Optimization of Micro-Pollutants’ Removal from Wastewater Using Agricultural Waste-Derived Sustainable Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. PKSB Adsorbent Preparation
2.3. Analytical Methods
2.4. Experimental Design via Response Surface Methodology
2.5. Regeneration Study
3. Results and Discussions
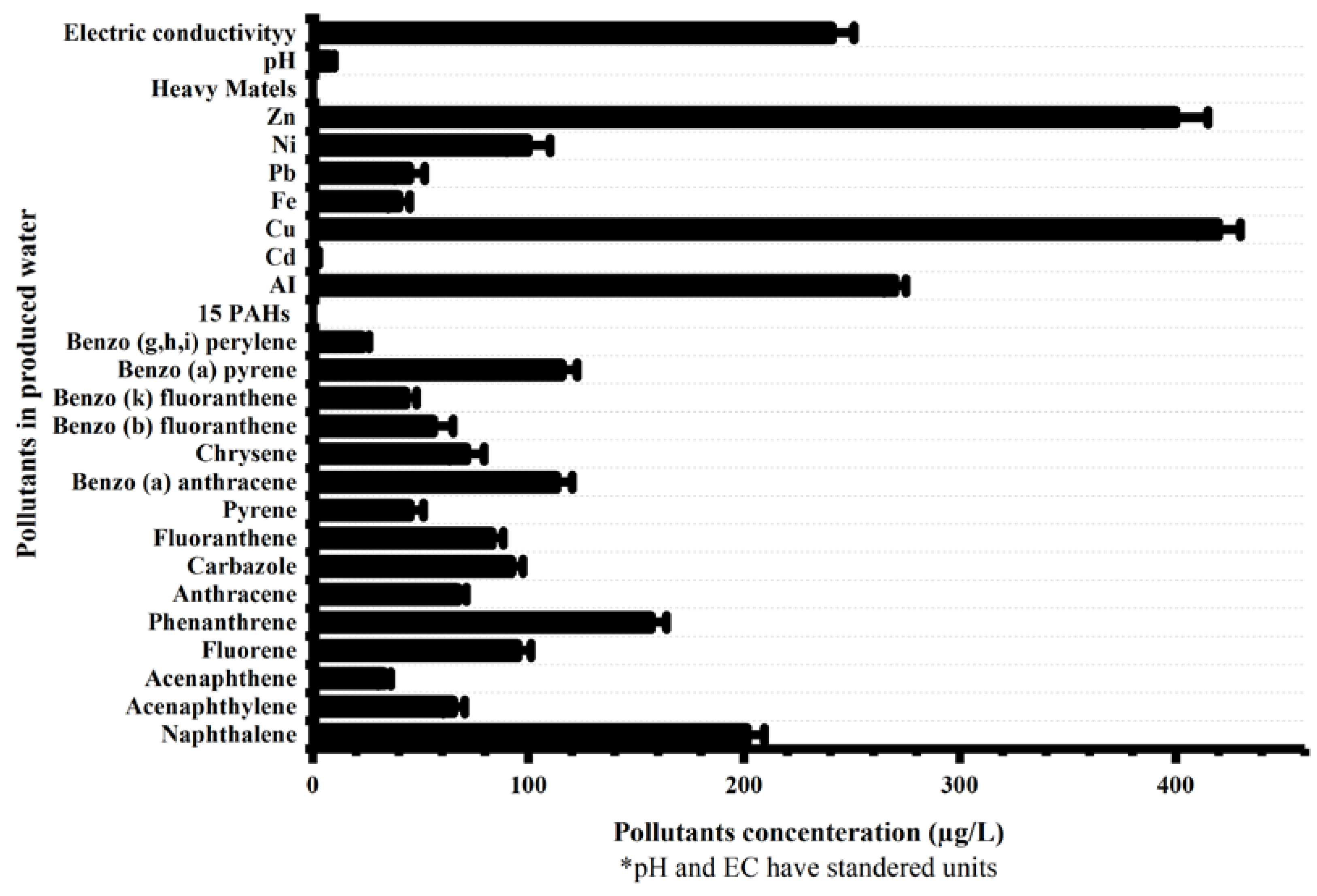
3.1. Produced Water Characterization and GC-MS Analysis for PAHs Concentration
3.2. Central Composite Design and Statistical Analysis
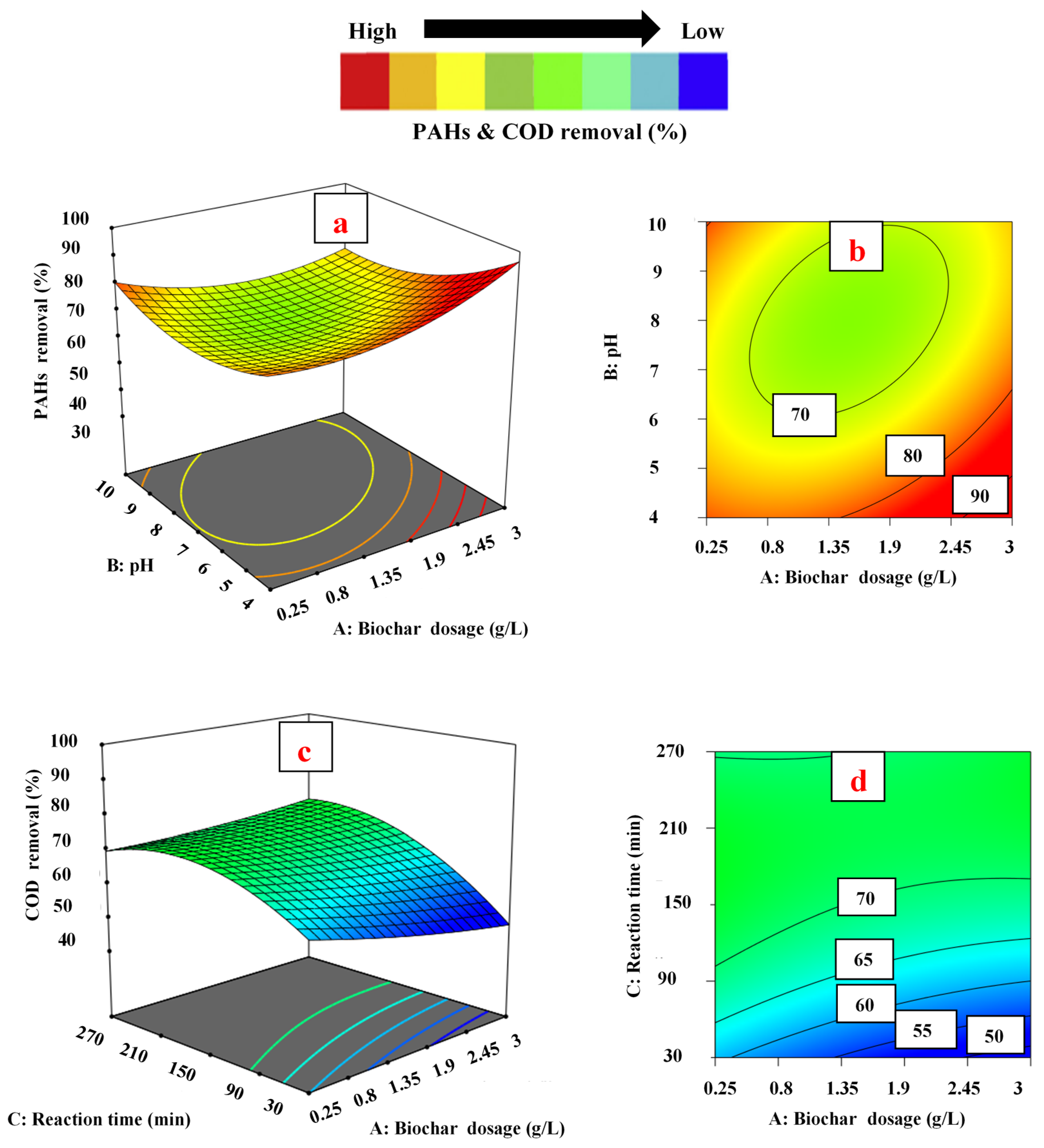
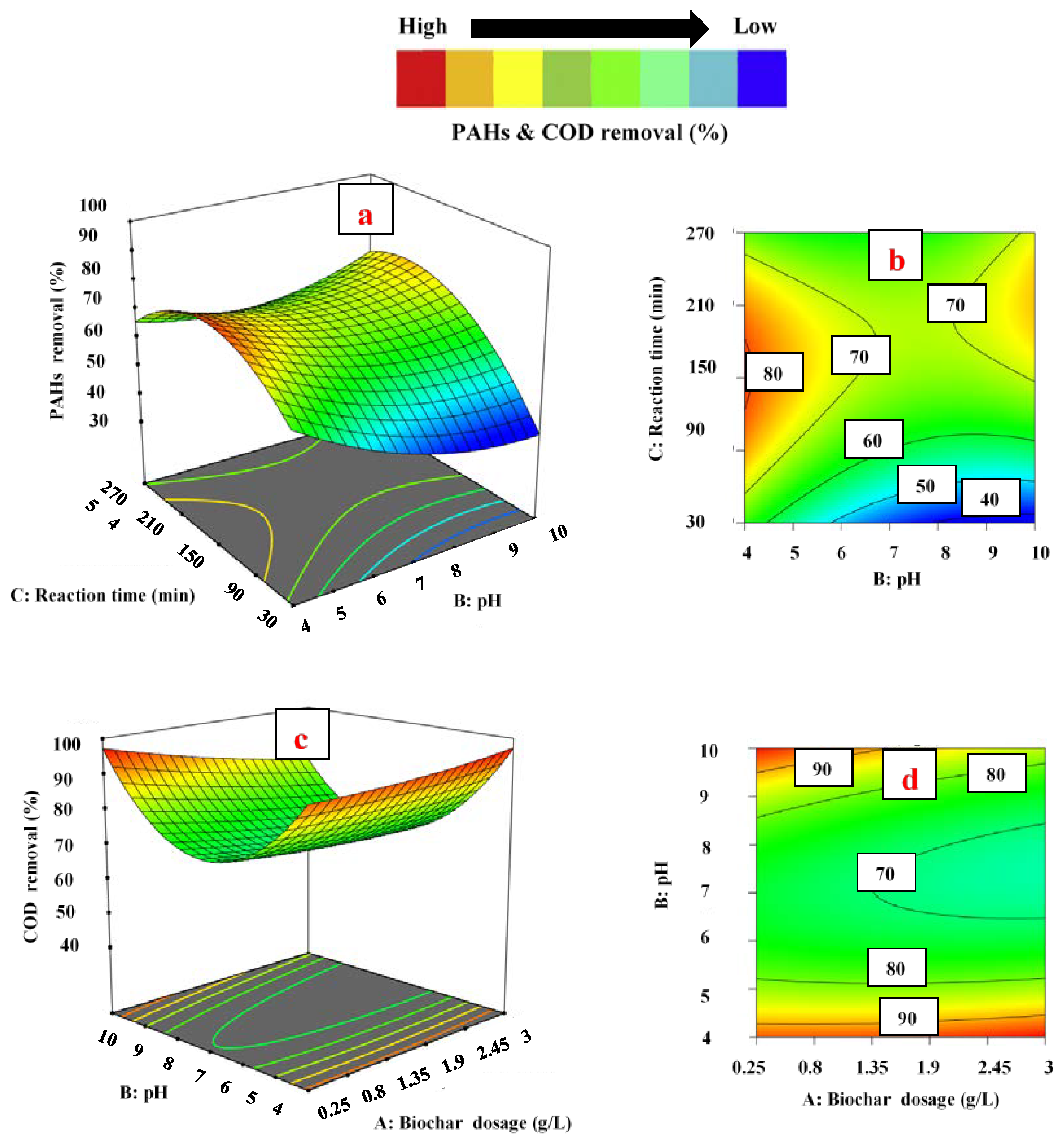
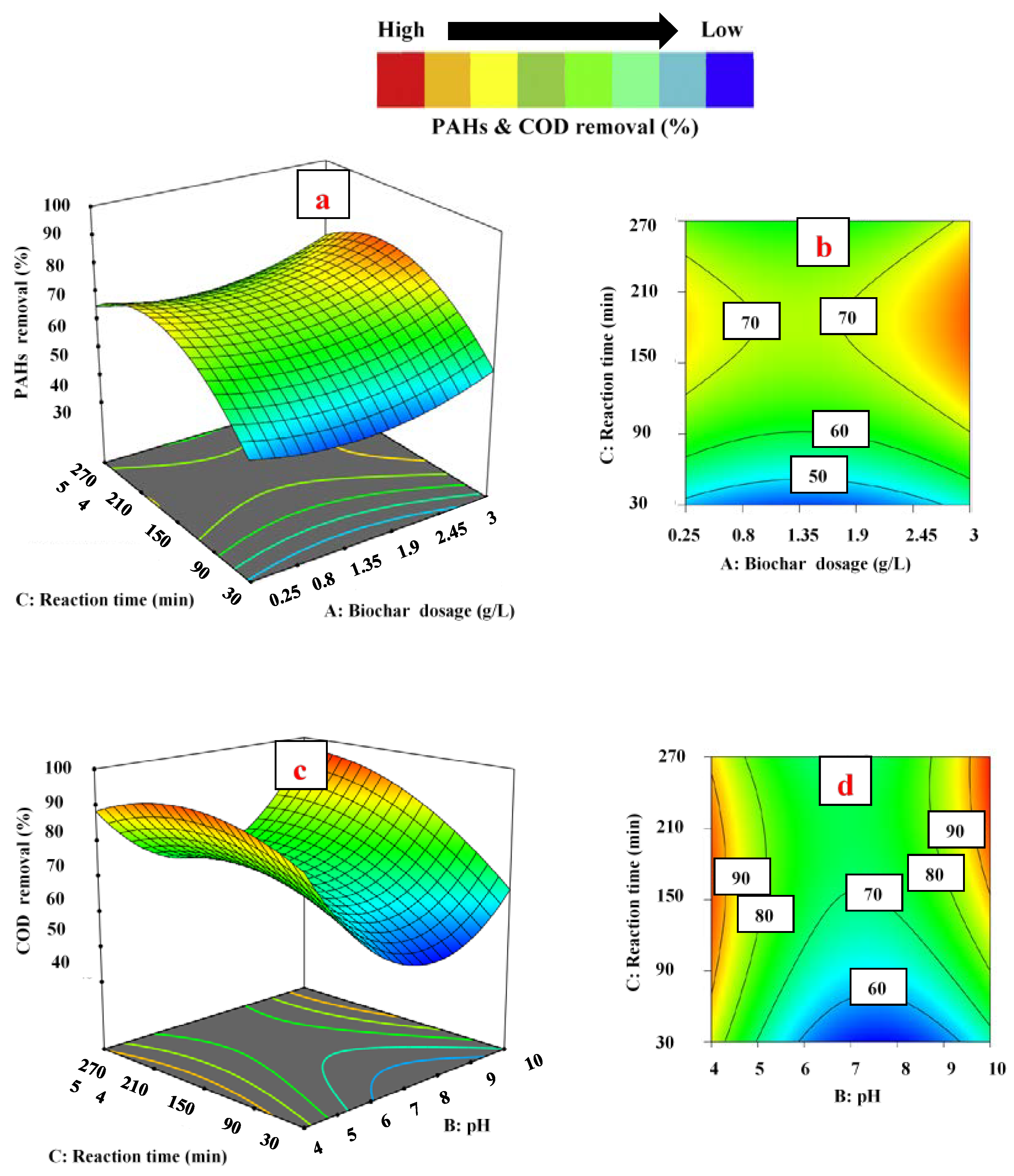
3.3. Effect of Operating Parameters on Responses
3.3.1. Effect of Biochar Dosage
3.3.2. Effect of pH
3.3.3. Effect of Contact Time
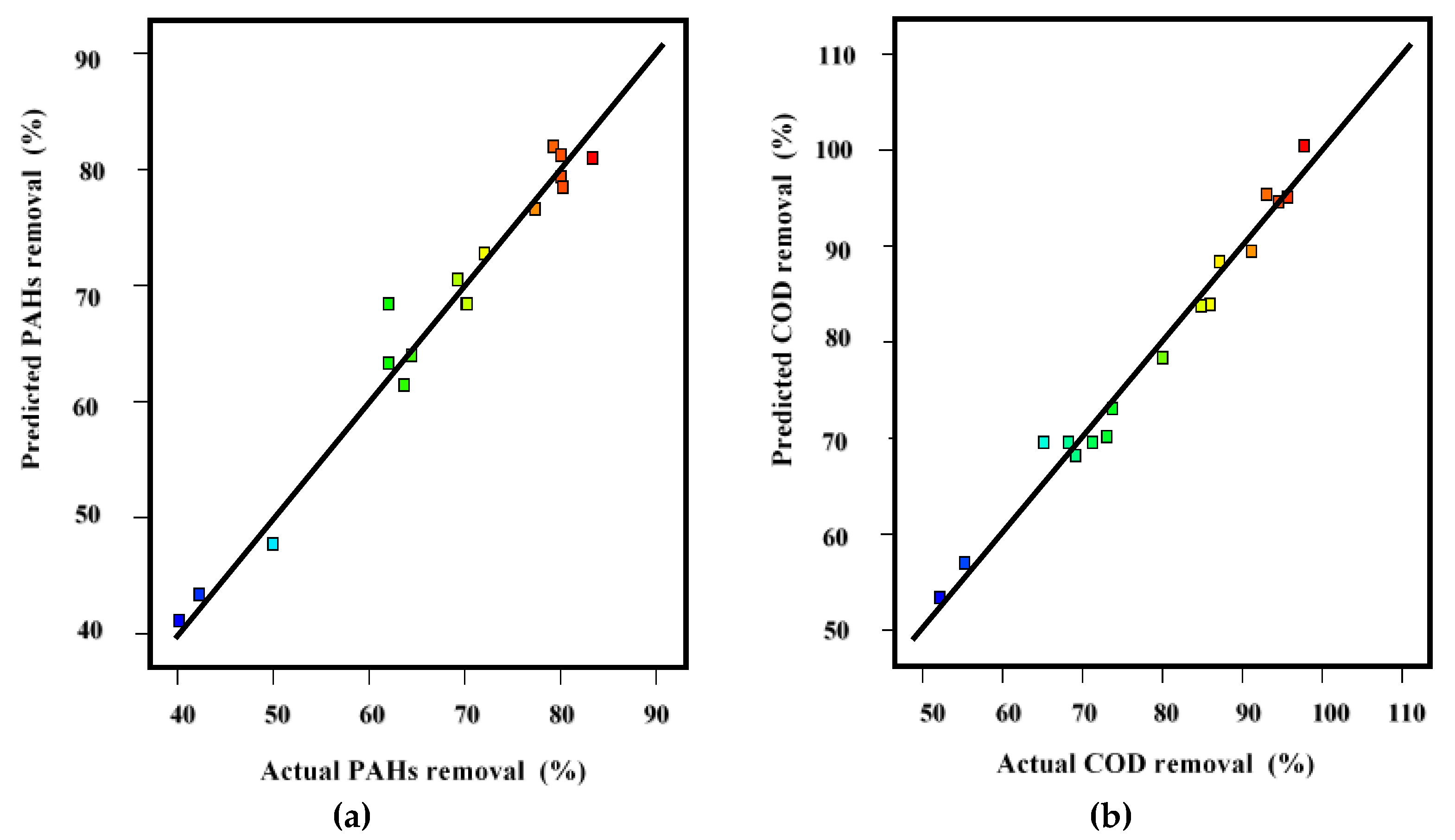
3.4. Validation of the Model
3.5. Regeneration Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Sahari, M.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Noman, E.; Naushad, M.; Rizuan, M.B.; Vo, D.-V.N.; Ismail, N. Green approach and strategies for wastewater treatment using bioelectrochemical systems: A critical review of fundamental concepts, applications, mechanism, and future trends. Chemosphere 2021, 285, 131373. [Google Scholar] [CrossRef]
- Verma, M.; Haritash, A. Photocatalytic degradation of Amoxicillin in pharmaceutical wastewater: A potential tool to manage residual antibiotics. Environ. Technol. Innov. 2020, 20, 101072. [Google Scholar] [CrossRef]
- Da Silva, F.C., Jr.; Felipe, M.B.M.C.; de Castro, D.E.F.; Araújo, S.C.D.S.; Sisenando, H.C.N.; de Medeiros, S.R.B. A look beyond the priority: A systematic review of the genotoxic, mutagenic, and carcinogenic endpoints of non-priority PAHs. Environ. Pollut. 2021, 278, 116838. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Haneef, T.; Mustafa, M.R.U.; Yasin, H.F.; Farooq, S.; Isa, M.H. Study of Ferrate(VI) oxidation for COD removal from wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 442. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, H.; Mustafa, M.R.U.; Rashid, U.; Isa, M.H.; Ho, Y.C.; Shah, M.M. Adsorptive removal of COD from produced water using tea waste biochar. Environ. Technol. Innov. 2021, 23, 101563. [Google Scholar] [CrossRef]
- Haneef, T.; Mustafa, M.R.U.; Rasool, K.; Ho, Y.C.; Kutty, S.R.M. Removal of Polycyclic Aromatic Hydrocarbons in a Heterogeneous Fenton Like Oxidation System Using Nanoscale Zero-Valent Iron as a Catalyst. Water 2020, 12, 2430. [Google Scholar] [CrossRef]
- Bertoz, V.; Purcaro, G.; Conchione, C.; Moret, S. A Review on the Occurrence and Analytical Determination of PAHs in Olive Oils. Foods 2021, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.; Chen, L.; Han, C.; Akutagawa, T.; Endo, O.; Yamauchi, M.; Neroda, A.; Toriba, A.; Tang, N. Polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons in five East Asian cities: Seasonal characteristics, health risks, and yearly variations. Environ. Pollut. 2021, 287, 117360. [Google Scholar] [CrossRef] [PubMed]
