Organochlorine Pesticides in Karst Soil: Levels, Distribution, and Source Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
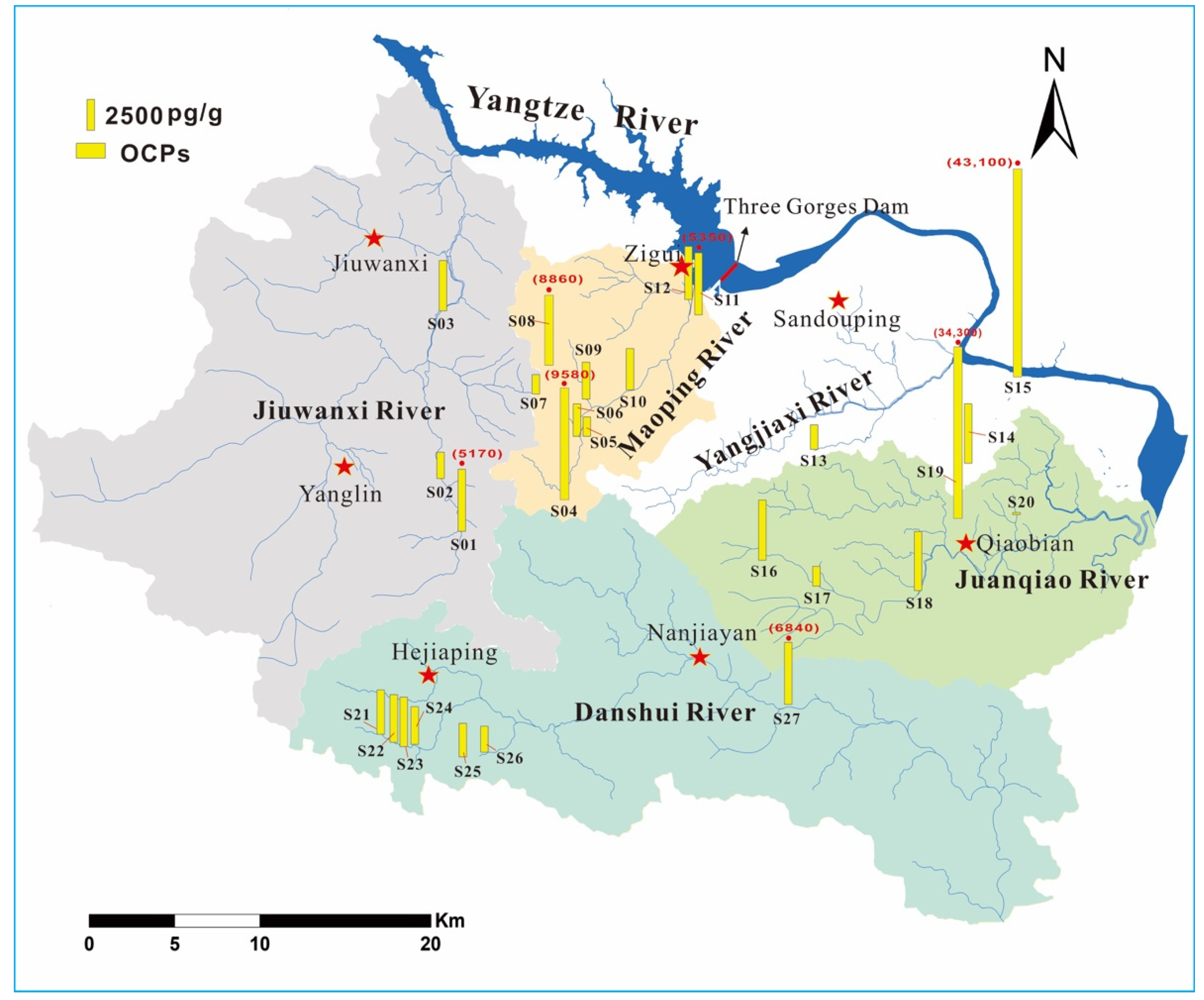
2.1. Study Area and Sample Collection
2.2. Sample Preparation and Analysis
2.3. Quality Assurance and Quality Control (QA/QC)
2.4. Data Analysis
