons Responsible for the Blooms in Chinese Coastal Waters; Ocean Press: Beijing, China, 2004; pp. 1–107. [Google Scholar]
- Chang, F.H.; McVeagh, M.; Gall, M.; Smith, P. Chattonella globosa is a member of Dictyochophyceae: Reassignment to Vicicitus gen. nov., based on molecular phylogeny, pigment composition, morphology and life history. Phycologia 2012, 51, 403–420. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Lin, S. Enhancement of Non-photochemical Quenching as an Adaptive Strategy under Phosphorus Deprivation in the Dinoflagellate Karlodinium veneficum. Front. Microbiol. 2017, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hirano, T.; Yoshimatsu, T.; Tanimoto, Y.; Matsumoto, T.; Suzuki, S.; Hayashi, Y.; Urabe, A.; Miyamura, K.; Sakamoto, S.; et al. Occurrence of Karenia papilionacea (Dinophyceae) and its novel sister phylotype in Japanese coastal waters. Harmful Algae 2016, 57, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, M.; Fukuyo, Y.; Matsuda, O.; Lee, S.G.; Lee, C.K.; Shulkin, V.; Orlova, T.; Kim, H.G.; Lu, S. Integrated Report on Harmful Algal Blooms (HABs) for the NOWPAP Region; NOWPAP CEARAC: Toyama, Japan, 2005. [Google Scholar]
- Chen, Z.; Sun, J.; Chen, D.; Wang, S.; Yu, H.; Chen, H.; Wang, M. Effects of Ocean Currents in the Western Pacific Ocean on Net-Phytoplankton Community Compositions. Diversity 2021, 13, 428. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, X.; Irwin, A.J.; Laws, E.A.; Wang, L.; Chen, B.; Zeng, Y.; Huang, B. Warming and eutrophication combine to restructure diatoms and dinoflagellates. Water Res. 2018, 128, 206–216. [Google Scholar] [CrossRef]
- Lin, S. Genomic understanding of dinoflagellates. Res. Microbiol. 2011, 162, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.D.; Jones, A.C.; Schnetzer, A.; Countway, P.D.; Tomas, C.R.; Kudela, R.M.; Hayashi, K.; Chia, P.; Caron, D.A. Quantitative Real-Time Polymerase Chain Reaction for Cochlodinium fulvescens (Dinophyceae), a Harmful Dinoflagellate from California Coastal Waters(1). J. Phycol. 2012, 48, 384–393. [Google Scholar] [CrossRef]
- Zhu, F.; Massana, R.; Not, F.; Marie, D.; Vaulot, D. Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol. Ecol. 2005, 52, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.R.; Harvey, E.L. Temporal Variability and Ecological Interactions of Parasitic Marine Syndiniales in Coastal Protist Communities. mSphere 2020, 5, e00209-20. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, H.J.; Kwon, J.E.; Kang, H.C.; Kim, J.H.; Jang, S.H.; Park, J.Y.; Yoon, E.Y.; Kim, J.S. Mixotrophic ability of the phototrophic dinoflagellates Alexandrium andersonii, A. affine, and A. fraterculus. Harmful Algae 2016, 59, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Fattorussoa, E.; Forinoa, M.; Montresorb, M. Saxitoxin and neosaxitoxin as toxic principles of Alexandrium andersoni (Dinophyceae) from the Gulf of Naples, Italy. Toxicon 2000, 38, 1871–1877. [Google Scholar] [CrossRef]
