Effect of Vibration Massage and Passive Rest on Recovery of Muscle Strength after Short-Term Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Vibration Sessions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogdanis, G.C. Effects of Physical Activity and Inactivity on Muscle Fatigue. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Żołądź, J. Co Warunkuje Siłę, Moc i Wytrzymałość Mięśni Szkieletowych Człowieka? StatSoft: Tulsa, OK, USA, 2013. [Google Scholar]
- Williams, C.A.; Ratel, S. Human Muscle Fatigue; Taylor& Francis: New York, NY, USA, 2009. [Google Scholar]
- Górski, J. Fizjologia wysiłku i treningu fizycznego. Wydaw. Lek. PZWL 2019, 28, 148. [Google Scholar]
- Neptune, R.R.; McGowan, C.P.; Fiandt, J.M. The influence of muscle physiology and advanced technology on sports performance. Annu. Rev. Biomed. Eng. 2009, 11, 81–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weippert, M.; Behrens, K.; Rieger, A.; Stoll, R.; Kreuzfeld, S. Heart Rate Variability and Blood Pressure during Dynamic and Static Exercise at Similar Heart Rate Levels. PLoS ONE 2013, 8, e83690. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [Green Version]
- Bull, R.K.; Davies, C.T.; Lind, A.R.; White, M.J. The human pressor response during and following voluntary and evoked isometric contraction with occluded local blood supply. J. Physiol. 1989, 411, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, M.H.; Bowles, D.K.; Duncker, D.J. The coronary circulation in exercise training. Am. J. Physiol. Circ. Physiol. 2012, 302, H10–H23. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, R.P.; Rietjens, G.J.; Tijdink, H.H.; Noakes, T.D.; Lambert, M.I. Measuring submaximal performance parameters to monitor fatigue and predict cycling performance: A case study of a world-class cyclo-cross cyclist. Eur. J. Appl. Physiol. 2010, 108, 183–190. [Google Scholar] [CrossRef]
- Rydzik, Ł.; Maciejczyk, M.; Czarny, W.; Kędra, A.; Ambroży, T. Physiological Responses and Bout Analysis in Elite Kickboxers During International K1 Competitions. Front. Physiol. 2021, 12, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A. Using Recovery Modalities between Training Sessions in Elite Athletes. Sport Med. 2006, 36, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.J. The role of massage in the management of the athlete: A review. Br. J. Sports Med. 1993, 27, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bellmunt, A.; Labata-Lezaun, N.; Llurda-Almuzara, L.; Rodríguez-Sanz, J.; González-Rueda, V.; Bueno-Gracia, E.; Celik, D.; López-de-Celis, C. Effects of a Massage Protocol in Tensiomyographic and Myotonometric Proprieties. Int. J. Environ. Res. Public Health 2021, 18, 3891. [Google Scholar] [CrossRef] [PubMed]