3. Results and Discussion
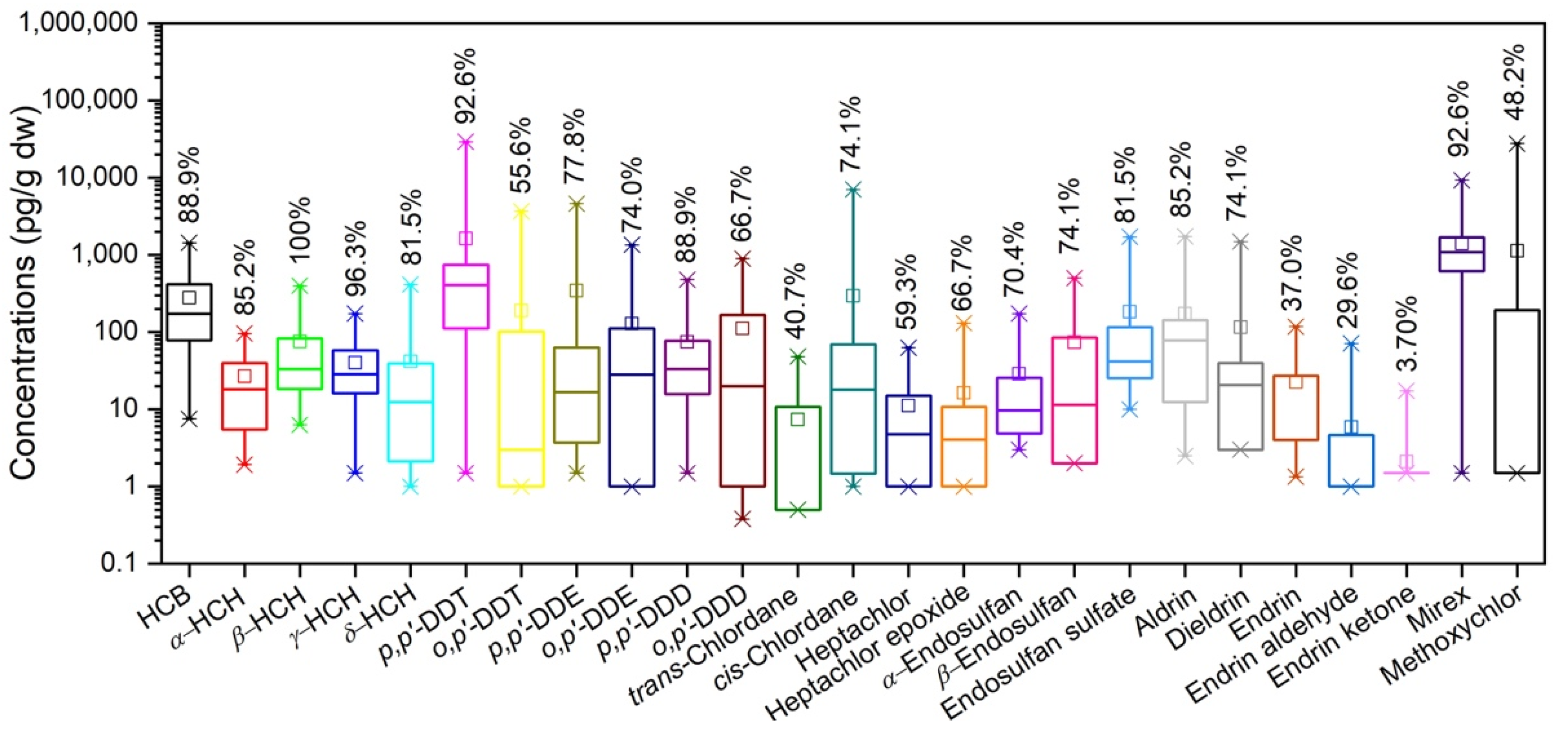
3.1. General Comments on OCP Concentrations
3.2. Influence of Land-Use Type and Water Transport on the OCP Spatial Variation
3.3. Source Diagnosis for OCPs by Composition Analysis
3.3.1. HCB
3.3.2. HCHs
3.3.3. DDTs
3.3.4. CHLs
3.3.5. ENDOs
3.3.6. DRINs
