Reactor Designs and Configurations for Biological and Bioelectrochemical C1 Gas Conversion: A Review
Abstract
:1. Introduction
2. Bioreactor Systems: Syngas Fermentation
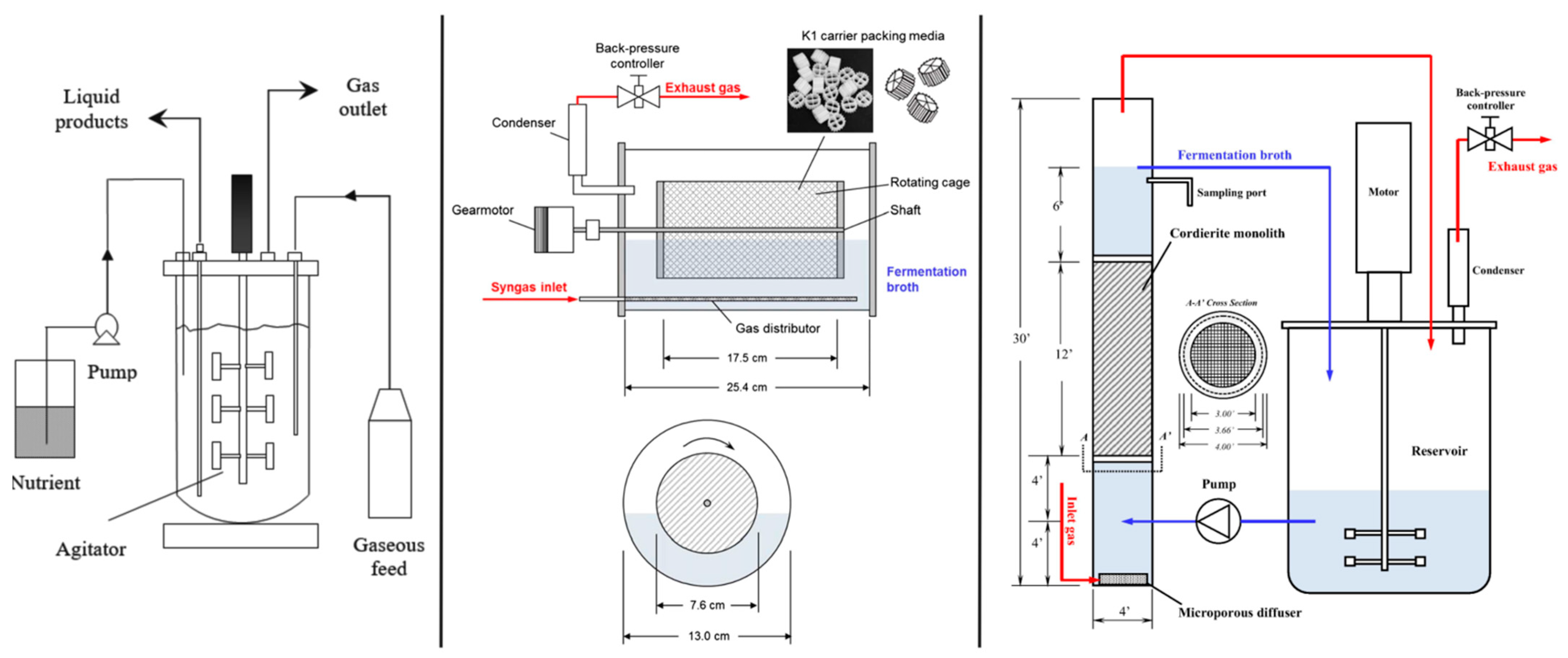
2.1. Rotating Packed Bed Biofilm Reactor
2.2. Monolithic Biofilm Reactor
2.3. Membrane Bioreactor (MBR)
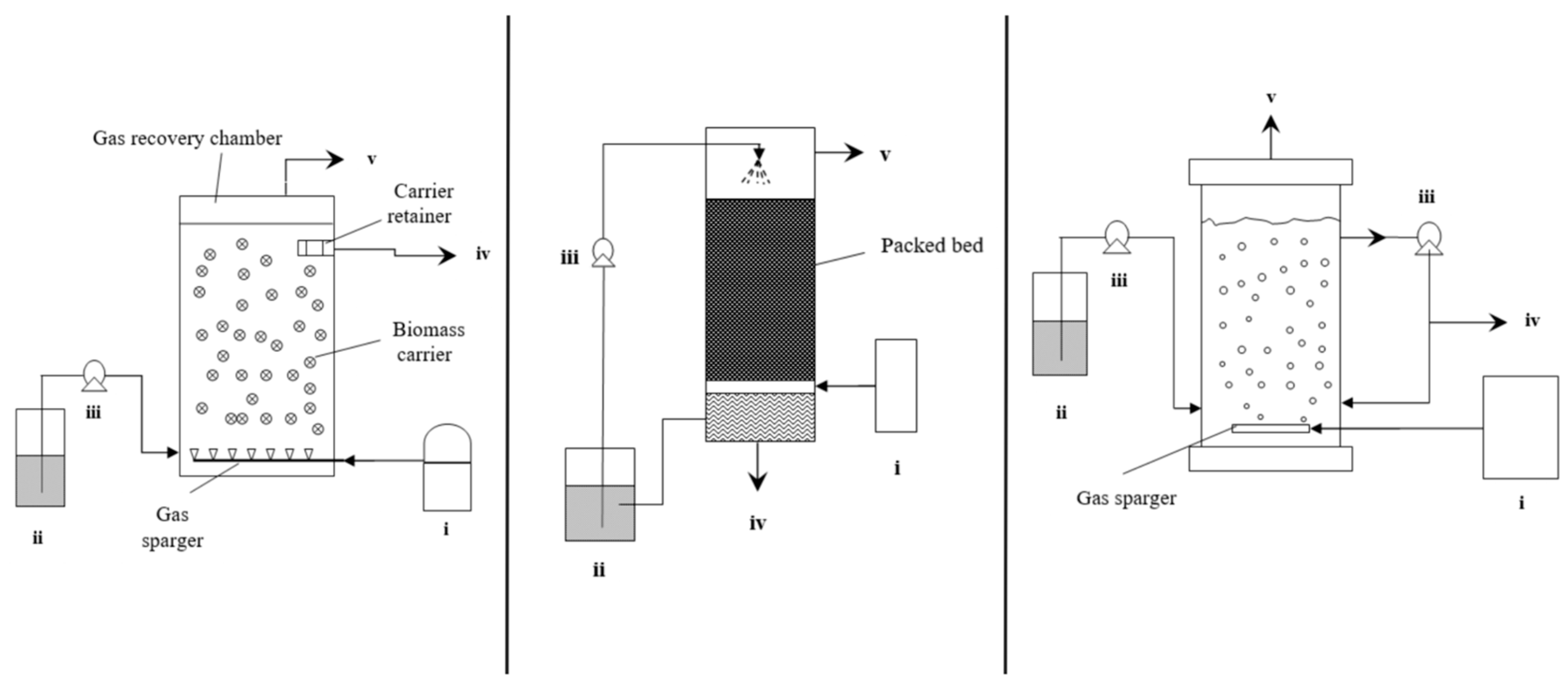
2.4. Moving Bed Biofilm Reactor (MBBR)
2.5. Trickle Bed Reactor (TBR)
2.6. Bubble Column Reactor (BCR)
3. Bioreactor Systems: Microbial Chain Elongation
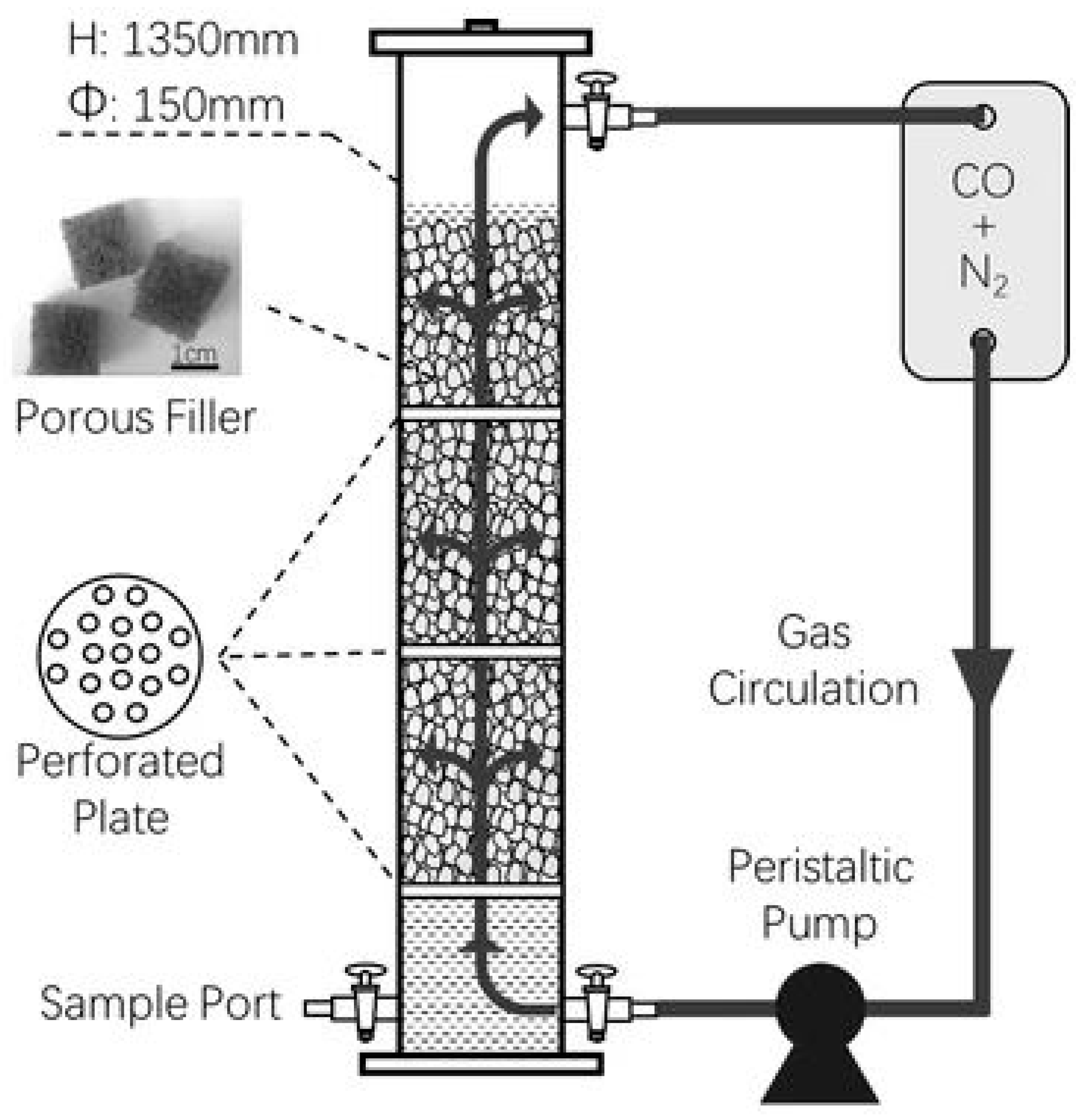
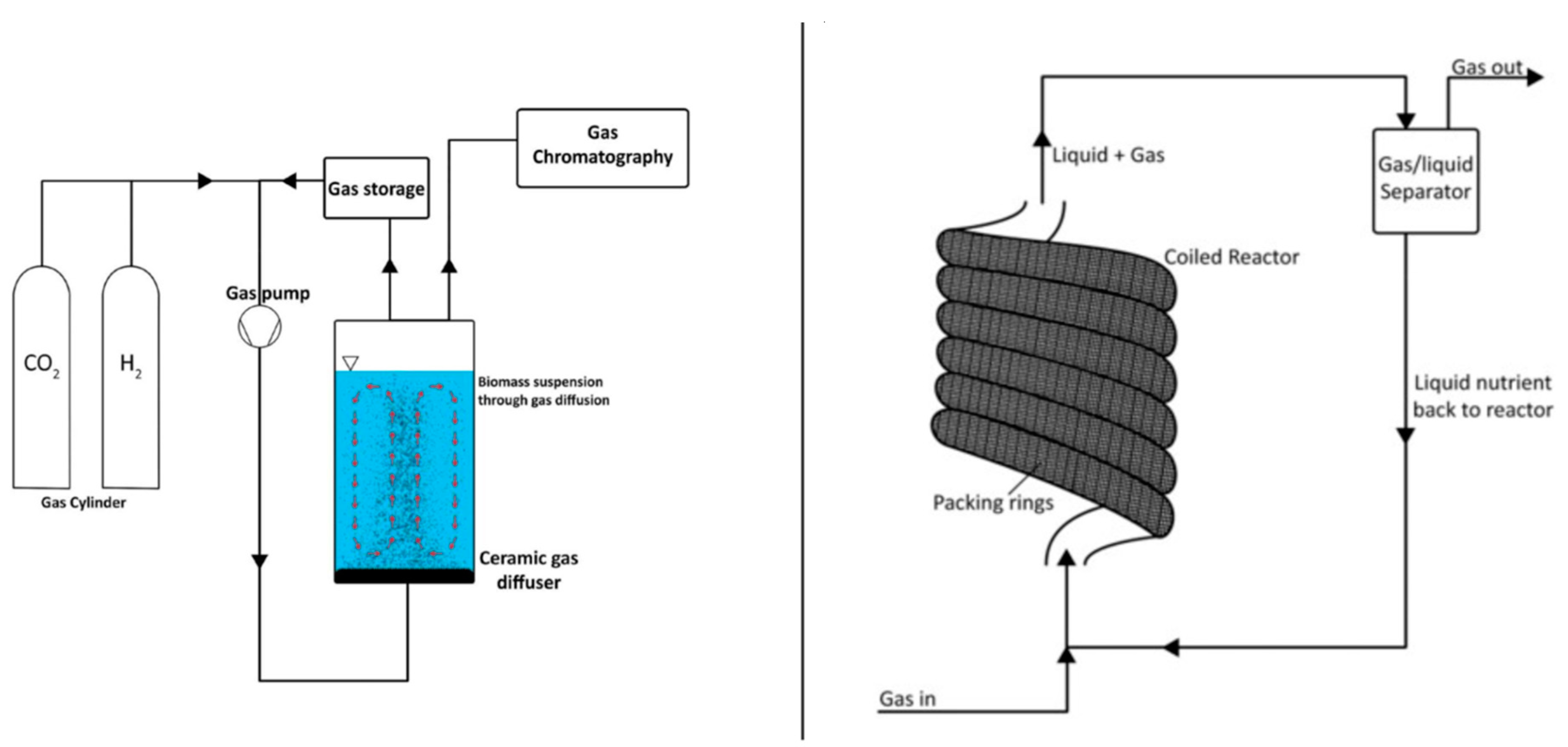
4. Bioreactor Systems: Hydrogenotrophic Methanation
5. Bioelectrochemical C1 Gas Conversion
6. Reactor Systems: Bio-Electrochemical Synthesis
6.1. Single-Chamber Bioelectrochemical Reactor
6.2. Tubular Bioelectrochemical Reactor
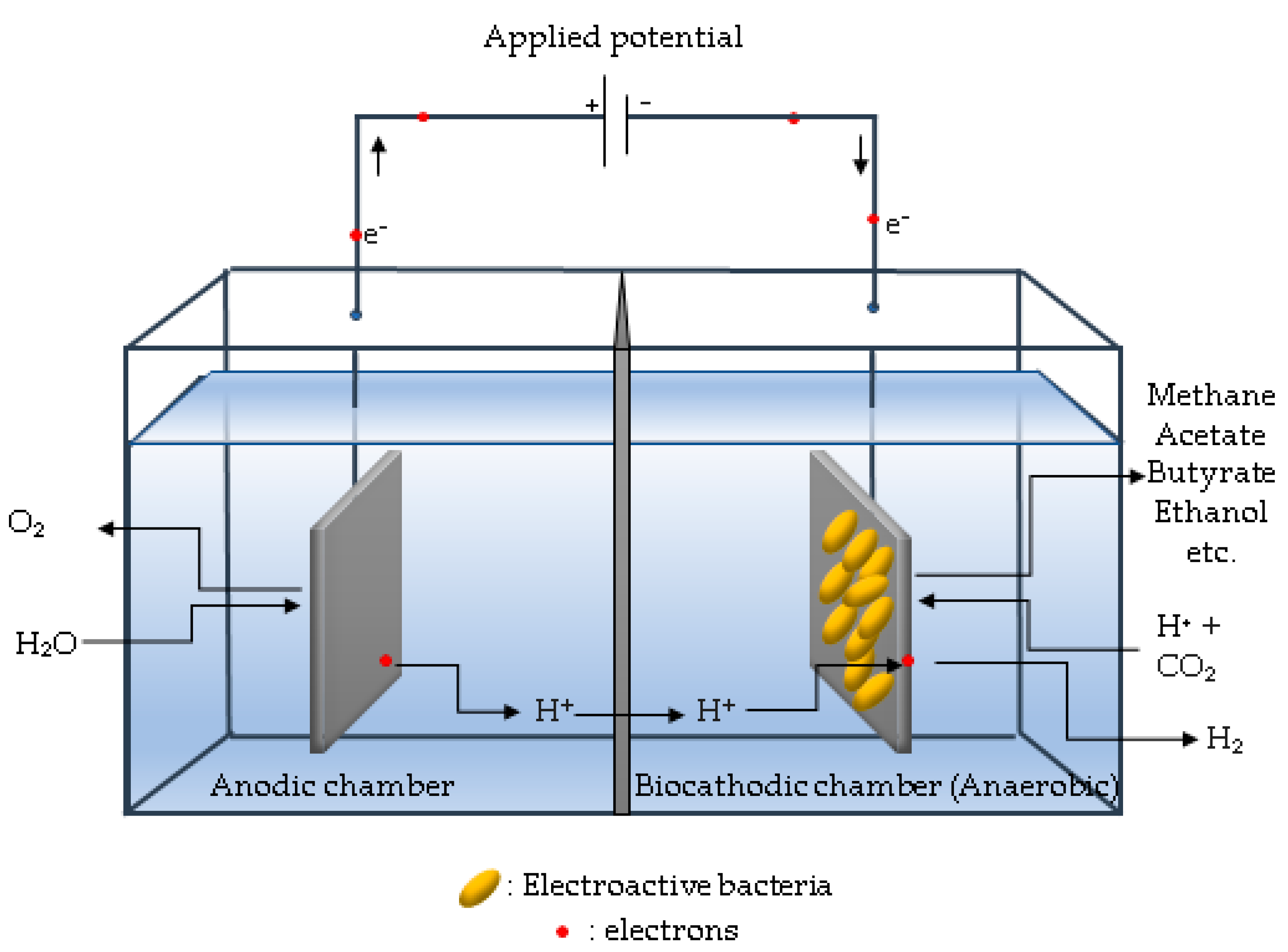
6.3. Dual-Chamber Bioelectrochemical Reactor
7. Biosensors in Bioelectrochemical Synthesis
8. Gas–Liquid Mass Transfer
9. Biofilm Formation
10. Kinetics
11. Electron Transfer Mechanism in Bioelectrochemical System
12. Industrialization and Patents
12.1. Syngas Fermentation
12.2. Microbial Chain Elongation
12.3. Hydrogenotrophic Methanation
12.4. Bioelectrochemical Synthesis
13. Techno-Economic Analysis and Life Cycle Analysis
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, J.; Song, Y.; Lee, H.; Shin, J.; Jin, S.; Kang, S.; Cho, B.K. Valorization of C1 gases to value-added chemicals using acetogenic biocatalysts. Chem. Eng. J. 2021, 428, 131325. [Google Scholar] [CrossRef]
- Gavala, H.N.; Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V. Gas Biological conversions: The potential of syngas and carbon dioxide as production platforms. Waste Biomass Valorization 2021, 12, 5303–5328. [Google Scholar] [CrossRef]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground bio-methanation: Concept and potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Bian, B.; Bajracharya, S.; Xu, J.; Pant, D.; Saikaly, P.E. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy. Bioresour. Technol. 2020, 302, 122863. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Chainese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Ciliberti, C.; Biundo, A.; Albergo, R.; Agrimi, G.; Braccio, G.; de Bari, I.; Pisano, I. Syngas derived from lignocellulosic biomass gasification as an alternative resource for innovative bioprocesses. Processes 2020, 8, 1567. [Google Scholar] [CrossRef]
- Molitor, B.; Richter, H.; Martin, M.E.; Jensen, R.O.; Juminaga, A.; Mihalcea, C.; Angenent, L.T. Carbon recovery by fermentation of CO-rich off gases–turning steel mills into biorefineries. Bioresour. Technol. 2016, 215, 386–396. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Beck, M.H.; Erz, C.; Hoffmeister, S.; Karl, M.M.; Riegler, P.; Wirth, S.; Poehlein, A.; Weuster-Botz, D.; Dürre, P. Bacterial anaerobic synthesis gas (Syngas) and CO2 + H2 fermentation. Adv. Appl. Microbiol. 2018, 103, 143–221. [Google Scholar] [CrossRef]
- Schuchmann, K.; Muller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Roghair, M.; Liu, Y.; Strik, D.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Development of an effective chain elongation process from acidified food waste and ethanol into n-Caproate. Front. Bioeng. Biotechnol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- Candry, P.; Ganigué, R. Chain elongators, friends, and foes. Curr. Opin. Biotechnol. 2021, 67, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, I.; Kracke, F.; Freguia, S.; Keller, J.; Krömer, J.O.; Ledezma, P.; Virdis, B. Microbial electrosynthesis system with dual biocathode arrangement for simultaneous acetogenesis, solventogenesis and carbon chain elongation. Chem. Commun. 2019, 55, 4351–4354. [Google Scholar] [CrossRef] [PubMed]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef] [Green Version]
- Yasri, N.; Roberts, E.P.L.; Gunasekaran, S. The electrochemical perspective of bioelectrocatalytic activities in microbial electrolysis and microbial fuel cells. Energy Rep. 2019, 5, 1116–1136. [Google Scholar] [CrossRef]
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N. Performance of exoelectrogenic bacteria used in microbial desalination cell technology. Int. J. Environ. Res. Public Health 2020, 17, 1121. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Md Jahim, J.; Ismail, M.; Lim, S.S. Biocathode in microbial electrolysis cell; present status and future prospects. Renew. Sustain. Energy Rev. 2015, 47, 23–33. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alexandria Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Ghangrekar, M.M.; Das, S.; Tiwari, B.R. Integration of bioelectrochemical systems with other existing wastewater treatment processes. In Integrated Microbial Fuel Cells for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 229–248. [Google Scholar] [CrossRef]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the edge of research and technological application: A critical review of electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Im, S.; Song, Y.-C.; Ahn, Y.; Kim, D.-H. Enhanced anaerobic digestion by stimulating DIET reaction. Processes 2020, 8, 424. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, A.; Shrestha, P.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.; Lovley, D. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef]