- Weerapong, P.; Hume, P.A.; Kolt, G.S. The Mechanisms of Massage and Effects on Performance, Muscle Recovery and Injury Prevention. Sport Med. 2005, 35, 235–256. [Google Scholar] [CrossRef]
- Hopper, D.; Conneely, M.; Chromiak, F.; Canini, E.; Berggren, J.; Briffa, K. Evaluation of the effect of two massage techniques on hamstring muscle length in competitive female hockey players. Phys. Ther. Sport 2005, 6, 137–145. [Google Scholar] [CrossRef]
- Garrison, N.A.; Yi, Z.; Cohen-Barak, O.; Huizing, M.; Hartnell, L.M.; Gahl, W.A.; Brilliant, M.H. P gene mutations in patients with oculocutaneous albinism and findings suggestive of Hermansky-Pudlak syndrome. J. Med. Genet. 2004, 41, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausswirth, C.; Le Meur, Y. Physiological and Nutritional Aspects of Post-Exercise Recovery. Sport Med. 2011, 41, 861–882. [Google Scholar] [CrossRef]
- Poppendieck, W.; Wegmann, M.; Ferrauti, A.; Kellmann, M.; Pfeiffer, M.; Meyer, T. Massage and Performance Recovery: A Meta-Analytical Review. Sport Med. 2016, 46, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Issurin, V.B. Vibrations and their applications in sport: A review. J. Sport Med. Phys. Fit. 2005, 45, 324–336. [Google Scholar]
- Oliveri, D.J.; Lynn, K.; Hong, C.-Z. Increased Skin Temperature after Vibratory Stimulation. Am. J. Phys. Med. Rehabil. 1989, 68, 81–85. [Google Scholar] [CrossRef]
- Marin, P.J.; Zarzuela, R.; Zarzosa, F.; Herrero, A.J.; Garatachea, N.; Rhea, M.R.; García-López, D. Whole-body vibration as a method of recovery for soccer players. Eur. J. Sport Sci. 2012, 12, 2–8. [Google Scholar] [CrossRef]
- Baloy, R.; Ogston, J. Efects of vibration training on muscle recovery and exercise induced soreness: A systematic review. Movement, Heal. Exerc. 2016, 5, 41–49. [Google Scholar]
- Petrofsky, J.; Schwab, E.; Lo, T.; Cúneo, M.; Lawson, D. The thermal effect on the blood flow response to electrical stimulation. Med. Sci. Monit. 2007, 13, 498–504. [Google Scholar]
- Kosar, A.C.; Candow, D.G.; Putland, J.T. Potential Beneficial Effects of Whole-Body Vibration for Muscle Recovery After Exercise. J. Strength Cond. Res. 2012, 26, 2907–2911. [Google Scholar] [CrossRef]