3.3.7. Mirex and Methoxychlor
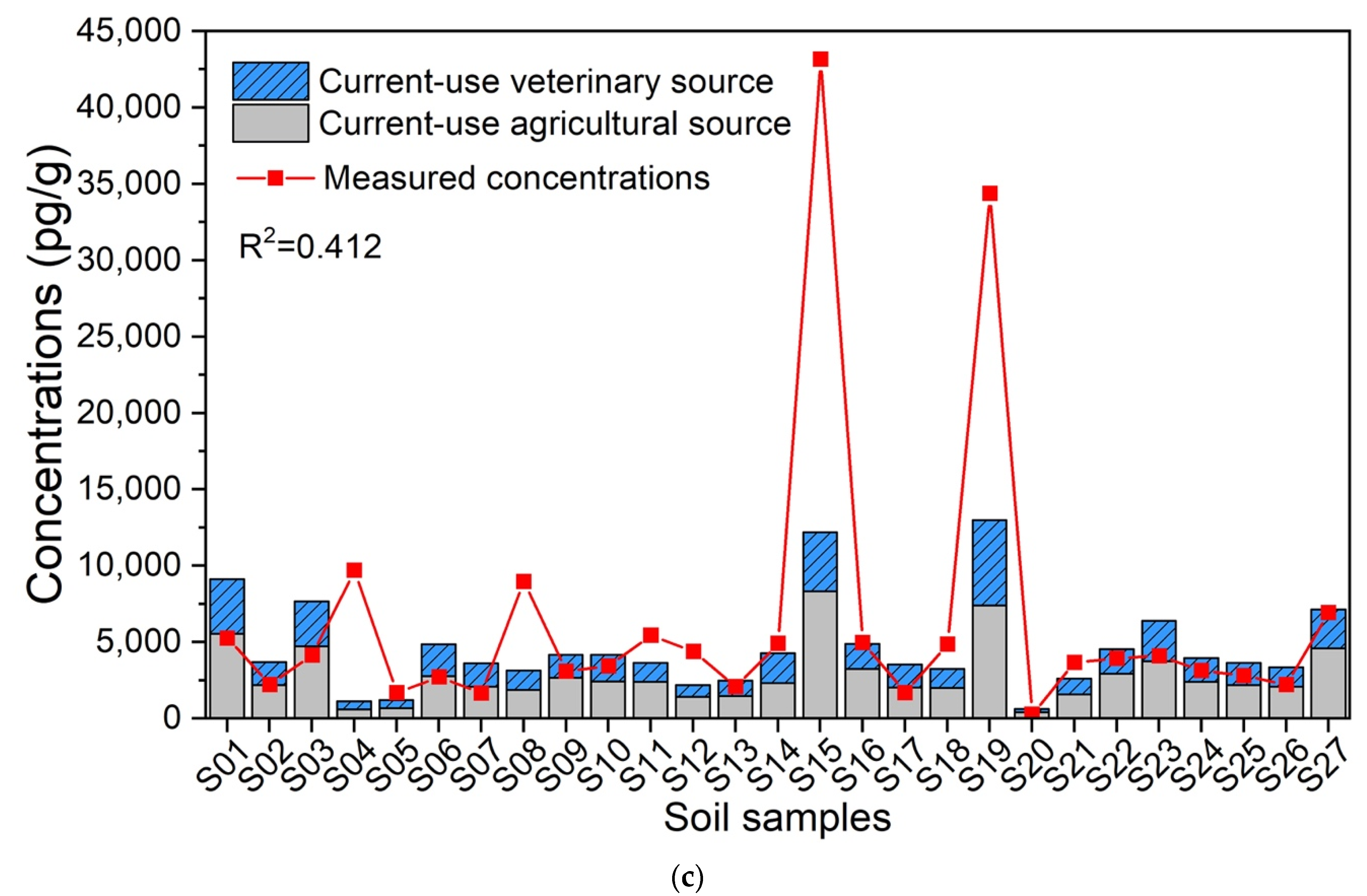
3.4. Characteristics and Contributions of Sources
3.5. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganzel, B. How Insecticides Work. Available online: https://livinghistoryfarm.org/farminginthe70s/pests_06.html (accessed on 15 March 2021).