- Nelabhotla, A.B.T.; Dinamarca, C. Bioelectrochemical CO2 reduction to methane: MES integration in biogas production processes. Appl. Sci. 2019, 9, 1056. [Google Scholar] [CrossRef] [Green Version]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial electrosynthesis: Feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 2010, 1, e00103-10. [Google Scholar] [CrossRef] [Green Version]
- Batlle-Vilanova, P.; Ganigue, R.; Ramio-Pujol, S.; Baneras, L.; Jimenez, G.; Hidalgo, M.; Balaguer, M.D.; Colprim, J.; Puig, S. Microbial electrosynthesis of butyrate from carbon dioxide: Production and extraction. Bioelectrochemistry 2017, 117, 57–64. [Google Scholar] [CrossRef]
- Doran, P.M. Mixing. In Bioprocess Engineering Principles, 2nd ed.; Doran, P.M., Ed.; Academic Press: London, UK, 2013; pp. 255–332. [Google Scholar] [CrossRef]
- Rodgers, M.; Zhan, X.M. Moving-Medium Biofilm Reactors. Rev. Environ. Sci. Biotechnol. 2003, 2, 213–224. [Google Scholar] [CrossRef]
- Abu Bakar, S.N.H.; Abu Hasan, H.; Mohammad, A.W.; Sheikh Abdullah, S.R.; Haan, T.Y.; Ngteni, R.; Yusof, K.M.M. A review of moving-bed biofilm reactor technology for palm oil mill effluent treatment. J. Clean. Prod. 2018, 171, 1532–1545. [Google Scholar] [CrossRef]
- Katuri, K.P.; Werner, C.M.; Jimenez-Sandoval, R.J.; Chen, W.; Jeon, S.; Logan, B.E.; Lai, Z.; Amy, G.L.; Saikaly, P.E. A novel anaerobic electrochemical membrane bioreactor (AnEMBR) with conductive hollow-fiber membrane for treatment of low-organic strength solutions. Environ. Sci. Technol. 2014, 48, 12833–12841. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.C.; Wen, Z. Syngas fermentation by Clostridium carboxidivorans P7 in a horizontal rotating packed bed biofilm reactor with enhanced ethanol production. Appl. Energy 2017, 187, 585–594. [Google Scholar] [CrossRef]
- Jin, Y.; Veiga, M.C.; Kennes, C. Development of a novel monolith-bioreactor for the treatment of VOC-polluted air. Environ. Technol. 2006, 27, 1271–1277. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Heiszwolf, J.J. Multiphase monolith reactors: Chemical reaction engineering of segmented flow in microchannels. Chem. Eng. Sci. 2005, 60, 5895–5916. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.; Wen, Z. Enhancing mass transfer and ethanol production in syngas fermentation of Clostridium carboxidivorans P7 through a monolithic biofilm reactor. Appl. Energy 2014, 136, 68–76. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Biological conversion of carbon monoxide: Rich syngas or waste gases to bioethanol. Biofuels Bioprod. Biorefining 2011, 5, 93–114. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dewulf, J.; Van Langenhove, H. Membrane-based biological waste gas treatment. Chem. Eng. J. 2008, 136, 82–91. [Google Scholar] [CrossRef]
- Pichardo-Romero, D.; Garcia-Arce, Z.; Zavala-Ramírez, A.; Castro-Muñoz, R. Current advances in biofouling mitigation in membranes for water treatment: An overview. Processes 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Brown, R.; Wen, Z. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: Evaluating the mass transfer coefficient and ethanol production performance. Biochem. Eng. J. 2014, 85, 21–29. [Google Scholar] [CrossRef]
- Di Biase, A.; Kowalski, M.S.; Devlin, T.R.; Oleszkiewicz, J.A. Moving bed biofilm reactor technology in municipal wastewater treatment: A review. J. Environ. Manag. 2019, 247, 849–866. [Google Scholar] [CrossRef]
- Sivalingam, V.; Dinamarca, C. High pressure moving bed biofilm reactor for syngas fermentation. Chem. Eng. Trans. 2021, 86, 1483–1488. [Google Scholar] [CrossRef]
- Jensen, M.B.; Ottosen, L.D.M.; Kofoed, M.V.W. H2 gas-liquid mass transfer: A key element in biological Power-to-Gas methanation. Renew. Sustain. Energy Rev. 2021, 147, 111209. [Google Scholar] [CrossRef]
- Devarapalli, M.; Lewis, R.S.; Atiyeh, H.K. Continuous ethanol production from synthesis gas by Clostridium ragsdalei in a trickle-bed reactor. Fermentation 2017, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Devarapalli, M.; Atiyeh, H.K.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. Ethanol production during semi-continuous syngas fermentation in a trickle bed reactor using Clostridium ragsdalei. Bioresour. Technol. 2016, 209, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Kantarci, N.; Borak, F.; Ulgen, K.O. Bubble column reactors. Process Biochem. 2005, 40, 2263–2283. [Google Scholar] [CrossRef]