- Lau, W.Y.; Nosaka, K. Effect of Vibration Treatment on Symptoms Associated with Eccentric Exercise-Induced Muscle Damage. Am. J. Phys. Med. Rehabil. 2011, 90, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, S.; Rousseau, J.J.; Thorp, R.M.; Choate, S.L.; Jackson, F.S.; Rowlands, D.S. Vibration therapy reduces plasma IL6 and muscle soreness after downhill running. Br. J. Sports Med. 2010, 44, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Aminian-Far, A.; Hadian, M.-R.; Olyaei, G.; Talebian, S.; Bakhtiary, A.H. Whole-Body Vibration and the Prevention and Treatment of Delayed-Onset Muscle Soreness. J. Athl. Train. 2011, 46, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imtiyaz, S.; Veqar, Z.; Shareef, M.Y. To Compare the Effect of Vibration Therapy and Massage in Prevention of Delayed Onset Muscle Soreness (DOMS). J. Clin. Diagn. Res. 2014, 8, 133. [Google Scholar] [CrossRef]
- Barnes, M.J.; Perry, B.G.; Mündel, T.; Cochrane, D.J. The effects of vibration therapy on muscle force loss following eccentrically induced muscle damage. Eur. J. Appl. Physiol. 2012, 112, 1189–1194. [Google Scholar] [CrossRef]
- Dabbs, N.C. Effects Of Whole Body Vibration On Vertical Jump Performance Following Exercise Induced Muscle Damage. Int. J. Kinesiol. Sport Sci. J. Kinesiol. Sport Sci. J. Kinesiol. Sport Sci. 2014, 2, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Annino, G.; Manzi, V.; Buselli, P.; Ruscello, B.; Franceschetti, F.; Romagnoli, C.; Cotelli, F.; Casasco, M.; Padua, E.; Iellamo, F. Acute effects of whole-body vibrations on the fatigue induced by multiple repeated sprint ability test in soccer players. J. Sports Med. Phys. Fit. 2021. [Google Scholar] [CrossRef]
- Edge, J.; Mündel, T.; Weir, K.; Cochrane, D.J. The effects of acute whole body vibration as a recovery modality following high-intensity interval training in well-trained, middle-aged runners. Eur. J. Appl. Physiol. 2009, 105, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Lim, J. The acute effects of two different whole body vibration frequencies on vertical jump performance. Med. Dello Sport 2003, 56, 287–292. [Google Scholar]
- Fuller, J.T.; Thomson, R.L.; Howe, P.R.C.; Buckley, J.D. Vibration Therapy Is No More Effective Than the Standard Practice of Massage and Stretching for Promoting Recovery From Muscle Damage After Eccentric Exercise. Clin. J. Sport Med. 2015, 25, 332–337. [Google Scholar] [CrossRef]