- Li, Y.; Cai, D.; Singh, A. Technical Hexachlorocyclohexane Use Trends in China and Their Impact on the Environment. Arch. Environ. Contam. Toxicol. 1998, 35, 688–697. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Breivik, K.; Münch, J.; Fudala, J. European Atmospheric Emissions of Selected Persistent Organic Pollutants, 1970–1995. Atmos. Environ. 2003, 37, 119–131. [Google Scholar] [CrossRef]
- WHO. Public Health Impact of Pesticides Used in Agriculture; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Ledirac, N.; Antherieu, S.; d’Uby, A.D.; Caron, J.C.; Rahmani, R. Effects of Organochlorine Insecticides on MAP Kinase Pathways in Human HaCaT Keratinocytes: Key Role of Reactive Oxygen Species. Toxicol. Sci. 2005, 86, 444–452. [Google Scholar] [CrossRef]
- Polanco-Rodríguez, A.G.; López, M.I.R.; Casillas, Á.D.; León, J.A.A.; Banik, S.D. Impact of Pesticides in Karst Groundwater. Review of Recent Trends in Yucatan, Mexico. Groundw. Sustain. Dev. 2018, 7, 20–29. [Google Scholar] [CrossRef]
- Tsygankov, V.Y. Organochlorine Pesticides in Marine Ecosystems of the Far Eastern Seas of Russia (2000–2017). Water Res. 2019, 161, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xing, X.; Qi, S.; Tan, L.; Yang, D.; Chen, W.; Yang, J.; Xu, M. Organochlorine Pesticides (OCPs) in Soils of the Coastal Areas along Sanduao Bay and Xinghua Bay, Southeast China. J. Geochem. Explor. 2013, 125, 153–158. [Google Scholar] [CrossRef]
- Van den Brink, N.W. Directed Transport of Volatile Organochlorine Pollutants to Polar Regions: The Effect on the Contamination Pattern of Antarctic Seabirds. Sci. Total Environ. 1997, 198, 43–50. [Google Scholar] [CrossRef]
- Wania, F.; Mackay, D. A Global Distribution Model for Persistent Organic Chemicals. Sci. Total Environ. 1995, 160–161, 211–232. [Google Scholar] [CrossRef]
- Gerber, R.; Smit, N.J.; Van Vuren, J.H.J.; Nakayama, S.M.M.; Yohannes, Y.B.; Ikenaka, Y.; Ishizuka, M.; Wepener, V. Bioaccumulation and Human Health Risk Assessment of DDT and Other Organochlorine Pesticides in an Apex Aquatic Predator from a Premier Conservation Area. Sci. Total Environ. 2016, 550, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Khuman, S.N.; Vinod, P.G.; Bharat, G.; Kumar, Y.S.M.; Chakraborty, P. Spatial Distribution and Compositional Profiles of Organochlorine Pesticides in the Surface Soil from the Agricultural, Coastal and Backwater Transects along the South-West Coast of India. Chemosphere 2020, 254, 126699. [Google Scholar] [CrossRef]
- Pan, H.; Lei, H.; He, X.; Xi, B.; Xu, Q. Spatial Distribution of Organochlorine and Organophosphorus Pesticides in Soil-Groundwater Systems and Their Associated Risks in the Middle Reaches of the Yangtze River Basin. Environ. Geochem. Health 2019, 41, 1833–1845. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, R.; Li, Y.; Wang, P.; Zhu, Y.; Yuan, G.; Zhang, Q.; Jiang, G. Accumulation and Fate Processes of Organochlorine Pesticides (OCPs) in Soil Profiles in Mt. Shergyla, Tibetan Plateau: A Comparison on Different Forest Types. Chemosphere 2019, 231, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Goldscheider, N.; Wagener, T.; Lange, J.; Weiler, M. Karst Water Resources in a Changing World: Review of Hydrological Modeling Approaches. Rev. Geophys. 2014, 52, 218–242. [Google Scholar] [CrossRef]
- Dan, X.; He, D.; Wu, X.; Wu, Z.; Li, M.; Tu, Z.; Dan, W. Ecological characteristics of karst areas in China and the hazard of rocky desertification. Cent. South For. Inventory Plan. 2018, 37, 62–66. (In Chinese) [Google Scholar]
- Zhu, J.; Nolte, A.M.; Jacobs, N.; Ye, M. Using Machine Learning to Identify Karst Sinkholes from LiDAR-Derived Topographic Depressions in the Bluegrass Region of Kentucky. J. Hydrol. 2020, 125049. [Google Scholar] [CrossRef]
- Zeng, F.; Jiang, Z.; Shen, L.; Chen, W.; Yang, Q.; Zhang, C. Assessment of Multiple and Interacting Modes of Soil Loss in the Karst Critical Zone, Southwest China (SWC). Geomorphology 2018, 322, 97–106. [Google Scholar] [CrossRef]