- Besagni, G.; Inzoli, F.; Ziegenhein, T. Two-phase bubble columns: A comprehensive review. ChemEngineering 2018, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Kommareddy, A.; Anderson, G. Analysis of currents and mixing in a modified bubble column reactor. In 2004 ASAE Annual Meeting; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2004; pp. 4105–4114. [Google Scholar]
- Doran, P.M. Reactor engineering. In Bioprocess Engineering Principles, 2nd ed.; Doran, P.M., Ed.; Academic Press: London, UK, 2013; pp. 761–852. [Google Scholar] [CrossRef]
- Richter, H.; Martin, M.E.; Angenent, L.T. A Two-stage continuous fermentation system for conversion of syngas into ethanol. Energies 2013, 6, 3987–4000. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, S.; Datar, R.P.; Lewis, R.S. Formation of ethanol from carbon monoxide via a new microbial catalyst. Biomass Bioenerg. 2002, 23, 487–493. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Ethanol and acetic acid production from carbon monoxide in a Clostridium strain in batch and continuous gas-fed bioreac-tors. Int. J. Environ. Res. Public Health 2015, 12, 1029–1043. [Google Scholar] [CrossRef] [Green Version]
- Gildemyn, S.; Molitor, B.; Usack, J.G.; Nguyen, M.; Rabaey, K.; Angenent, L.T. Upgrading syngas fermentation effluent using Clostridium kluyveri in a continuous fermentation. Biotechnol. Biofuels 2017, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Kucek, L.A.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain elongation with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494. [Google Scholar] [CrossRef]
- Ghysels, S.; Buffel, S.; Rabaey, K.; Ronsse, F.; Ganigué, R. Biochar and activated carbon enhance ethanol conversion and selectivity to caproic acid by Clostridium kluyveri. Bioresour. Technol. 2021, 319, 124236. [Google Scholar] [CrossRef]
- Candry, P.; Ulcar, B.; Petrognani, C.; Rabaey, K.; Ganigué, R. Ethanol:propionate ratio drives product selectivity in odd-chain elongation with Clostridium kluyveri and mixed communities. Bioresour. Technol. 2020, 313, 123651. [Google Scholar] [CrossRef] [PubMed]
- Roghair, M.; Hoogstad, T.; Strik, D.P.; Plugge, C.M.; Timmers, P.H.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J. Controlling ethanol use in chain elongation by CO2 loading rate. Environ. Sci. Technol. 2018, 52, 1496–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grootscholten, T.I.M.; Steinbusch, K.J.J.; Hamelers, H.V.M.; Buisman, C.J.N. High rate heptanoate production from propionate and ethanol using chain elongation. Bioresour. Technol. 2013, 136, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Grootscholten, T.I.M.; Kinsky dal Borgo, F.; Hamelers, H.V.M.; Buisman, C.J.N. Promoting chain elongation in mixed culture acidification reactors by addition of ethanol. Biomass Bioenergy 2013, 48, 10–16. [Google Scholar] [CrossRef]
- Grootscholten, T.I.; Steinbusch, K.J.; Hamelers, H.V.; Buisman, C.J. Improving medium chain fatty acid productivity using chain elongation by reducing the hydraulic retention time in an upflow anaerobic filter. Bioresour. Technol. 2013, 136, 735–738. [Google Scholar] [CrossRef]
- Grootscholten, T.I.; Steinbusch, K.J.; Hamelers, H.V.; Buisman, C.J. Chain elongation of acetate and ethanol in an upflow anaerobic filter for high rate MCFA production. Bioresour. Technol. 2013, 1325, 440–445. [Google Scholar] [CrossRef]
- Candry, P.; Van Daele, T.; Denis, K.; Amerlinck, Y.; Andersen, S.J.; Ganigue, R.; Arends, J.B.A.; Nopens, I.; Rabaey, K. A novel high-throughput method for kinetic characterisation of anaerobic bioproduction strains, applied to Clostridium kluyveri. Sci. Rep. 2018, 8, 9724. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, J.; Zhang, Y.; Chen, M.; Ding, Z.W.; van Loosdrecht, M.C.; Zeng, R.J. Fatty acids production from hydrogen and carbon dioxide by mixed culture in the membrane biofilm reactor. Water Res. 2013, 47, 6122–6129. [Google Scholar] [CrossRef]
- San-Valero, P.; Abubackar, H.N.; Veiga, M.C.; Kennes, C. Effect of pH, yeast extract and inorganic carbon on chain elongation for hexanoic acid production. Bioresour. Technol. 2020, 300, 122659. [Google Scholar] [CrossRef]
- Diender, M.; Stams, A.J.; Sousa, D.Z. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels 2016, 9, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, J.M.; Richter, H.; Loftus, S.E.; Angenent, L.T. Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol. Bioeng. 2013, 110, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Molitor, B.; Diender, M.; Sousa, D.Z.; Angenent, L.T. A narrow pH range supports butanol, hexanol, and octanol production from syngas in a continuous co-culture of Clostridium ljungdahlii and Clostridium kluyveri with In-line product extraction. Front. Microbiol. 2016, 7, 1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.; Han, W.; Shao, L.; Lu, F. One-step production of C6–C8 carboxylates by mixed culture solely grown on CO. Biotechnol. Biofuels 2018, 11, 4. [Google Scholar] [CrossRef]