- Mukhtar, A.M.; Ali, K.A.; Mohd, F. Effects of vibratory massage therapy on grip strength, endurance time and forearm muscle performance. Work 2021, 68, 619–632. [Google Scholar] [CrossRef]
- Albasini, A.; Kruse, M.; Rembitzki, I. Using Whole Body Vibration in Physical Therapy and Sport-Clinical Practice and Treatment Exercises; Elsevier: London, UK, 2010. [Google Scholar]
- Alam, M.M.; Khan, A.A.; Farooq, M.; Bhardwaj, S. Effect of one week intervention of vibratory massage therapy on forearm grip strength and endurance. Age 2016, 28, 5. [Google Scholar]
- Kang, S.R.; Min, J.-Y.; Yu, C.; Kwon, T.-K. Effect of whole body vibration on lactate level recovery and heart rate recovery in rest after intense exercise. Technol. Heal. Care 2017, 25, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, P.J.; Rhea, M.R. Effects of Vibration Training on Muscle Power: A Meta-Analysis. J. Strength Cond. Res. 2010, 24, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Rhea, M.R.; Kenn, J.G. The Effect of Acute Applications of Whole-Body Vibration on the iTonic Platform on Subsequent Lower-Body Power Output During the Back Squat. J. Strength Cond. Res. 2009, 23, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Bedient, A.M.; Adams, J.B.; Edwards, D.A.; Serravite, D.H.; Huntsman, E.; Mow, S.E.; Roos, B.A.; Signorile, J.F. Displacement and Frequency for Maximizing Power Output Resulting From A Bout of Whole-Body Vibration. J. Strength Cond. Res. 2009, 23, 1683–1687. [Google Scholar] [CrossRef]
- Cochrane, D.J.; Booker, H. Does acute vibration exercise enhance horizontal jump performance? J. Sports Sci. Med. 2014, 13, 315–320. [Google Scholar]
- Park, S.-Y.; Son, W.-M.; Kwon, O.-S. Effects of whole body vibration training on body composition, skeletal muscle strength, and cardiovascular health. J. Exerc. Rehabil. 2015, 11, 289–295. [Google Scholar] [CrossRef]
- Rittweger, J.; Mutschelknauss, M.; Felsenberg, D. Acute changes in neuromuscular excitability after exhaustive whole body vibration exercise as compared to exhaustion by squatting exercise. Clin. Physiol. Funct. Imaging 2003, 23, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; McNamara, B.; Moran, K. The Use of Vibration Training to Enhance Muscle Strength and Power. Sport Med. 2005, 35, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, B.R. Acute