- Sampedro, N.; Franco, J.M.; Zapata, M.; Riobó, P.; Garcés, E.; Penna, A.; Caillaud, A.; Diogène, J.; Cacho, E.; Camp, J. The toxicity and intraspecific variability of Alexandrium andersonii Balech. Harmful Algae 2013, 25, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Zeng, N.; Liu, T.; Yang, W.; Müller, A.; Krock, B. Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae 2013, 27, 68–81. [Google Scholar] [CrossRef]
- Razali, R.M.; Leaw, C.-P.; Lim, H.-C.; Ayub, M.N.A.; Rahim, M.; Lim, P.T. First report of a marine dinoflagellate, Alexandrium andersonii (Dinophyceae) in Malaysian waters. Malays. J. Sci. 2016, 35, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.K.; Chun, S.J.; Kim, J.H.; Park, B.S.; Baek, S.H. Short-term response of pelagic planktonic communities after inoculation with the mass cultured dinoflagellate Alexandrium affine in a large-scale mesocosm experiment. J. Appl. Phycol. 2021, 33, 3123–3137. [Google Scholar] [CrossRef]
- Shang, L.; Xu, Y.; Leaw, C.P.; Lim, P.T.; Wang, J.; Chen, J.; Deng, Y.; Hu, Z.; Tang, Y.Z. Potent allelopathy and non-PSTs, non-spirolides toxicity of the dinoflagellate Alexandrium leei to phytoplankton, finfish and zooplankton observed from laboratory bioassays. Sci. Total Environ. 2021, 780, 146484. [Google Scholar] [CrossRef] [PubMed]
- Usup, G.; Pin, L.C.; Ahmad, A.; Teen, L.P. Alexandrium (Dinophyceae) species in Malaysian waters. Harmful Algae 2002, 1, 265–275. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Kong, L.; Holmes, M.J. Dinoflagellate Alexandrium leei (Dinophyceae) from Singapore coastal waters produces a water-soluble ichthyotoxin. Mar. Biol. 2006, 150, 541–549. [Google Scholar] [CrossRef]
- Shikata, T.; Taniguchi, E.; Sakamoto, S.; Kitatsuji, S.; Yamasaki, Y.; Yoshida, M.; Oikawa, H. Phylogeny, growth and toxicity of the noxious red-tide dinoflagellate Alexandrium leei in Japan. Reg. Stud. Mar. Sci. 2020, 36, 101265. [Google Scholar] [CrossRef]
- Gobler, C.J.; Sunda, W.G. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae 2012, 14, 36–45. [Google Scholar] [CrossRef]
- Nézan, E.; Siano, R.; Boulben, S.; Six, C.; Bilien, G.; Chèze, K.; Duval, A.; Le Panse, S.; Quéré, J.; Chomérat, N. Genetic diversity of the harmful family Kareniaceae (Gymnodiniales, Dinophyceae) in France, with the description of Karlodinium gentienii sp. nov.: A new potentially toxic dinoflagellate. Harmful Algae 2014, 40, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.D.; Jeanette, G. Five red tide species in genus Prorocentrum including the description of Prorocentrum donghaiense Lu SP. nov. from the East China Sea. Chin. J. Oceanol. Limnol. 2001, 19, 337–344. [Google Scholar]
- Li, J.; Glibert, P.M.; Zhou, M. Temporal and spatial variability in nitrogen uptake kinetics during harmful dinoflagellate blooms in the East China Sea. Harmful Algae 2010, 9, 531–539. [Google Scholar] [CrossRef]
- Shin, H.H.; Li, Z.; Mertens, K.N.; Seo, M.H.; Gu, H.; Lim, W.A.; Yoon, Y.H.; Soh, H.Y.; Matsuoka, K. Prorocentrum shikokuense Hada and P. donghaiense Lu are junior synonyms of P. obtusidens Schiller, but not of P. dentatum Stein (Prorocentrales, Dinophyceae). Harmful Algae 2019, 89, 101686. [Google Scholar] [CrossRef]