- Rusmanis, D.; O’Shea, R.; Wall, D.M.; Murphy, J.D. Biological hydrogen methanation systems: An overview of design and efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhardt, M.; Busch, G. Methanation of hydrogen and carbon dioxide. Appl. Energy 2013, 111, 74–79. [Google Scholar] [CrossRef]
- Strübing, D.; Huber, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. High performance biological methanation in a thermophilic anaerobic trickle bed reactor. Bioresour. Technol. 2017, 245, 1176–1183. [Google Scholar] [CrossRef]
- Dupnock, T.L.; Deshusses, M.A. High-performance biogas upgrading using a biotrickling filter and hydrogenotrophic methanogens. Appl. Biochem. Biotechnol. 2017, 183, 488–502. [Google Scholar] [CrossRef]
- Yang, H.J.; Yang, Z.M.; Xu, X.H.; Guo, R.B. Increasing the methane production rate of hydrogenotrophic methanogens using biochar as a biocarrier. Bioresour. Technol. 2020, 302, 122829. [Google Scholar] [CrossRef]
- Daglioglu, S.T.; Ogut, T.C.; Ozdemir, G.; Azbar, N. Comparative evaluation of two packing materials (glass pipe and ceramic ball) for hydrogenothrophic biomethanation (BHM) of CO2. Waste Biomass Valorization 2021, 12, 3717–3726. [Google Scholar] [CrossRef]
- Miehle, M.; Hackbarth, M.; Gescher, J.; Horn, H.; Hille-Reichel, A. Biological biogas upgrading in a membrane biofilm reactor with and without organic carbon source. Bioresour. Technol. 2021, 335, 125287. [Google Scholar] [CrossRef]
- Pratofiorito, G.; Hackbarth, M.; Mandel, C.; Madlanga, S.; West, S.; Horn, H.; Hille-Reichel, A. A membrane biofilm reactor for hydrogenotrophic methanation. Bioresour. Technol. 2021, 321, 124444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Dong, H.; Zhang, H.; Yuan, L.; Li, H.; Yue, L.; Hua, J.; Zhou, J. Improving CH4 production and energy conversion from CO2 and H2 feedstock gases with mixed methanogenic community over Fe nanoparticles. Bioresour. Technol. 2020, 314, 123799. [Google Scholar] [CrossRef]
- Thema, M.; Weidlich, T.; Hörl, M.; Bellack, A.; Mörs, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T.; et al. Biological CO2-methanation: An approach to standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, W.; Bockrath, R.; De Wever, H. Effects of moderately elevated pressure on gas fermentation processes. Bioresour. Technol. 2019, 293, 122129. [Google Scholar] [CrossRef] [PubMed]
- Oswald, F.; Stoll, I.K.; Zwick, M.; Herbig, S.; Sauer, J.; Boukis, N.; Neumann, A. Formic acid formation by Clostridium ljungdahlii at elevated pressures of carbon dioxide and hydrogen. Front. Bioeng. Biotechnol. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Ayol, A.; Biryol, İ.; Taşkan, E.; Hasar, H. Enhanced sludge stabilization coupled with microbial fuel cells (MFCs). Int. J. Hydrogen Energy 2021, 46, 29529–29540. [Google Scholar] [CrossRef]
- Peixoto, L.; Rodrigues, A.L.; Martins, G.; Nicolau, A.; Brito, A.G.; Silva, M.M.; Parpot, P.; Nogueira, R. A flat microbial fuel cell for decentralized wastewater valorization: Process performance and optimization potential. Environ. Technol. 2013, 34, 1947–1956. [Google Scholar] [CrossRef]
- Peixoto, L.; Min, B.; Martins, G.; Brito, A.G.; Kroff, P.; Parpot, P.; Angelidaki, I.; Nogueira, R. In situ microbial fuel cell-based biosensor for organic carbon. Bioelectrochemistry 2011, 81, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Peixoto, L.; Ribeiro, D.C.; Parpot, P.; Brito, A.G.; Nogueira, R. Towards implementation of a benthic microbial fuel cell in lake Furnas (Azores): Phylogenetic affiliation and electrochemical activity of sediment bacteria. Bioelectrochemistry 2010, 78, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-fermentation: How to drive fermentation using electrochemical systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Schievano, A.; Sciarria, T.P.; Vanbroekhoven, K.; De Wever, H.; Puig, S.; Andersen, S.J.; Rabaey, K.; Pant, D. Electro-fermentation—Merging electrochemistry with fermentation in industrial applications. Trends Biotechnol. 2016, 34, 866–878. [Google Scholar] [CrossRef]
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Sci. Rep. 2014, 4, 6961. [Google Scholar] [CrossRef]
- Prévoteau, A.; Carvajal-Arroyo, J.M.; Ganigué, R.; Rabaey, K. Microbial electrosynthesis from CO2: Forever a promise? Curr. Opin. Biotechnol. 2020, 62, 48–57. [Google Scholar] [CrossRef]
- Jabeen, G.; Farooq, R. Bio-electrochemical synthesis of commodity chemicals by autotrophic acetogens utilizing CO2 for environmental remediation. J. Biosci. 2016, 41, 367–380. [Google Scholar] [CrossRef]