- Jimene, S.; Andreozzi, M.; Maria, M. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Rosińska, A.; Dąbrowska, L. Influence of type and dose of coagulants on effectiveness of PAH removal in coagulation water treatment. Water Sci. Eng. 2021, 14, 193–200. [Google Scholar] [CrossRef]
- Gong, C.; Ren, X.; Han, J.; Wu, Y.; Gou, Y.; Zhang, Z.; He, P. Toxicity reduction of reverse osmosis concentrates from petrochemical wastewater by electrocoagulation and Fered-Fenton treatments. Chemosphere 2022, 286, 131582. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Zhu, N.; Yuan, H.; Shen, Y. The extent of sludge solubilization allows to estimate the efficacy of ozonation for removal of polycyclic aromatic hydrocarbons (PAHs) in municipal sewage sludge. J. Hazard. Mater. 2022, 413, 125404. [Google Scholar] [CrossRef]
- Ajab, H.; Isa, M.H.; Yaqub, A. Electrochemical oxidation using Ti/RuO2 anode for COD and PAHs removal from aqueous solution. Sustain. Mater. Technol. 2020, 26. [Google Scholar] [CrossRef]
- Nas, B.; Argun, M.; Dolu, T.; Ateş, H.; Yel, E.; Koyuncu, S.; Dinç, S.; Kara, M. Occurrence, loadings and removal of EU-priority polycyclic aromatic hydrocarbons (PAHs) in wastewater and sludge by advanced biological treatment, stabilization pond and constructed wetland. J. Environ. Manag. 2020, 268, 110580. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, M.; Lorgeoux, C.; Biache, C.; Saada, A.; Faure, P. Fenton-like and potassium permanganate oxidations of PAH-contaminated soils: Impact of oxidant doses on PAH and polar PAC (polycyclic aromatic compound) behavior. Chemosphere 2019, 224, 437–444. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Hang, X.; Zhao, S.; Wan, Y. Removal of polycyclic aromatic hydrocarbons by nanofiltration membranes: Rejection and fouling mechanisms. J. Membr. Sci. 2019, 582, 264–273. [Google Scholar] [CrossRef]
- Bao, H.; Wang, J.; Li, J.; Zhang, H.; Wu, F. Effects of corn straw on dissipation of polycyclic aromatic hydrocarbons and potential application of backpropagation artificial neural network prediction model for PAHs bioremediation. Ecotoxicol. Environ. Saf. 2019, 186, 109745. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Catalfo, A.; Finocchiaro, G.; Vagliasindi, F.G.; Romano, S.; De Guidi, G. Microwave heating coupled with UV-A irradiation for PAH removal from highly contaminated marine sediments and subsequent photo-degradation of the generated vaporized organic compounds. Chem. Eng. J. 2018, 334, 172–183. [Google Scholar] [CrossRef]