- Fenton, O.; Mellander, P.E.; Daly, K.; Wall, D.P.; Jahangir, M.M.R.; Jordan, P.; Hennessey, D.; Huebsch, M.; Blum, P.; Vero, S.; et al. Integrated Assessment of Agricultural Nutrient Pressures and Legacies in Karst Landscapes. Agric. Ecosyst. Environ. 2017, 239, 246–256. [Google Scholar] [CrossRef]
- Li, S.; Xu, S.; Wang, T.; Yue, F.; Peng, T.; Zhong, J.; Wang, L.; Chen, J.; Wang, S.; Chen, X.; et al. Effects of Agricultural Activities Coupled with Karst Structures on Riverine Biogeochemical Cycles and Environmental Quality in the Karst Region. Agric. Ecosyst. Environ. 2020, 303, 107120. [Google Scholar] [CrossRef]
- Coxon, C. Agriculture and Karst. In Karst Management; van Beynen, P.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 103–138. ISBN 978-94-007-1207-2. [Google Scholar]
- Wang, Z.; Li, S.; Yue, F.; Qin, C.; Buckerfield, S.; Zeng, J. Rainfall Driven Nitrate Transport in Agricultural Karst Surface River System: Insight from High Resolution Hydrochemistry and Nitrate Isotopes. Agric. Ecosyst. Environ. 2020, 291, 106787. [Google Scholar] [CrossRef]
- Xu, S.; Lang, Y.; Zhong, J.; Xiao, M.; Ding, H. Coupled Controls of Climate, Lithology and Land Use on Dissolved Trace Elements in a Karst River System. J. Hydrol. 2020, 591, 125328. [Google Scholar] [CrossRef]
- Gillieson, D.S. Management of Caves. In Karst Management; van Beynen, P.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 141–158. ISBN 978-94-007-1206-5. [Google Scholar]
- Xu, X.; Sun, Y.; Wang, P.; Alam, M. The Comparison of Organochlorine Pesticides between Underground Water and Surface Water in Karst Area. China Environ. Sci. 2013, 33, 1630–1637. (In Chinese) [Google Scholar]
- Guo, F.; Yuan, D.; Qin, Z. Groundwater Contamination in Karst Areas of Southwestern China and Recommended Countermeasures. Acta Carsologica 2010, 39. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Xiong, S.; Zeng, F.; Bu, J.; Zhang, B.; Liu, W.; Zhou, H.; Qi, S.; Xu, L.; et al. Rapid Transport of Organochlorine Pesticides (OCPs) in Multimedia Environment from Karst Area. Sci. Total Environ. 2021, 775, 145698. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Mao, Y.; Xiong, S.; Peng, B.; Liu, W.; Liu, H.; Zhang, Y.; Chen, W.; Zhou, H.; Qi, S. Historical Residues of Organochlorine Pesticides (OCPs) and Polycyclic Aromatic Hydrocarbons (PAHs) in a Flood Sediment Profile from the Longwang Cave in Yichang, China. Ecotoxicol. Environ. Saf. 2020, 196, 110542. [Google Scholar] [CrossRef] [PubMed]
- Green, S.M.; Dungait, J.A.J.; Tu, C.; Buss, H.L.; Sanderson, N.; Hawkes, S.J.; Xing, K.; Yue, F.; Hussey, V.L.; Peng, J.; et al. Soil Functions and Ecosystem Services Research in the Chinese Karst Critical Zone. Chem. Geol. 2019, 527, 119107. [Google Scholar] [CrossRef] [Green Version]
- Polanco-Rodríguez, A.G.; Alberto, J.A.N.; Sánchez, J.S.; Rejón, G.J.M.; Gómez, J.M.; Del Valls Casillas, T.A. Contamination by Organochlorine Pesticides in the Aquifer of the Ring of Cenotes in Yucatán, México: Contamination by Organochlorine Pesticides. Water Environ. J. 2015, 29, 140–150. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, S.; Xue, R.; Qi, S.; Xu, Y.; Xue, B.; Yuan, D. Organochlorine Pesticides in the Soil of a Karst Cave in Guilin, China. Environ. Monit. Assess. 2011, 180, 489–500. [Google Scholar] [CrossRef]
- Sun, Y. Study on Migration and Transformation Characteristics of OCPs and PAHs in Epikarst System. Ph.D. Thesis, Southwest University, Chongqing, China, 2012. [Google Scholar]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.C.; García-Río, L. The Mobility and Degradation of Pesticides in Soils and the Pollution of Groundwater Resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Teng, Y.; Su, L.; Li, W. Searching Resolutions for the Water Resources Dilemma in the Karst Area of Southwest China. Available online: https://www.cgs.gov.cn/xwl/ddyw/201610/t20161018_409605.html (accessed on 16 February 2021).