- Bajracharya, S.; Yuliasni, R.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Long-term operation of microbial electrosynthesis cell reducing CO2 to multi-carbon chemicals with a mixed culture avoiding methanogenesis. Bioelectrochemistry 2017, 113, 26–34. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Liu, Y.; Harnisch, F.; Fricke, K.; Schröder, U.; Climent, V.; Feliu, J.M. The study of electrochemically active microbial biofilms on different carbon-based anode materials in microbial fuel cells. Biosens. Bioelectron. 2010, 25, 2167–2171. [Google Scholar] [CrossRef]
- You, S.J.; Ren, N.Q.; Zhao, Q.L.; Wang, J.Y.; Yang, F.L. Power generation and electrochemical analysis of biocathode microbial fuel cell using graphite fibre brush as cathode material. Fuel Cells 2009, 9, 588–596. [Google Scholar] [CrossRef]
- Logan, B. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef]
- Sharma, M.; Alvarez-Gallego, Y.; Achouak, W.; Pant, D.; Sarma, P.M.; Dominguez-Benetton, X. Electrode material properties for designing effective microbial electrosynthesis systems. J. Mater. Chem. A 2019, 7, 24420–24436. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jourdin, L.; Freguia, S.; Flexer, V.; Keller, J. Bringing high-rate, CO2-based microbial electrosynthesis closer to practical implementation through improved electrode design and operating conditions. Environ. Sci. Technol. 2016, 50, 1982–1989. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.; Angenent, L. Electricity generation from artificial wastewater using upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Cheng, S.; Oh, S.-E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Ter Heijne, A.; Hamelers, H.V.; De Wilde, V.; Rozendal, R.A.; Buisman, C.J. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Krieg, T.; Madjarov, J.; Rosa, L.F.M.; Enzmann, F.; Harnisch, F.; Holtmann, D.; Rabaey, K. Reactors for microbial electrobiotechnology. In Bioelectrosynthesis. Advances in Biochemical Engineering/Biotechnology; Harnisch, F., Holtmann, D., Eds.; Springer: Cham, Switzerland, 2017; Volume 167, pp. 127–141. [Google Scholar] [CrossRef] [Green Version]
- Koroglu, E.O.; Yoruklu, H.C.; Demir, A.; Ozkaya, B. Chapter 3.9-Scale-up and commercialization issues of the MFCs: Challenges and implications. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 565–583. [Google Scholar] [CrossRef]
- Korth, B.; Harnisch, F. Modeling Microbial Electrosynthesis. In Bioelectrosynthesis. Advances in Biochemical Engineering/Biotechnology; Harnisch, F., Holtmann, D., Eds.; Springer: Cham, Switzerland, 2017; Volume 167, pp. 273–325. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Ho, G.; Cord-Ruwisch, R. Novel methanogenic rotatable bioelectrochemical system operated with polarity inversion. Environ. Sci. Technol. 2011, 45, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Paiano, P.; Torres, C.; Pant, D. A critical evaluation of the pH split and associated effects in bioelectrochemical processes. Chem. Eng. J. 2021, 422, 130155. [Google Scholar] [CrossRef]
- Guo, K.; Tang, X.; Du, Z.; Li, H. Hydrogen production from acetate in a cathode-on-top single-chamber microbial electrolysis cell with a mipor cathode. Biochem. Eng. J. 2010, 51, 48–52. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; He, W.; Yang, W.; Feng, Y.; Logan, B.E. A microbial fluidized electrode electrolysis cell (MFEEC) for enhanced hydrogen production. J. Power Sources 2014, 271, 530–533. [Google Scholar] [CrossRef]
- Liang, D.W.; Peng, S.K.; Lu, S.F.; Liu, Y.Y.; Lan, F.; Xiang, Y. Enhancement of hydrogen production in a single chamber microbial electrolysis cell through anode arrangement optimization. Bioresour. Technol. 2011, 102, 10881–10885. [Google Scholar] [CrossRef]
- Guo, K.; Prévoteau, A.; Rabaey, K. A novel tubular microbial electrolysis cell for high rate hydrogen production. J. Power Sources 2017, 356, 484–490. [Google Scholar] [CrossRef]
- Blasco-Gómez, R.; Ramió-Pujol, S.; Bañeras, L.; Colprim, J.; Balaguer, M.D.; Puig, S. Unravelling the factors that influence the bio-electrorecycling of carbon dioxide towards biofuels. Green Chem. 2019, 21, 684–691. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapa, A.; Moreno, R.; Morán, A. Reduced energy consumption during low strength domestic wastewater treatment in a semi-pilot tubular microbial electrolysis cell. J. Environ. Manag. 2013, 122, 1–7. [Google Scholar] [CrossRef]
- Vassilev, I.; Hernandez, P.A.; Batlle-Vilanova, P.; Freguia, S.; Krömer, J.O.; Keller, J.; Ledezma, P.; Virdis, B. Microbial electrosynthesis of isobutyric, butyric, caproic acids, and corresponding alcohols from carbon dioxide. ACS Sustain. Chem. Eng. 2018, 6, 8485–8493. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Dong, F.; Chun-Yuan, B.; Si-Rong, Z.; Jian-Guo, S. Recent progress of commercially available biosensors in China and their applications in fermentation processes. J. Northeast Agric. Univ. 2014, 21, 73–85. [Google Scholar] [CrossRef]