- Man, X.; Ning, X.-A.; Zou, H.; Liang, J.; Sun, J.; Lu, X.; Sun, J. Removal of polycyclic aromatic hydrocarbons (PAHs) from textile dyeing sludge by ultrasound combined zero-valent iron/EDTA/Air system. Chemosphere 2018, 191, 839–847. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M. Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: Isotherm and kinetic modelling. J. Mol. Liq. 2020, 310, 113025. [Google Scholar] [CrossRef]
- Kocsis, T.; Kotroczó, Z.; Kardos, L.; Biró, B. Optimization of increasing biochar doses with soil–plant–microbial functioning and nutrient uptake of maize. Environ. Technol. Innov. 2020, 20, 101191. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Dong, J.; Zhao, J.; Gong, X.; Liu, S. Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol. Environ. Saf. 2018, 149, 80–87. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhao, X.; Tysklind, M.; Hao, F. Typical agricultural diffuse herbicide sorption with agricultural waste-derived biochars amended soil of high organic matter content. Water Res. 2016, 92, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Bianco, F.; Race, M.; Papirio, S.; Oleszczuk, P.; Esposito, G. The addition of biochar as a sustainable strategy for the remediation of PAH–contaminated sediments. Chemosphere 2020, 263, 128274. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tang, J.; Wang, L.; Sun, H. Combined Effects of Microplastics and Biochar on the Removal of Polycyclic Aromatic Hydrocarbons and Phthalate Esters and Its Potential Microbial Ecological Mechanism. Front. Microbiol. 2021, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, A.H.; Abdullah, L.C.; Zahri, N.A.M.; Alkhatib, M. Column Efficiency of Fluoride Removal Using Quaternized Palm Kernel Shell (QPKS). Int. J. Chem. Eng. 2019, 2019, 5743590. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Palm Kernel Shell as an effective adsorbent for the treatment of heavy metal contaminated water. Sci. Rep. 2019, 9, 18955. [Google Scholar] [CrossRef] [Green Version]
- Razavi, M.M.; Fekri, M.H.; Omidali, F.; Eftekhari, N.; Akbari-adergani, B. Removal of chromium (VI) from wastewater by palm kernel shell-based on a green method. J. Chem. Health Risks 2019, 9, 75–86. [Google Scholar]
- Kyi, P.P.; Quansah, J.O.; Lee, C.-G.; Moon, J.-K.; Park, S.-J. The Removal of Crystal Violet from Textile Wastewater Using Palm Kernel Shell-Derived Biochar. Appl. Sci. 2020, 10, 2251. [Google Scholar] [CrossRef] [Green Version]
- Biswal, B.K.; Vijayaraghavan, K.; Tsen-Tieng, D.L.; Balasubramanian, R. Biochar-based bioretention systems for removal of chemical and microbial pollutants from stormwater: A critical review. J. Hazard. Mater. 2021, 422, 126886. [Google Scholar] [CrossRef] [PubMed]