Effects of Various Whole-Body Vibration Frequencies on Lower-Body Power in Trained and Untrained Subjects. J. Strength Cond. Res. 2009, 23, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Lamont, H.S.; Cramer, J.T.; Bemben, D.A.; Shehab, R.L.; Anderson, M.A.; Bemben, M.G. Effects of a 6-Week Periodized Squat Training Program With or Without Whole-Body Vibration on Jump Height and Power Output Following Acute Vibration Exposure. J. Strength Cond. Res. 2009, 23, 2317–2325. [Google Scholar] [CrossRef]
- Bullock, N.; Martin, D.T.; Ross, A.; Rosemond, C.D.; Jordan, M.J.; Marino, F.E. Acute Effect of Whole-Body Vibration on Sprint and Jumping Performance in Elite Skeleton Athletes. J. Strength Cond. Res. 2008, 22, 1371–1374. [Google Scholar] [CrossRef]
- Da Silva-Grigoletto, M.E.; Vaamonde, D.M.; Castillo, E.; Poblador, M.S.; García-Manso, J.M.; Lancho, J.L. Acute and Cumulative Effects of Different Times of Recovery From Whole Body Vibration Exposure on Muscle Performance. J. Strength Cond. Res. 2009, 23, 2073–2082. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Grampp, S.; Henk, C.; Resch, H.; Preisinger, E.; Fialka-Moser, V.; Imhof, H. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin. Physiol. 2001, 21, 377–382. [Google Scholar] [CrossRef]
- Maloney-Hinds, C.; Petrofsky, J.; Zimmerman, G. The effect of 30 Hz vs. 50 Hz passive vibration and duration of vibration on skin blood flow in the arm. Med. Sci. Monit. 2008, 14, 112–116. [Google Scholar]
- Da Silva, M.E.; Fernandez, J.M.; Castillo, E.; Nuñez, V.M.; Vaamonde, D.M.; Poblador, M.S.; Lancho, J.L. Influence of vibration training on energy expenditure in active men. J. Strength Cond. Res. 2007, 21, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafarelli, E.; Sim, J.; Carolan, B.; Liebesman, J. Vibratory Massage and Short-Term Recovery from Muscular Fatigue. Int. J. Sports Med. 1990, 11, 474–478. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Rademaker, A.C.H.J.; Sargeant, A.J. Human Muscle Power Generating Capability During Cycling at Different Pedalling Rates. Exp. Physiol. 2000, 85, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kenney, W.; Wilmore, J.; Costill, D. Physiology of Sport and Exercise. Hum. Kinet. 2012. [Google Scholar]
- Karatzaferi, C.; de Haan, A.; Ferguson, R.; van Mechelen, W.; Sargeant, A. Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflügers Arch. 2001, 442, 467–474. [Google Scholar] [CrossRef]