- Heiskanen, A.; Yakovleva, J.; Spégel, C.; Taboryski, R.; Koudelka-Hep, M.; Emnéus, J.; Ruzgas, T. Amperometric monitoring of redox activity in living yeast cells: Comparison of menadione and menadione sodium bisulfite as electron transfer mediators. Electrochem. Commun. 2004, 6, 219–224. [Google Scholar] [CrossRef]
- Sun, H.; Angelidaki, I.; Wu, S.; Dong, R.; Zhang, Y. The potential of bioelectrochemical sensor for monitoring of acetate during anaerobic digestion: Focusing on novel reactor design. Front. Microbiol. 2019, 9, 3357. [Google Scholar] [CrossRef]
- Huang, S.; Shen, M.; Ren, Z.J.; Wu, H.; Yang, H.; Si, B.; Lin, J.; Liu, Z. Long-term in situ bioelectrochemical monitoring of biohythane process: Metabolic interactions and microbial evolution. Bioresour. Technol. 2021, 332, 125119. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.Z.; Fang, Z.; Yu, Y.Y.; Yong, Y.C. Bioelectrochemical biosensor for water toxicity detection: Generation of dual signals for electrochemical assay confirmation. Anal. Bioanal. Chem. 2018, 410, 1231–1236. [Google Scholar] [CrossRef]
- Bouaifi, M.; Hebrard, G.; Bastoul, D.; Roustan, M. A comparative study of gas hold-up, bubble size, interfacial area and mass transfer coefficients in stirred gas-liquid reactors and bubble columns. Chem. Eng. Process. Process. Intensif. 2001, 40, 97–111. [Google Scholar] [CrossRef]
- Yasin, M.; Jeong, Y.; Park, S.; Jeong, J.; Lee, E.Y.; Lovitt, R.W.; Kim, B.H.; Lee, J.; Chang, I.S. Microbial synthesis gas utilization and ways to resolve kinetic and mass-transfer limitations. Bioresour. Technol. 2015, 177, 361–374. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial conversion process of gaseous substrates to various products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Yasin, M.; Park, S.; Jeong, Y.; Lee, E.Y.; Lee, J.; Chang, I.S. Effect of internal pressure and gas/liquid interface area on the CO mass transfer coefficient using hollow fibre membranes as a high mass transfer gas diffusing system for microbial syngas fermentation. Bioresour. Technol. 2014, 169, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.; Yasin, M.; Kang, H.; Lee, Y.; Park, G.W.; Park, S.; Chang, I.S. Bubble coalescence suppression driven carbon monoxide (CO)-water mass transfer increase by electrolyte addition in a hollow fiber membrane bioreactor (HFMBR) for microbial CO conversion to ethanol. Bioresour. Technol. 2018, 263, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Sathish, A.; Sharma, A.; Gable, P.; Skiadas, I.; Brown, R.; Wen, Z. A novel bulk-gas-to-atomized-liquid reactor for enhanced mass transfer efficiency and its application to syngas fermentation. Chem. Eng. J. 2019, 370, 60–70. [Google Scholar] [CrossRef]
- De Medeiros, E.M.; Posada, J.A.; Noorman, H.; Filho, R.M. Dynamic modeling of syngas fermentation in a continuous stirred–tank reactor: Multi–response parameter estimation and process optimization. Biotechnol. Bioeng. 2019, 116, 2473–2487. [Google Scholar] [CrossRef]
- Orgill, J.J.; Atiyeh, H.K.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour. Technol. 2013, 133, 340–346. [Google Scholar] [CrossRef]
- Liu, K.; Phillips, J.R.; Sun, X.; Mohammad, S.; Huhnke, R.L.; Atiyeh, H.K. Investigation and modeling of gas-liquid mass transfer in a sparged and non-Sparged continuous stirred tank reactor with potential application in syngas fermentation. Fermentation 2019, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Benalcázar, E.A.; Noorman, H.; Maciel Filho, R.; Posada, J.A. Modeling ethanol production through gas fermentation: A biothermodynamics and mass transfer-based hybrid model for microbial growth in a large-scale bubble column bioreactor. Biotechnol. Biofuels 2020, 13, 59. [Google Scholar] [CrossRef]
- Atiyeh, H.K.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. Method Improving Producer Gas Fermentation. U.S. Patent Application 20160215303A1, 2016. [Google Scholar]
- Kim, Y.-K.; Lee, H. Use of magnetic nanoparticles to enhance bioethanol production in syngas fermentation. Bioresour. Technol. 2016, 204, 139–144. [Google Scholar] [CrossRef]