- Hairuddin, M.N.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Walvekar, R.; Karri, R.R. Magnetic palm kernel biochar potential route for phenol removal from wastewater. Environ. Sci. Pollut. Res. 2019, 26, 35183–35197. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Shrestha, S.; Athar, S.; Ali, B.S.; Siddiqui, M.A. A critical analysis on palm kernel shell from oil palm industry as a feedstock for solid char production. Rev. Chem. Eng. 2016, 32. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Zhang, J.; Yu, J. Formation of hollow carbon nanofibers on bio-char during microwave pyrolysis of palm kernel shell. Energy Convers. Manag. 2017, 148, 583–592. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bachmann, R.; Loh, S.K.; Manroshan, S.; Ong, S. Effect of Pyrolysis Temperature and Time on Properties of Palm Kernel Shell-Based Biochar. IOP Conf. Series: Mater. Sci. Eng. 2019, 548, 012020. [Google Scholar] [CrossRef]
- Yeboah, M.L.; Li, X.; Zhou, S. Facile Fabrication of Biochar from Palm Kernel Shell Waste and Its Novel Application to Magnesium-Based Materials for Hydrogen Storage. Materials 2020, 13, 625. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, R.F.; Rashid, U.; Ibrahim, M.L.; Hazmi, B.; Alharthi, F.A.; Nehdi, I.A. Bifunctional nano-catalyst produced from palm kernel shell via hydrothermal-assisted carbonization for biodiesel production from waste cooking oil. Renew. Sustain. Energy Rev. 2021, 137, 110638. [Google Scholar] [CrossRef]
- Goh, C.M.H.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Rashid, U.; Khalid, M.; Walvekar, R. Synthesis of magnetic basic palm kernel shell catalyst for biodiesel production and characterisation and optimisation by Taguchi method. Appl. Nanosci. 2021, 1–13. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF): Washington, DC, USA, 2012; Volume 1496. [Google Scholar]
- Iberahim, N.; Sethupathi, S.; Goh, C.L.; Bashir, M.J.; Ahmad, W. Optimization of activated palm oil sludge biochar preparation for sulphur dioxide adsorption. J. Environ. Manag. 2019, 248, 109302. [Google Scholar] [CrossRef]
- Barman, S.R.; Das, P.; Mukhopadhayay, A.J. Biochar from waste Sterculia foetida and its application as adsorbent for the treatment of PAH compounds: Batch and optimization. Fuel 2021, 306, 121623. [Google Scholar] [CrossRef]
- Çalışkan, E.; Bermúdez, J.; Parra, J.; Menéndez, J.; Mahramanlıoğlu, M.; Ania, C. Low temperature regeneration of activated carbons using microwaves: Revising conventional wisdom. J. Environ. Manag. 2012, 102, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, A.; Zheng, X.; Wang, S.; Peng, J.; Zhang, L.; Li, Z. Study on dechlorination kinetics from zinc oxide dust by clean metallurgy technology. Green Process. Synth. 2016, 5, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, B.; Dominguez, A.; Pis, J.; Menéndez, J.A. Microwave-assisted dry reforming of methane. Int. J. Hydrogen Energy 2008, 33, 4337–4344. [Google Scholar] [CrossRef]