- Hultman, E.; Sjoholm, H. Substrate availability. Biochem. Exerc. 1983, 13, 63–75. [Google Scholar]
- Hargreaves, M. Skeletal muscle metabolism during exercise in humans. Clin. Exp. Pharmacol Physiol. 2000, 27, 225–228. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Nevill, M.E.; Lakomy, H.K.A.; Boobis, L.H. Power output and muscle metabolism during and following recovery from 10 and 20 s of maximal sprint exercise in humans. Acta Physiol. Scand. 1998, 163, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Abe, D.; Fukuoka, Y. Phosphocreatine resynthesis during recovery in different muscles of the exercising leg by 31 P-MRS. Scand. J. Med. Sci. Sports 2013, 23, e313–e319. [Google Scholar] [CrossRef]
- Dupont, G.; Blondel, N.; Berthoin, S. Performance for short intermittent runs: Active recovery vs. passive recovery. Eur. J. Appl. Physiol. 2003, 89, 548–554. [Google Scholar] [CrossRef]
- Glaister, M. Multiple Sprint Work. Sport Med. 2005, 35, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Villanueva, A.; Hamer, P.; Bishop, D. Physical fitness and performance. Fatigue responses during repeated sprints matched for initial mechanical output. Med. Sci. Sport Exerc. 2007, 39, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S. Fizjologia Człowieka: Podręcznik dla Studentów Medycyny, 2nd ed.; Elsevier Urban & Partner: Wrocław, Poland, 2013. [Google Scholar]
- Ortega, J.O.; Lindstedt, S.L.; Nelson, F.E.; Jubrias, S.A.; Kushmerick, M.J.; Conley, K.E. Muscle force, work and cost: A novel technique to revisit the Fenn Effect. J. Exp. Biol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Girard, O.; Mendez-Villanueva, A.; Bishop, D. Repeated-Sprint Ability—Part I. Sport Med. 2011, 41, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Padulo, J.; Di Giminiani, R.; Ibba, G.; Zarrouk, N.; Moalla, W.; Attene, G.; Migliaccio, M.G.; Pizzolato, F.; Bishop, D.; Chamari, K. The Acute Effect of Whole Body Vibration on Repeated Shuttle-Running in Young Soccer Players. Int. J. Sports Med. 2013, 35, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattier, G.; Millet, G.Y.; Martin, A.; Martin, V. Fatigue and Recovery After High-Intensity Exercise Part II: Recovery Interventions. Int. J. Sports Med. 2004, 25, 509–515. [Google Scholar] [CrossRef]