- Van den Heuvel, J.C.; Vredenbregt, L.H.; Portegies-Zwart, I.; Ottengraf, S.P. Acceleration of mass transfer in methane-producing loop reactors. Antonie van Leeuwenhoek 1995, 67, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Philips, J.; Rabaey, K.; Lovley, D.R.; Vargas, M. Biofilm formation by Clostridium ljungdahlii is induced by sodium chloride stress: Experimental evaluation and transcriptome analysis. PLoS ONE 2017, 12, e0170406. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Kleerebezem, R.; Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Heijnen, J.J.; Van Loosdrecht, M.C.M. Potential application of monolith packed columns as bioreactors, control of biofilm formation. Biotechnol. Bioeng. 2006, 93, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ammam, F.; Tremblay, P.-L.; Lizak, D.M.; Zhang, T. Effect of tungstate on acetate and ethanol production by the electrosynthetic bacterium Sporomusa ovata. Biotechnol. Biofuels 2016, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Haas, T.; Krause, R.; Weber, R.; Demler, M.; Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 2018, 1, 32–39. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Mailier, J.; Martin, C.; Rodríguez, J.; Aceves-Lara, C.A.; Wouwer, A.V. Model selection, identification and validation in anaerobic digestion: A review. Water Res. 2011, 45, 5347–5364. [Google Scholar] [CrossRef] [PubMed]
- Lanzillo, F.; Ruggiero, G.; Raganati, F.; Russo, M.E.; Marzocchella, A. Batch syngas fermentation by Clostridium carboxidivorans for production of acids and alcohols. Processes 2020, 8, 1075. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mohamed, A.R.; Najafpour, G.D.; Younesi, H.; Uzir, M.H. Kinetic studies on fermentative production of biofuel from synthesis gas using Clostridium ljungdahlii. Sci. World J. 2014, 2014, 910590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sertkaya, S.; Azbar, N.; Abubackar, H.N.; Gundogdu, T.K. Design of low-cost ethanol production medium from syngas: An optimization of trace metals for Clostridium ljungdahlii. Energies 2021, 14, 6981. [Google Scholar] [CrossRef]
- Ripoll, E.; López, I.; Borzacconi, L. Hydrogenotrophic activity: A tool to evaluate the kinetics of methanogens. J. Environ. Manage. 2020, 270, 110937. [Google Scholar] [CrossRef]
- Kim, M.S.; Moon, C.; Kang, S.; Kim, D.H. Continuous performance of hydrogenotrophic methanogenic mixed cultures: Kinetic and SMP analysis. Int. J. Hydrogen Energy 2017, 42, 27767–27773. [Google Scholar] [CrossRef]
- Choi, O.; Sang, B.-I. Extracellular electron transfer from cathode to microbes: Application for biofuel production. Biotechnol. Biofuels 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Campos Rodrigues, T.; Rosenbaum, M.A. Microbial electroreduction: Screening for new cathodic biocatalysts. ChemElectroChem 2014, 1, 1916–1922. [Google Scholar] [CrossRef]
- Patil, S.A.; Hagerhall, C.; Gorton, L. Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal. Rev. 2012, 4, 159–192. [Google Scholar] [CrossRef]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Nat. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [Green Version]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Croese, E.; Pereira, M.A.; Euverink, G.-J.W.; Stams, A.J.M.; Geelhoed, J.S. Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2011, 92, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Squier, T.C.; Zachara, J.M.; Fredrickson, J.K. Respiration of metal (hydr) oxides by Shewanella and Geobacter: A key role for multihaem c-type cytochromes. Mol. Microbiol. 2007, 65, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutzmann, J.S.; Spormann, A.M. Enhanced microbial electrosynthesis by using defined co-cultures. ISME J. 2017, 11, 704–714. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, P.-L.; Zhang, T. Electrifying microbes for the production of chemicals. Front. Microbiol. 2015, 6, 201. [Google Scholar] [CrossRef] [Green Version]
- Aulenta, F.; Catervi, A.; Majone, M.; Panero, S.; Reale, P.; Rossetti, S. Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of TCE. Environ. Sci. Technol. 2007, 41, 2554–2559. [Google Scholar] [CrossRef]
- Hatch, J.L.; Finneran, K.T. Influence of reduced electron shuttling compounds on biological H2 production in the fermentative pure culture Clostridium beijerinckii. Curr. Microbiol. 2008, 56, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Zeikus, J.G. Utilization of electrically reduced neutral red by Actinobacillus succinogenes: Physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J. Bacteriol. 1999, 181, 2403–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlson, B.; Bellavitis, C.; France, N. Commercializing LanzaTech, from waste to fuel: An effectuation case. J. Manag. Organ. 2021, 27, 175–196. [Google Scholar] [CrossRef]
- ArcelorMittal Belgium Lifts Bioreactors into Place at Its Groundbreaking Steelanol Plant for Carbon-Neutral Steelmaking. Available online: http://www.steelanol.eu/en/news/lifting-bioreactors-steelanol (accessed on 1 September 2021).
- LanzaTech and Lululemon Partner to Create the First Fabric Using Recycled Carbon Emissions. Available online: https://www.lanzatech.com/2021/07/13/lanzatech-and-lululemon-partner-to-create-the-first-fabric-using-recycled-carbon-emissions/ (accessed on 5 September 2021).
- LanzaTech and BASF Achieve First Milestone in Utilizing Industrial Off-Gases for Chemical Production. Available online: https://www.basf.com/global/en/media/news-releases/2021/05/p-21-206.html (accessed on 7 September 2021).