- Brunner, P. Methods of Analysis of Sewage Sludge, Solid Wastes and Compost; WHO International Reference Centre for Wastes Disposal: Dübendorf, Switzerland, 1978. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Pollution Control Federation; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1912; Volume 2. [Google Scholar]
- Cerda, V.; Estela, J.M. Nutrient control. In Wastewater Quality Monitoring and Treatment; Wiley: Chichester, UK, 2006; p. 219. [Google Scholar]
- Mansourri, G.; Madani, M. Examination of the level of heavy metals in wastewater of Bandar Abbas Wastewater Treatment Plant. Open J. Ecol. 2016, 6, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Baumann, F.J. Dichromate reflux chemical oxygen demand. Proposed method for chloride correction in highly saline wastes. Anal. Chem. 1974, 46, 1336–1338. [Google Scholar] [CrossRef]
- McFarlane, J.; Bostick, D.T.; Luo, H. Characterization and modeling of produced water. In Proceedings of the Ground Water Protection Council Produced Water Conference, Colorado Springs, CO, USA, 16–17 October 2002. [Google Scholar]
- Zhang, L.; Jiao, X.; Wu, S.; Song, X.; Yao, R. Study on Optimization of Tungsten Ore Flotation Wastewater Treatment by Response Surface Method (RSM). Minerals 2021, 11, 184. [Google Scholar] [CrossRef]
- Deng, S.; Chen, J.; Chang, J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits. J. Clean. Prod. 2021, 293, 126156. [Google Scholar] [CrossRef]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, M.; Li, L.Y.; Grace, J.R. Effect of Co-existing Heavy Metals and Natural Organic Matter on Sorption/Desorption of Polycyclic Aromatic Hydrocarbons in Soil: A Review. Pollution 2020, 6, 1–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and beneficial impact of biochar for environmental application: A comprehensive review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, S.; Zhang, C.; Zeng, G.; Tan, X.; Song, B.; Zhang, P.; Yang, H.; Li, M.; Chen, Q. Application of biochar for the remediation of polluted sediments. J. Hazard. Mater. 2021, 404, 124052. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Luo, K.; Pang, Y.; Wang, D.; Li, X.; Wang, L.; Lei, M.; Huang, Q.; Yang, Q. A critical review on the application of biochar in environmental pollution remediation: Role of persistent free radicals (PFRs). J. Environ. Sci. 2021, 108, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, H. Activated carbon from sawdust for naphthalene removal from contaminated water. Environ. Technol. Innov. 2020, 20, 101080. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Lam, S.S.; Yek, P.N.Y.; Ok, Y.S.; Chong, C.C.; Liew, R.K.; Tsang, D.C.; Park, Y.-K.; Liu, Z.; Wong, C.S.; Peng, W. Engineering pyrolysis biochar via single-step microwave steam activation for hazardous landfill leachate treatment. J. Hazard. Mater. 2019, 390, 121649. [Google Scholar] [CrossRef]