- Yu, D.; Su, L. Revealing the Secrets of Karst in Southwest China: Perspective of Karst Dynamics and Global Change. Available online: https://www.cgs.gov.cn/ddztt/jdqr/49dqr/kpzs/201804/t20180426_456338.html (accessed on 16 February 2021).
- Liu, W.; Wang, Z.; Chen, Q.; Yan, Z.; Zhang, T.; Han, Z.; Chen, W.; Zhou, H. An Interpretation of Water Recharge in Karst Trough Zone as Determined by High-Resolution Tracer Experiments in Western Hubei, China. Environ. Earth Sci. 2020, 79, 357. [Google Scholar] [CrossRef]
- Zhou, L.; Duan, Z.; Han, Q.; Xia, L.; Zhang, G. Comprehensive evaluation of soil fertility in citrus orchards in Zigui County. Jiangsu J. Agric. Sci. 2019, 35, 1346–1353. (In Chinese) [Google Scholar]
- Yichang Bureau of Statistics. Yichang Statistical Yearbook (2020); China Statistics Press: Beijing, China, 2020. [Google Scholar]
- Wu, Q. The Benefit Evaluation of Converting Farmland to Forest in Zigui. Master’s Thesis, Beijing Forestry University, Beijing, China, 2011. [Google Scholar]
- Zheng, J.; Xiang, C.; Hu, D.; Yi, J.; Li, L. Current situation and countermeasures of agricultural non-point source pollution in Zigui County. Hubei Plant Prot. 2013, 6, 3–5. (In Chinese) [Google Scholar]
- Huang, Y.; Zhang, R.; Li, K.; Cheng, Z.; Zhong, G.; Zhang, G.; Li, J. Experimental Study on the Role of Sedimentation and Degradation Processes on Atmospheric Deposition of Persistent Organic Pollutants in a Subtropical Water Column. Environ. Sci. Technol. 2017, 51, 4424–4433. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Riaz, R.; Sweetman, A.J.; Jones, K.C.; Li, J.; Zhang, G.; Malik, R.N. Role of Black Carbon in Soil Distribution of Organochlorines in Lesser Himalayan Region of Pakistan. Environ. Pollut. 2018, 236, 971–982. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Zhang, Y.; Chen, W.; Ding, Y.; Chen, W.; Qi, S. How Persistent Are POPs in Remote Areas? A Case Study of DDT Degradation in the Qinghai-Tibet Plateau, China. Environ. Pollut. 2020, 263, 114574. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jing, M.; Bu, J.; Burnet, J.E.; Qi, S.; Song, Q.; Ke, Y.; Miao, J.; Liu, M.; Yang, C. Organochlorine Pesticides in the Surface Water and Sediments from the Peacock River Drainage Basin in Xinjiang, China: A Study of an Arid Zone in Central Asia. Environ. Monit. Assess. 2011, 177, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ding, Y.; Chen, W.; Zhang, Y.; Chen, W.; Chen, Y.; Mao, Y.; Qi, S. Two-Way Long-Range Atmospheric Transport of Organochlorine Pesticides (OCPs) between the Yellow River Source and the Sichuan Basin, Western China. Sci. Total Environ. 2019, 651, 3230–3240. [Google Scholar] [CrossRef]
- U.S. EPA. Exposure Factors Handbook (1997, Final Report); EPA/600/P-95/002F a-c; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Sultana, J.; Syed, J.H.; Mahmood, A.; Ali, U.; Rehman, M.Y.A.; Malik, R.N.; Li, J.; Zhang, G. Investigation of Organochlorine Pesticides from the Indus Basin, Pakistan: Sources, Air–Soil Exchange Fluxes and Risk Assessment. Sci. Total Environ. 2014, 497–498, 113–122. [Google Scholar] [CrossRef]