- Zarauz, L.; Irigoien, X. Effects of Lugol’s fixation on the size structure of natural nano-microplankton samples, analyzed by means of an automatic counting method. J. Plankton Res. 2008, 30, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Gomez, F.; Takayama, H.; Moreira, D.; Lopez-Garcia, P. Unarmoured dinoflagellates with a small hyposome: Torodinium and Lebouridinium gen. nov. for Katodinium glaucum (Gymnodiniales, Dinophyceae). Eur. J. Phycol. 2016, 51, 226–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.; Moita, M.T.; Bashmachnikov, I.; Menezes, G.M.; Carmo, V.; Loureiro, C.M.; Mendonça, A.; Silva, A.F.; Martins, A. Phytoplankton variability and oceanographic conditions at Condor seamount, Azores (NE Atlantic). Deep. Sea Res. Part II Top. Stud. Oceanogr. 2013, 98, 52–62. [Google Scholar] [CrossRef]
- Yu, R.C.; Zhang, Q.C.; Kong, F.Z.; Zhou, Z.X.; Chen, Z.F.; Zhou, Y.; Geng, H.X.; Dai, L.; Yan, T.; Zhou, M.J. Status, impacts and long-term changes of harmful algal blooms in the sea area adjacent to the Changjiang river estuary. Oceanol. Et Limnol. Sinica 2017, 48, 1178–1186. [Google Scholar]



| Group | HAB Species | ASV Number | ASV Id | Division | Class | Accession | PID (%) | Literature Report | HAB Evidence |
|---|---|---|---|---|---|---|---|---|---|
| G1 | Katodinium glaucum | 1 | ASV_163 | Dinoflagellata | Dinophyceae | KP790162 | 100.00 | N | [38] |
| G1 | Margalefidinium polykrikoides | 1 | ASV_571 | Dinoflagellata | Dinophyceae | AY347309 | 100.00 | N | [39] |
| G1 | Alexandrium andersonii | 1 | ASV_703 | Dinoflagellata | Dinophyceae | KF925334 | 99.74 | N | [40] |
| G1 | Akashiwo sanguinea | 1 | ASV_1238 | Dinoflagellata | Dinophyceae | AY421770 | 100.00 | N | [41] |
| G1 | Alexandrium affine | 1 | ASV_1484 | Dinoflagellata | Dinophyceae | AY421778 | 100.00 | N | [42] |
| G1 | Alexandrium ostenfeldii | 1 | ASV_1588 | Dinoflagellata | Dinophyceae | KJ361986 | 100.00 | N | [40] |
| G1 | Protoceratium reticulatum | 1 | ASV_1699 | Dinoflagellata | Dinophyceae | MK995623 | 99.74 | N | [40] |
| G1 | Pseudochattonella verruculosa | 1 | ASV_1973 | Ochrophyta | Dictyochophyceae | AB217629 | 100.00 | N | [40] |
| G1 | Phalacroma rotundatum | 1 | ASV_2352 | Dinoflagellata | Dinophyceae | EU780657 | 100.00 | N | [42] |
| G1 | Alexandrium leei | 1 | ASV_3247 | Dinoflagellata | Dinophyceae | AY641565 | 100.00 | N | [42] |
| G1 | Amphisolenia bidentata | 1 | ASV_3266 | Dinoflagellata | Dinophyceae | GU196149 | 100.00 | [13] | [41] |
| G1 | Eucampia cornuta | 1 | ASV_3344 | Ochrophyta | Bacillariophyta | KJ577856 | 100.00 | [13] | [41] |
| G1 | Cochlodinium polykrikoides | 1 | ASV_4020 | Dinoflagellata | Dinophyceae | EU418971 | 99.21 | N | [40] |
| G1 | Pseudo-nitzschia seriata | 1 | ASV_4169 | Ochrophyta | Bacillariophyta | AY485490 | 100.00 | N | [40] |
| G1 | Chaetoceros peruvianus | 1 | ASV_4180 | Ochrophyta | Bacillariophyta | HQ912650 | 99.48 | [14] | [42] |
| G1 | Pseudo-nitzschia galaxiae | 1 | ASV_4889 | Ochrophyta | Bacillariophyta | KJ608079 | 100.00 | N | [40] |
| G1 | Vicicitus globosus | 1 | ASV_5088 | Ochrophyta | Dictyochophyceae | HQ646558 | 99.49 | N | [43] |