- Khan, T.A.; Nazir, M.; Ali, I.; Kumar, A. Removal of Chromium(VI) from aqueous solution using guar gum–nano zinc oxide biocomposite adsorbent. Arab. J. Chem. 2017, 10, S2388–S2398. [Google Scholar] [CrossRef]
- Payel, S.; Hashem, A.; Hasan, A. Recycling biochar derived from tannery liming sludge for chromium adsorption in static and dynamic conditions. Environ. Technol. Innov. 2021, 102010. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Kao, C.-M.; Chien, C.-C.; Hung, C.-M. Wood-Biochar-Supported Magnetite Nanoparticles for Remediation of PAH-Contaminated Estuary Sediment. Catalysts 2018, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Zhang, J.; Xie, H.; Hu, Z.; Liang, S.; Ngo, H.H.; Guo, W.; Chen, X.; Fan, J.; Zhao, C. Intensive removal of PAHs in constructed wetland filled with copper biochar. Ecotoxicol. Environ. Saf. 2020, 205, 111028. [Google Scholar] [CrossRef] [PubMed]
- Kaetzl, K.; Lübken, M.; Uzun, G.; Gehring, T.; Nettmann, E.; Stenchly, K.; Wichern, M. On-farm wastewater treatment using biochar from local agroresidues reduces pathogens from irrigation water for safer food production in developing countries. Sci. Total Environ. 2019, 682, 601–610. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, S.; Zheng, F.; Huang, L.; Pan, J.; Jiang, Y.; Hou, W.; Xiao, L. Organics removal, nitrogen removal and N2O emission in subsurface wastewater infiltration systems amended with/without biochar and sludge. Bioresour. Technol. 2018, 249, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Huang, C.-P.; Chen, C.-W.; Wu, C.-H.; Lin, Y.-L.; Dong, C.-D. Activation of percarbonate by water treatment sludge–derived biochar for the remediation of PAH-contaminated sediments. Environ. Pollut. 2020, 265, 114914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, L.; Guo, X.; Han, Z.; Ji, L.; He, Q.; Han, L.; Sun, K. Mechanism of biochar as a biostimulation strategy to remove polycyclic aromatic hydrocarbons from heavily contaminated soil in a coking plant. Geoderma 2020, 375, 114497. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sridhar, K.; Chen, B.-H. Removal of polycyclic aromatic hydrocarbons from water by magnetic activated carbon nanocomposite from green tea waste. J. Hazard. Mater. 2021, 415, 125701. [Google Scholar] [CrossRef]
- Manyuchi, M.; Mbohwa, C.; Muzenda, E. Potential to use municipal waste bio char in wastewater treatment for nutrients recovery. Phys. Chem. Earth Parts A/B/C 2018, 107, 92–95. [Google Scholar] [CrossRef]

| Methods | Pollutants | Year | References |
|---|---|---|---|
| Coagulation process | 16 PAHs | 2021 | [11] |
| Electrocoagulation | PAHs | 2021 | [12] |
| Ozonation | 16 PAHs | 2021 | [13] |
| Heterogeneous Fenton oxidation | 15 PAHs | 2020 | [14] |
| Electrochemical oxidation | PAHs | 2020 | [15] |
| Advanced biological treatment | 08 PAHs | 2020 | [16] |