- Manz, M.; Wenzel, K.D.; Dietze, U.; Schüürmann, G. Persistent Organic Pollutants in Agricultural Soils of Central Germany. Sci. Total Environ. 2001, 277, 187–198. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Qi, S.; Li, X.; Peng, X. Concentrations, Enantiomeric Compositions, and Sources of HCH, DDT and Chlordane in Soils from the Pearl River Delta, South China. Sci. Total Environ. 2006, 372, 215–224. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Chen, W.; Chen, W.; Yuen, D.A.; Ding, Y.; Chen, Y.; Mao, Y.; Qi, S. Sources and Transformation Pathways for Dichlorodiphenyltrichloroethane (DDT) and Metabolites in Soils from Northwest Fujian, China. Environ. Pollut. 2018, 235, 560–570. [Google Scholar] [CrossRef]
- Taylor, J.; Wilson, J.D. Toxicological Profile for Hexachlorobenzene; U.S. Department of Health and Human Services: Washington, DC, USA, 2002. [Google Scholar]
- Ministry of Ecology and Environment. PRC Announcement on the Prohibition of the Production, Circulation, Use and Import and Export of DDT, Chlordane, Mirex and Hexachlorobenzene; Ministry of Ecology and Environment: Beijing, China, 2009. [Google Scholar]
- Tong, M.; Yuan, S. Physiochemical Technologies for HCB Remediation and Disposal: A Review. J. Hazard. Mater. 2012, 229–230, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Dong, L.; Shi, S.; Zhou, L.; Zhang, T.; Mi, F.; Zeng, L.; Shao, D. Levels, Seasonal Patterns, and Potential Sources of Organochlorine Pesticides in the Urban Atmosphere of Beijing, China. Arch. Environ. Contam. Toxicol. 2011, 61, 159–165. [Google Scholar] [CrossRef]
- Buser, H.R.; Mueller, M.D. Isomer and Enantioselective Degradation of Hexachlorocyclohexane Isomers in Sewage Sludge under Anaerobic Conditions. Environ. Sci. Technol. 1995, 29, 664–672. [Google Scholar] [CrossRef]
- Li, Y. Global Technical Hexachlorocyclohexane Usage and Its Contamination Consequences in the Environment: From 1948 to 1997. Sci. Total Environ. 1999, 232, 121–158. [Google Scholar] [CrossRef]
- Benezet, H.J.; Matsumura, F. Isomerization of γ-BHC to α-BHC in the Environment. Nature 1973, 243, 480–481. [Google Scholar] [CrossRef]
- Malaiyandi, M.; Shah, S.M. Evidence of Photoisomerization of Hexachlorocyclohexane Isomers in the Ecosphere. J. Environ. Sci. Health 1984, 19, 887–910. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, S.; Xu, Y.; Wang, W.; Qi, S.; Xing, X.; Yuan, D. The Concentration and Distribution of Organochlorine Pesticides in the Air from the Karst Cave, South China. Environ. Geochem. Health 2012, 34, 493–502. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, B.; Mei, P.; Fu, L.; Tan, C. Problems and countermeasures of pesticide supervision in Zigui County. Hubei Plant Prot. 2016, 53–54, 59. [Google Scholar]
- Bidleman, T.F.; Jantunen, L.M.M.; Helm, P.A.; Brorström-Lundén, E.; Juntto, S. Chlordane Enantiomers and Temporal Trends of Chlordane Isomers in Arctic Air. Environ. Sci. Technol. 2002, 36, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, G.; Li, J.; Yu, L.; Xu, Y.; Li, X.; Kobara, Y.; Jones, K.C. Seasonal Patterns and Current Sources of DDTs, Chlordanes, Hexachlorobenzene, and Endosulfan in the Atmosphere of 37 Chinese Cities. Environ. Sci. Technol. 2009, 43, 1316–1321. [Google Scholar] [CrossRef]
- Cuozzo, S.A.; Fuentes, M.S.; Bourguignon, N.; Benimeli, C.S.; Amoroso, M.J. Chlordane Biodegradation under Aerobic Conditions by Indigenous Streptomyces Strains. Int. Biodeterior. Biodegrad. 2012, 66, 19–24. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment. PRC Announcement on Banning the Production, Circulation, Use, Import and Export of Lindane and Other POPs (000014672/2019-00287). Available online: http://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/201903/t20190312_695462.html (accessed on 16 May 2020).