- Maughan, R.; Gleeson, M.; Greenhaff, P. Biochemistry of Exercise and Training; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- MacClaren, D.; Gibson, H.; Parry-Billings, M.; Edwards, R. A review of the metabolic and physiological factors in fatigue. Exerc. Sport Sci. Rev. 1989, 17, 29–66. [Google Scholar] [CrossRef]
- Larivière, C.; Gravel, D.; Arsenault, A.B.; Gagnon, D.; Loisel, P. Muscle recovery from a short fatigue test and consequence on the reliability of EMG indices of fatigue. Eur. J. Appl. Physiol. 2003, 89, 171–176. [Google Scholar] [CrossRef]
- Mika, A.; Mika, P.; Fernhall, B.; Unnithan, V.B. Comparison of Recovery Strategies on Muscle Performance After Fatiguing Exercise. Am. J. Phys. Med. Rehabil. 2007, 86, 474–481. [Google Scholar] [CrossRef]
- Dolgener, F.; Morien, A. The effect of massage on lactate disappearance. J. Strength Cond. Res. 1993, 7, 159–162. [Google Scholar]
- Ahmaidi, S.; Granier, P.; Taoutaou, Z.; Mercier, J.; Dubouchaud, H.; Prefaut, C. Effects of active recovery on plasma lactate and anaerobic power following repeated intensive exercise. Med. Sci. Sport Exerc. 1996, 28, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Thiriet, P.; Gozal, D.; Wouassi, D.; Oumarou, T.; Gelas, H.; Lacour, J.R. The effect of various recovery modalities on subsequent performance, in consecutive supramaximal exercise. J. Sports Med. Phys. Fit. 1993, 33, 118–129. [Google Scholar]
- Busch, A.J.; van der Spuy, I.; Tupper, S.; Kim, S.Y.; Bidonde, J.; Overend, T.J. Whole body vibration exercise for fibromyalgia. In Cochrane Database of Systematic Reviews; Busch, A.J., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2015. [Google Scholar]


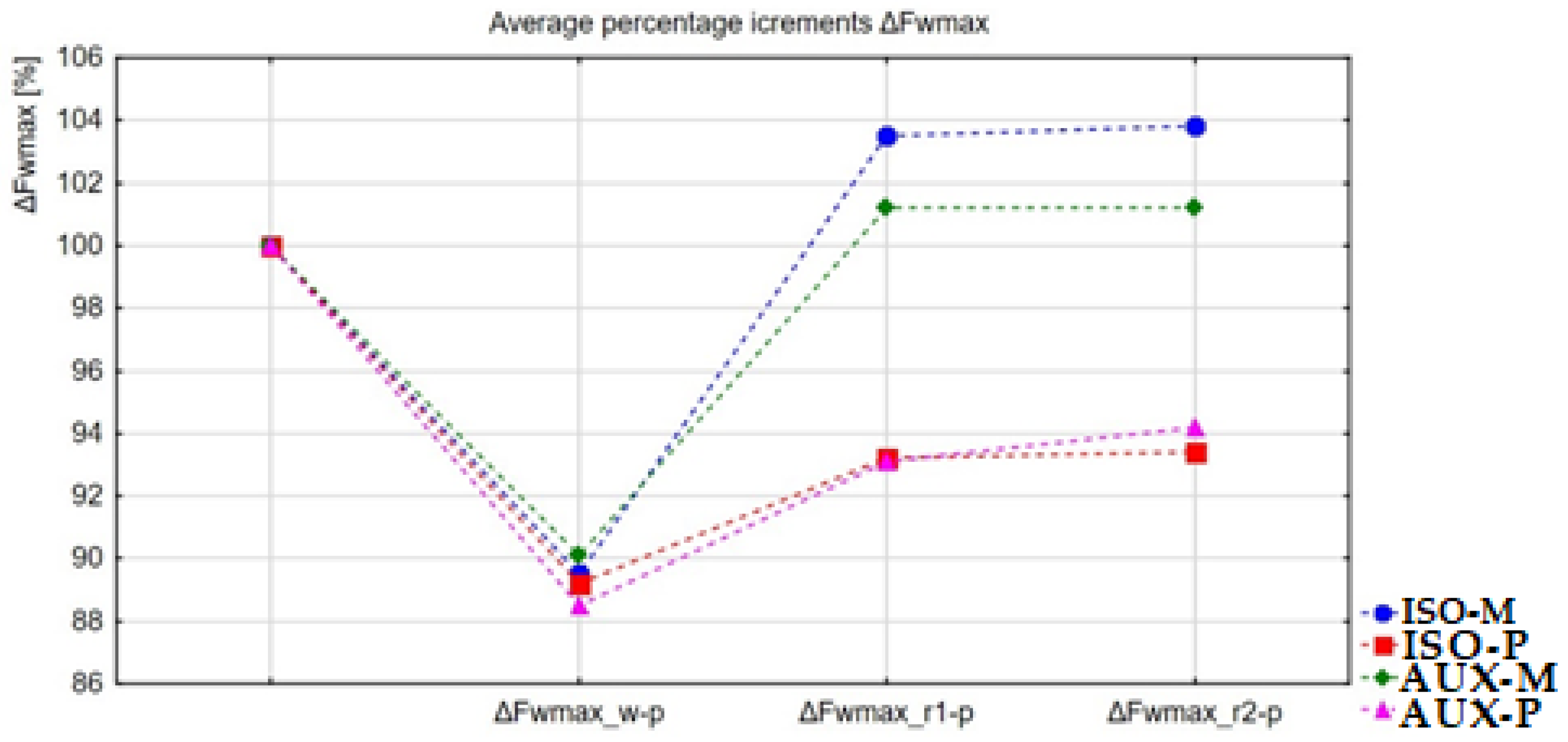
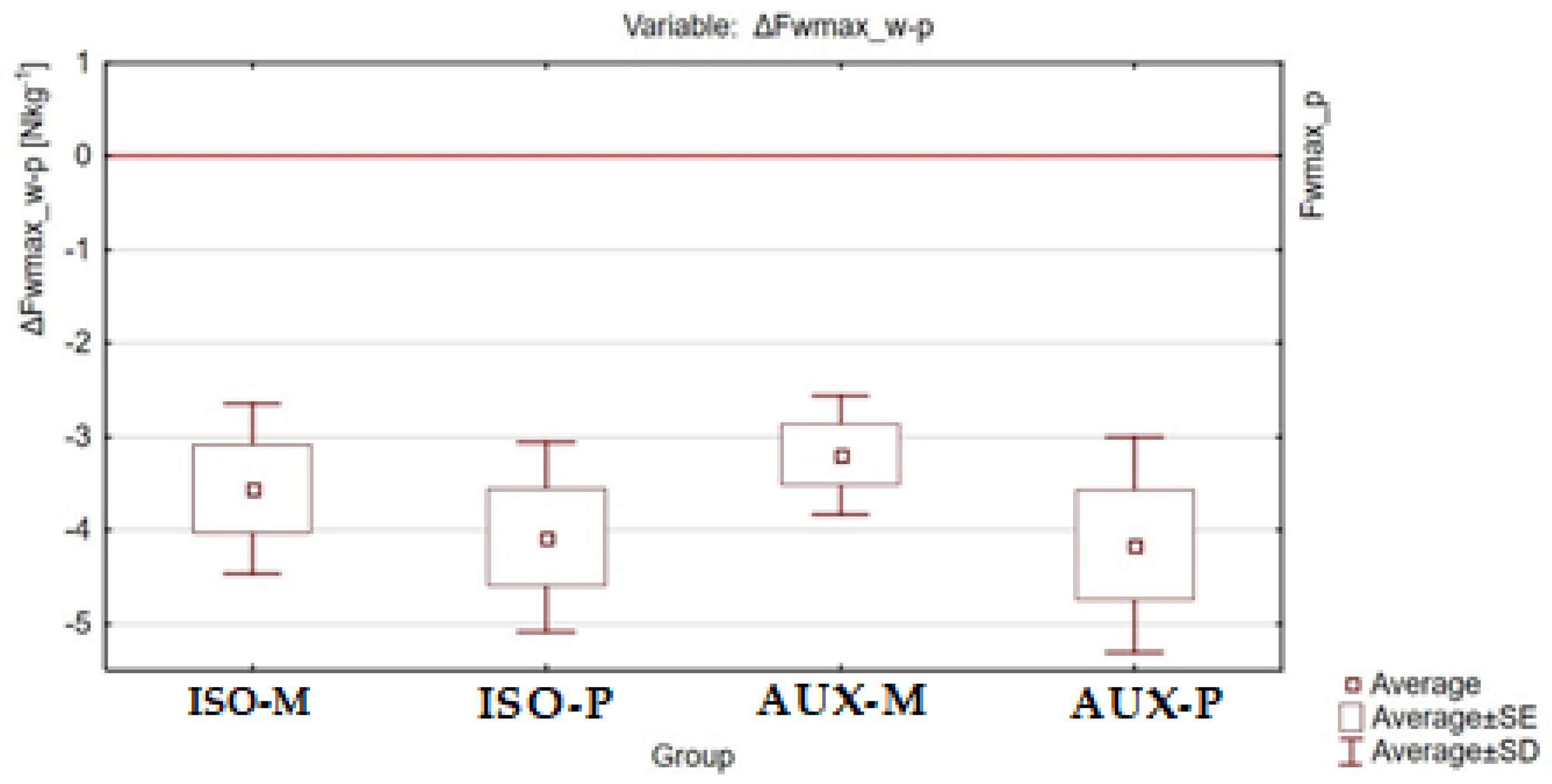


| Group/Variable | ISO-M | ISO-P | AUX-M | AUX-P |
|---|---|---|---|---|
| Number [n] | 21 | 21 | 21 | 21 |
| Age [years] | 20.4 ± 1.78 | 19.9 ± 1.91 | 21.1 ± 1.24 | 20.7 ± 1.55 |
| Body mass [kg] | 79.1 ± 11.33 | 74.9 ± 5.67 | 72.4 ± 8.34 | 78.6 ± 8.31 |
| Body height [m] | 1.81 ± 0.97 | 1.79 ± 0.81 | 1.78 ± 0.86 | 1.81 ± 0.10 |
| BMI [kgm−2] | 24.1 ± 1.26 | 23.2 ± 1.96 | 22.9 ± 2.04 | 23.9 ± 1.13 |
| Variables/Parameters/Group | ISO-M | ISO-P | AUX-M | AUX-P | |
|---|---|---|---|---|---|
| ΔFwmax_w-p [Nkg−1] | ± s | −3.6 ± 2.13 | −4.1 ± 2.38 | −3.2 ± 1.49 | −4.2 ± 2.69 |
| Me ± Q | - | −4.2 ± 1.17 | - | −2.9 ± 2.22 | |
| Min. | −8.1 | −11.4 | −5.9 | −8.7 | |
| Max. | −0.4 | −1 | −0.6 | −0.6 | |
| ΔFwmax_r1-p [Nkg−1] | ± s | 1.2 ± 2.65 | −2.6 ± 4.64 | 0.4 ± 2.27 | −2.5 ± 2.10 |