| Fenton-like oxidations | 16 PAHs | 2019 | [17] |
| Nano-filtration membranes | 03 PAHs | 2019 | [18] |
| Bioremediation | 14 PAHs | 2019 | [19] |
| UV photo-degradation | 08 PAHs | 2018 | [20] |
| Ultrasound-Fenton | PAHs | 2018 | [21] |
| No | Factors | PAHs Removal (%) | COD Removal (%) | ||||
|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | |||||
| Biochar Dosage (g/L) | pH (-) | Contact Time (min) | Actual Responses | Predicted Responses | Actual Responses | Predicted Responses | |
| 1 | 1.62 | 4.0 | 150 | 83.40 | 80.95 | 94.60 | 94.54 |
| 2 | 3.00 | 10.0 | 270 | 77.40 | 76.55 | 95.70 | 95.04 |
| 3 | 1.62 | 10.0 | 150 | 69.30 | 70.47 | 91.20 | 89.40 |
| 4 | 0.25 | 10.0 | 270 | 80.10 | 81.20 | 97.80 | 100.39 |
| 5 | 1.62 | 7.0 | 150 | 70.30 | 68.38 | 68.30 | 69.51 |
| 6 | 3.00 | 10.0 | 30 | 40.20 | 41.09 | 55.30 | 56.93 |
| 7 | 1.62 | 7.0 | 150 | 62.10 | 68.38 | 71.30 | 69.51 |
| 8 | 0.25 | 4.0 | 30 | 62.10 | 63.27 | 87.20 | 88.32 |
| 9 | 0.25 | 7.0 | 150 | 72.10 | 72.71 | 73.80 | 73.02 |
| 10 | 1.62 | 7.0 | 270 | 63.70 | 61.39 | 73.10 | 70.10 |
| 11 | 1.62 | 7.0 | 150 | 70.20 | 68.38 | 65.20 | 69.51 |
| 12 | 1.62 | 7.0 | 30 | 42.30 | 43.33 | 52.20 | 53.34 |
| 13 | 3.00 | 7.0 | 150 | 80.30 | 78.41 | 69.20 | 68.12 |
| 14 | 3.00 | 4.0 | 270 | 79.30 | 81.93 | 93.10 | 95.33 |
| 15 | 0.25 | 10.0 | 30 | 50.00 | 47.69 | 80.10 | 78.33 |
| 16 | 3.00 | 4.0 | 30 | 80.10 | 79.32 | 86.00 | 83.87 |
| 17 | 0.25 | 4.0 | 270 | 64.50 | 63.93 | 84.90 | 83.73 |
| Source | SS | DF | MS | F-Value | p-Value | Status |
|---|---|---|---|---|---|---|
| PAHs | ||||||
| Model | 2697.87 | 9 | 299.76 | 25.77 | 0.0001 | Significant |
| x1 | 81.22 | 1 | 81.22 | 6.98 | 0.0333 | |
| x2 | 274.58 | 1 | 274.58 | 23.61 | 0.0018 | |
| x3 | 815.41 | 1 | 815.41 | 70.10 | 0.0001 | |
| x1x2 | 256.51 | 1 | 256.51 | 22.05 | 0.0022 | |
| x2x3 | 539.56 | 1 | 539.56 | 46.39 | 0.0003 | |
| x12 | 138.11 | 1 | 138.11 | 11.87 | 0.0108 | |
| x22 | 143.94 | 1 | 143.94 | 12.37 | 0.0098 | |
| x32 | 687.64 | 1 | 687.64 | 59.12 | 0.0001 | |
| Residual | 81.42 | 7 | 11.63 | |||
| Lack of Fit | 37.14 | 5 | 7.43 | 0.3354 | 0.8595 | Non-significant |
| COD | ||||||
| Model | 3113.08 | 9 | 345.90 | 38.06 | 0.0001 | Significant |
| x1 | 60.02 | 1 | 60.02 | 6.60 | 0.0370 | |
| x2 | 66.05 | 1 | 66.05 | 7.27 | 0.0308 | |
| X3 | 702.24 | 1 | 702.24 | 77.27 | 0.0001 | |
| x1x2 | 143.65 | 1 | 143.65 | 15.81 | 0.0054 | |
| x1x3 | 128.80 | 1 | 128.80 | 14.17 | 0.0070 | |
| x2x3 | 355.11 | 1 | 355.11 | 39.07 | 0.0004 | |
| x22 | 1351.87 | 1 | 1351.87 | 148.75 | 0.0001 | |
| x32 | 162.48 | 1 | 162.48 | 17.88 | 0.0039 | |
| Residual | 63.62 | 7 | 9.09 | |||
| Lack of Fit | 45.01 | 5 | 9.00 | 0.9677 | 0.5789 | Non-significant |
| Statistical Factors | Abbreviated as | PAHs | COD |
|---|---|---|---|
| Standard deviation | St. Dev. | 3.41 | 3.01 |