| G1 | Lingulodinium polyedra | 1 | ASV_5301 | Dinoflagellata | Dinophyceae | AB693194 | 100.00 | N | [41] |
| G1 | Rhizosolenia setigera | 1 | ASV_5960 | Ochrophyta | Bacillariophyta | KY980291 | 100.00 | [13] | [41] |
| G1 | Cerataulina pelagica | 1 | ASV_6058 | Ochrophyta | Bacillariophyta | HQ912669 | 99.74 | [13] | [41] |
| G1 | Gymnodinium impudicum | 1 | ASV_6327 | Dinoflagellata | Dinophyceae | AF022197 | 100.00 | N | [41] |
| G1 | Heterosigma akashiwo | 1 | ASV_6494 | Ochrophyta | Raphidophyceae | AB001287 | 100.00 | N | [40] |
| G1 | Guinardia striata | 1 | ASV_7307 | Ochrophyta | Bacillariophyta | KT861015 | 99.74 | [13] | [42] |
| G1 | Odontella aurita | 1 | ASV_7967 | Ochrophyta | Bacillariophyta | JX413551 | 100.00 | N | [41] |
| G1 | Chaetoceros curvisetus | 1 | ASV_8665 | Ochrophyta | Bacillariophyta | MG972241 | 99.48 | N | [42] |
| G2 | Karlodinium veneficum | 9 | ASV_11 | Dinoflagellata | Syndiniales | KY979983 | 100.00 | N | [44] |
| G2 | Karenia papilionacea | 15 | ASV_21 | Dinoflagellata | Dinophyceae | HM067005 | 100.00 | N | [45] |
| G2 | Heterocapsa rotundata | 14 | ASV_23 | Dinoflagellata | Dinophyceae | KY980288 | 100.00 | N | [46] |
| G2 | Amphidoma languida | 3 | ASV_71 | Dinoflagellata | Dinophyceae | LS974149 | 99.21 | N | [40] |
| G2 | Noctiluca scintillans | 2 | ASV_107 | Dinoflagellata | Noctilucophyceae | AF022200 | 100.00 | N | [42] |
| G2 | Azadinium dexteroporum | 2 | ASV_184 | Dinoflagellata | Dinophyceae | KR362889 | 100.00 | N | [40] |
| G2 | Gymnodinium aureolum | 2 | ASV_404 | Dinoflagellata | Dinophyceae | KR362891 | 99.48 | N | [42] |
| G2 | Aureococcus anophagefferens | 5 | ASV_1565 | Ochrophyta | Pelagophyceae | KY980308 | 99.74 | N | [6] |
| G2 | Gonyaulax polygramma | 2 | ASV_1958 | Dinoflagellata | Dinophyceae | AY775287 | 99.74 | [13] | [42] |
| G2 | Karenia selliformis | 2 | ASV_2204 | Dinoflagellata | Dinophyceae | HM067007 | 99.74 | N | [41] |
| G2 | Thalassiosira diporocyclus | 2 | ASV_2450 | Ochrophyta | Bacillariophyta | MF405351 | 100.00 | N | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Wang, C.; Xu, K.; Chen, N. Metabarcoding Analysis of Harmful Algal Bloom Species in the Western Pacific Seamount Regions. Int. J. Environ. Res. Public Health 2021, 18, 11470. https://doi.org/10.3390/ijerph182111470
Xu Q, Wang C, Xu K, Chen N. Metabarcoding Analysis of Harmful Algal Bloom Species in the Western Pacific Seamount Regions. International Journal of Environmental Research and Public Health. 2021; 18(21):11470. https://doi.org/10.3390/ijerph182111470
Chicago/Turabian StyleXu, Qing, Chunzhi Wang, Kuidong Xu, and Nansheng Chen. 2021. "Metabarcoding Analysis of Harmful Algal Bloom Species in the Western Pacific Seamount Regions" International Journal of Environmental Research and Public Health 18, no. 21: 11470. https://doi.org/10.3390/ijerph182111470
APA StyleXu, Q., Wang, C., Xu, K., & Chen, N. (2021). Metabarcoding Analysis of Harmful Algal Bloom Species in the Western Pacific Seamount Regions. International Journal of Environmental Research and Public Health, 18(21), 11470. https://doi.org/10.3390/ijerph182111470