- Chakraborty, P.; Zhang, G.; Li, J.; Xu, Y.; Liu, X.; Tanabe, S.; Jones, K.C. Selected Organochlorine Pesticides in the Atmosphere of Major Indian Cities: Levels, Regional versus Local Variations, and Sources. Environ. Sci. Technol. 2010, 44, 8038–8043. [Google Scholar] [CrossRef]
- Jia, H.; Liu, L.; Sun, Y.; Sun, B.; Wang, D.; Su, Y.; Kannan, K.; Li, Y. Monitoring and Modeling Endosulfan in Chinese Surface Soil. Environ. Sci. Technol. 2010, 44, 9279–9284. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs. PRC Announcement of the Ministry of Agriculture of the People’s Republic of China No.199; Ministry of Agriculture and Rural Affairs: Beijing, China, 2002. [Google Scholar]
- Stockholm Convention. All POPs Listed in the Stockholm Convention. Available online: http://www.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 12 May 2020).
- Dong, Y. Investigation of termite damage in the Three Gorges Reservoir Area (Zigui County). Hubei Plant Prot. 2017, 29–30, 40. (In Chinese) [Google Scholar]
- Stockholm Convention. UN Environment Chemicals Proposed for Listing under the Convention. Available online: http://www.pops.int/TheConvention/ThePOPs/ChemicalsProposedforListing/tabid/2510/Default.aspx (accessed on 1 February 2021).
- U.S. EPA. Methoxychlor Reregistration Eligibility Decision (RED) (No. EPA 738-R-04-010). Available online: https://archive.epa.gov/pesticides/reregistration/web/html/methoxychlor_red.html (accessed on 1 February 2021).
- Cheng, L.; Song, W.; Rao, Q.; Zhou, J.; Zhao, Z. Bioaccumulation and Toxicity of Methoxychlor on Chinese Mitten Crab (Eriocheir Sinensis). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 221, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Zheng, Q.; Bing, H.; Zhang, R.; Wang, Y.; Luo, C.; Liu, X.; Wu, Y.; Pan, S.; et al. Forest Filter Effect versus Cold Trapping Effect on the Altitudinal Distribution of PCBs: A Case Study of Mt. Gongga, Eastern Tibetan Plateau. Environ. Sci. Technol. 2014, 48, 14377–14385. [Google Scholar] [CrossRef] [PubMed]
- State Administration for Market Regulation; Ministry of Ecology and Environment. PRC Soil Environmental Quality–Risk Control Standard for Soil Contamination of Agricultural Land (GB 15618-2018); State Administration for Market Regulation: Beijing, China; Ministry of Ecology and Environment: Beijing, China, 2018. [Google Scholar]
- Ministry of Housing, Spatial Planning and Environmental Management. Soil Remediation Circular 2009; Ministry of Housing, Spatial Planning and Environmental Management: Amsterdam, The Netherlands, 2009. [Google Scholar]
- U.S. EPA. Guidelines for Carcinogen Risk Assessment. Available online: https://www.epa.gov/risk/guidelines-carcinogen-risk-assessment (accessed on 15 January 2021).
- Zhou, P.; Zhao, Y.; Li, J.; Wu, G.; Zhang, L.; Liu, Q.; Fan, S.; Yang, X.; Li, X.; Wu, Y. Dietary Exposure to Persistent Organochlorine Pesticides in 2007 Chinese Total Diet Study. Environ. Int. 2012, 42, 152–159. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zeng, F.; Liu, W.; Bu, J.; Hu, G.; Xie, S.; Yao, H.; Zhou, H.; Qi, S.; Huang, H. Organochlorine Pesticides in Karst Soil: Levels, Distribution, and Source Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 11589. https://doi.org/10.3390/ijerph182111589
Chen W, Zeng F, Liu W, Bu J, Hu G, Xie S, Yao H, Zhou H, Qi S, Huang H. Organochlorine Pesticides in Karst Soil: Levels, Distribution, and Source Diagnosis. International Journal of Environmental Research and Public Health. 2021; 18(21):11589. https://doi.org/10.3390/ijerph182111589
Chicago/Turabian StyleChen, Wei, Faming Zeng, Wei Liu, Jianwei Bu, Guofeng Hu, Songshi Xie, Hongyan Yao, Hong Zhou, Shihua Qi, and Huanfang Huang. 2021. "Organochlorine Pesticides in Karst Soil: Levels, Distribution, and Source Diagnosis" International Journal of Environmental Research and Public Health 18, no. 21: 11589. https://doi.org/10.3390/ijerph182111589