| Coefficient of determination | R2 | 0.97 | 0.96 |
| Mean | Mean | 67.49 | 78.76 |
| Predicted R2 | Pre. R2 | 0.80 | 0.78 |
| Adjusted R2 | Adj. R2 | 0.93 | 0.95 |
| Coefficient of variance | C.V.% | 5.05 | 3.83 |
| Adequate precision | A.P. | 15.61 | 20.35 |
| Dependent Variable | Biochar Dosage. (g L−1) | pH | Reaction Time (min) | Predicted Solution | Lab Experiments | Error (%) |
|---|---|---|---|---|---|---|
| PAHs removal (%) | 2.99 | 4.0 | 208.89 | 93.16 | 95.34 | 2.28 |
| COD removal (%) | 2.99 | 4.0 | 208.89 | 97.84 | 98.21 | 0.37 |
| Adsorbent | Source of Pollutants | Pollutants | Removal (%) | References |
|---|---|---|---|---|
| Tea waste biochar | Oil and gas wastewater | COD | 95.5 | [6] |
| Magnetic wood biochar (Fe3O4-WB) | Estuary sediment | PAHs | 87.0 | [65] |
| Biochar-loading copper ions (Cu-BC) | Constructed wetland | 1 PAH | >90 | [66] |
| Rice husk biochar | Municipal wastewater | Organic pollutants/COD | 94.0 | [67] |
| Corn straw biochar | Synthetic wastewater | COD | 95.4 | [68] |
| Water treatment sludge–derived biochar | Aquatic sediments | 3 PAHs | 87 | [69] |
| Rice straw-derived biochar | Coking plant soil | PAHs | 58.4 | [70] |
| Magnetic biochar from tea waste | Synthetic wastewater | 4 PAHs | 89 | [71] |
| Municipal waste biochar | Municipal wastewater | COD | 90.0 | [72] |
| Palm kernel shell biochar | Produced water | PAHs and COD | 95.3 and 98.21 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhothali, A.; Haneef, T.; Mustafa, M.R.U.; Moria, K.M.; Rashid, U.; Rasool, K.; Bamasag, O.O. Optimization of Micro-Pollutants’ Removal from Wastewater Using Agricultural Waste-Derived Sustainable Adsorbent. Int. J. Environ. Res. Public Health 2021, 18, 11506. https://doi.org/10.3390/ijerph182111506
Alhothali A, Haneef T, Mustafa MRU, Moria KM, Rashid U, Rasool K, Bamasag OO. Optimization of Micro-Pollutants’ Removal from Wastewater Using Agricultural Waste-Derived Sustainable Adsorbent. International Journal of Environmental Research and Public Health. 2021; 18(21):11506. https://doi.org/10.3390/ijerph182111506
Chicago/Turabian StyleAlhothali, Areej, Tahir Haneef, Muhammad Raza Ul Mustafa, Kawthar Mostafa Moria, Umer Rashid, Kashif Rasool, and Omaimah Omar Bamasag. 2021. "Optimization of Micro-Pollutants’ Removal from Wastewater Using Agricultural Waste-Derived Sustainable Adsorbent" International Journal of Environmental Research and Public Health 18, no. 21: 11506. https://doi.org/10.3390/ijerph182111506
APA StyleAlhothali, A., Haneef, T., Mustafa, M. R. U., Moria, K. M., Rashid, U., Rasool, K., & Bamasag, O. O. (2021). Optimization of Micro-Pollutants’ Removal from Wastewater Using Agricultural Waste-Derived Sustainable Adsorbent. International Journal of Environmental Research and Public Health, 18(21), 11506. https://doi.org/10.3390/ijerph182111506