| Me ± Q | 1.3 ± 0.74 | - | - | - | |
| Min. | −6.1 | −16.7 | −4.5 | −7.4 | |
| Max. | 8.7 | 5.1 | 7.2 | 0.3 | |
| ΔFwmax_r2-p [Nkg−1] | ± s | 1.3 ± 3.73 | −2.5 ± 3.35 | 0.3 ± 2.04 | −2.1 ± 2.78 |
| Me ± Q | - | - | - | - | |
| Min. | −6 | −9.1 | −3.2 | −6.5 | |
| Max. | 8.5 | 3.62 | 4.6 | 4 | |
| Variable/Group | ΔFwmax_w-p [Nkg−1] vs. ΔFwmax_r1-p [Nkg−1] | ΔFwmax_w-p [Nkg−1] vs. ΔFwmax_r2-p [Nkg−1] | ΔFwmax_r1-p [Nkg−1] vs. ΔFwmax_r2-p [Nkg−1] |
|---|---|---|---|
| ISO-M | p < 0.001 N η2 = 0.82 | p < 0.001 N η2 = 0.83 | p = 0.82 N η2 = 0.18 |
| ISO-P | p < 0.01 N η2 = 0.76 | p < 0.05 N η2 = 0.76 | p = 0.79 N η2 = 0.38 |
| AUX-M | p < 0.001 P η2 = 0.85 | p < 0.001 P η2 = 0.85 | p = 0.98 P η2 = 0.05 |
| AUX-P | p = 0.097 N | ||
| Variable/Group | ΔFwmax_w-p [Nkg−1] | ΔFwmax_r1-p [Nkg−1] | ΔFwmax_r2-p [Nkg−1] |
|---|---|---|---|
| ISO-M/ISO-P | p = 0.71 N | p < 0.005 N η2 = 0.37 | p < 0.001 P η2 = 0.39 |
| ISO-M/AUX-M | p = 1.00 N η2 = 0.87 | p = 0.73 P η2 = 0.08 | |
| ISO-M/AUX-P | p < 0.001 N η2 = 0.62 | p < 0.005 P η2 = 0.40 | |
| ISO-P/AUX-M | p = 0.055 N η2 = 0.27 | p < 0.05 P η2 = 0.37 | |
| ISO-P/AUX-P | p = 1.00 N η2 = 0.61 | p = 0.98 P η2 = 0.48 | |
| AUX-M/AUX-P | p < 0.005 N η2 = 0.61 | p = 0.05 P η2 = 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chwała, W.; Pogwizd, P.; Rydzik, Ł.; Ambroży, T. Effect of Vibration Massage and Passive Rest on Recovery of Muscle Strength after Short-Term Exercise. Int. J. Environ. Res. Public Health 2021, 18, 11680. https://doi.org/10.3390/ijerph182111680
Chwała W, Pogwizd P, Rydzik Ł, Ambroży T. Effect of Vibration Massage and Passive Rest on Recovery of Muscle Strength after Short-Term Exercise. International Journal of Environmental Research and Public Health. 2021; 18(21):11680. https://doi.org/10.3390/ijerph182111680
Chicago/Turabian StyleChwała, Wiesław, Paweł Pogwizd, Łukasz Rydzik, and Tadeusz Ambroży. 2021. "Effect of Vibration Massage and Passive Rest on Recovery of Muscle Strength after Short-Term Exercise" International Journal of Environmental Research and Public Health 18, no. 21: 11680. https://doi.org/10.3390/ijerph182111680
APA StyleChwała, W., Pogwizd, P., Rydzik, Ł., & Ambroży, T. (2021). Effect of Vibration Massage and Passive Rest on Recovery of Muscle Strength after Short-Term Exercise. International Journal of Environmental Research and Public Health, 18(21), 11680. https://doi.org/10.3390/ijerph182111680