- World-First Laundry Capsule in Market Made from Industrial Carbon Emissions. Available online: https://www.unilever.com/news/press-releases/2021/world-first-laundry-capsule-in-market-made-from-industrial-carbon-emissions.html (accessed on 2 September 2021).
- Coty to Partner with LanzaTech to Pioneer New Sustainable Fragrance Production. Available online: https://www.coty.com/in-the-news/press-release/coty-partner-lanzatech-pioneer-new-sustainable-fragrance-production (accessed on 5 September 2021).
- Lanzatech, Total and L’Oréal: The First Cosmetic Plastic Bottle Made from Industrial Carbon Emissions. Available online: https://www.loreal.com/en/news/group/lanzatech-total-and-loreal/ (accessed on 25 August 2021).
- Proven and Proprietary Global Technology Solution for Sustainable Fuel from Waste Sources. Available online: https://www.lanzajet.com/what-we-do/ (accessed on 20 August 2021).
- Virgin Atlantic Conducts Biofuel-Powered Flight. Available online: https://www.aerospace-technology.com/news/virgin-atlantic-conducts-biofuel-powered-flight/ (accessed on 7 September 2021).
- Chen, W.S.; Strik, D.; Buisman, C.J.N.; Kroeze, C. Production of caproic acid from mixed organic waste: An environmental life cycle perspective. Environ. Sci. Technol. 2017, 51, 7159–7168. [Google Scholar] [CrossRef]
- Upgrading Steel Mill off Gas to Caproic Acid and Derivatives Using Anaerobic Technology. Available online: https://catalisti.be/project/capra/ (accessed on 5 September 2021).
- Chaincraft Technology. Available online: https://www.chaincraft.nl/technology/ (accessed on 10 October 2021).
- Barbosa, S.G.; Peixoto, L.; Alves, J.I.; Alves, M.M. Bioelectrochemical systems (BESs) towards conversion of carbon monoxide/syngas: A mini-review. Renew. Sustain. Energy Rev. 2021, 135, 110358. [Google Scholar] [CrossRef]
- Ganigué, R.; Puig, S.; Batlle-Vilanova, P.; Balaguer, M.D.; Colprim, J. Microbial electrosynthesis of butyrate from carbon dioxide. Chem. Commun. 2015, 51, 3235–3238. [Google Scholar] [CrossRef]
- Patil, S.A.; Arends, J.B.; Vanwonterghem, I.; Van Meerbergen, J.; Guo, K.; Tyson, G.W.; Rabaey, K. Selective enrichment establishes a stable performing community for microbial electrosynthesis of acetate from CO2. Environ. Sci. Technol. 2015, 49, 8833–8843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Balaguer, M.D.; Colprim, J. Continuous acetate production through microbial electrosynthesis from CO2 with microbial mixed culture. J. Chem. Technol. Biotechnol. 2016, 91, 921–927. [Google Scholar] [CrossRef]
- Kongjan, P.; Min, B.; Angelidaki, I. Biohydrogen production from xylose at extreme thermophilic temperatures (70 °C) by mixed culture fermentation. Water Res. 2009, 43, 1414–1424. [Google Scholar] [CrossRef]
- Dessì, P.; Rovira-Alsina, L.; Sánchez, C.; Dinesh, G.K.; Tong, W.; Chatterjee, P.; Tedesco, M.; Farràs, P.; Hamelers, H.M.; Puig, S. Microbial electrosynthesis: Towards sustainable biorefineries for production of green chemicals from CO2 emissions. Biotechnol. Adv. 2021, 46, 107675. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapa, A.; Mehta, P.; Santoyo, G.; Guiot, S.R.; Moran, A.; Tartakovsky, B. Microbial electrolysis cell scale-up for combined wastewater treatment and hydrogen production. Bioresour. Technol. 2013, 130, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Enzmann, F.; Holtmann, D. Rational Scale-Up of a methane producing bioelectrochemical reactor to 50 L pilot scale. Chem. Eng. Sci. 2019, 207, 1148–1158. [Google Scholar] [CrossRef]
- Evonik and Siemens to Generate High-Value Specialty Chemicals from Carbon Dioxide and Eco-Electricity. Available online: https://corporate.evonik.com/en/media/press-releases/corporate/evonik-and-siemens-to-generate-high-value-specialty-chemicals-from-carbon-dioxide-and-eco-electricit-106259.html (accessed on 7 September 2021).
- De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 2019, 364, 6438. [Google Scholar] [CrossRef] [Green Version]
- Villanueva Perales, A.L.; Reyes Valle, C.; Ollero, P.; Gómez-Barea, A. Technoeconomic assessment of ethanol production via thermochemical conversion of biomass by entrained flow gasification. Energy 2011, 36, 4097–4108. [Google Scholar] [CrossRef]
- International Energy Agency, Production Costs of Alternative Transportation Fuels. Available online: https://www.ourenergypolicy.org/wp-content/uploads/2015/04/FeaturedInsights_AlternativeFuel_FINAL.pdf (accessed on 7 September 2021).
- Hossain, M.S.; Theodoropoulos, C.; Yousuf, A. Techno-economic evaluation of heat integrated second generation bioethanol and furfural coproduction. Biochem. Eng. J. 2019, 144, 89–103. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Haro, P.; Ollero, P.; Perales, A.V.; Valle, C.R. Technoeconomic assessment of lignocellulosic ethanol production via DME (dimethyl ether) hydrocarbonylation. Energy 2012, 44, 891–901. [Google Scholar] [CrossRef]
- Haro, P.; Ollero, P.; Trippe, F. Technoeconomic assessment of potential processes for bio-ethylene production. Fuel Process. Technol. 2013, 114, 35–48. [Google Scholar] [CrossRef]
- Müller, L.J.; Kätelhön, A.; Bringezu, S.; McCoy, S.; Suh, S.; Edwards, R.; Sick, V.; Kaiser, S.; Cuéllar-Franca, R.; El Khamlichi, A.; et al. The carbon footprint of the carbon feedstock CO2. Energy Environ. Sci. 2020, 13, 2979–2992. [Google Scholar] [CrossRef]
- Wainaina, S.; Horvath, I.S.; Taherzadeh, M.J. Biochemicals from food waste and recalcitrant biomass via syngas fermentation: A review. Bioresour. Technol. 2018, 248, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Chipman, D.C.; Bents, S.C.; Brown, R.C. A techno-economic analysis of polyhydroxyalkanoate and hydrogen production from syngas fermentation of gasified biomass. Appl. Biochem. Biotechnol. 2010, 160, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, O.P.R.; Faaij, A.P.C.; Turkenburg, W.C. Fischer–Tropsch diesel production in a well-to-wheel perspective: A carbon, energy flow and cost analysis. Energy Convers. Manag. 2009, 50, 855–876. [Google Scholar] [CrossRef]
- Akiyama, M.; Tsuge, T.; Doi, Y. Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable carbon resources by bacterial fermentation. Polym. Degrad. Stab. 2003, 80, 183–194. [Google Scholar] [CrossRef]
- Sternberg, A.; Jensa, C.M.; Bardow, A. Life cycle assessment of CO2-based C1-chemicals. Green Chem. 2017, 19, 2244–2259. [Google Scholar] [CrossRef]


| Bioreactor Type | Process | Merits | Drawbacks | ||
|---|---|---|---|---|---|
| Syngas Fermentation | Chain Elongation | Hydrogenotrophic Methanation | |||
| Continuous Stirred Tank Reactors (CSTR) [31,67,68,69,71] | ✓ | ✓ | ✓ | Flexible for many bioprocesses Control on gas–liquid mass transfer | Commercialization is not cost effective Scale up increases energy requirements |
| Biofilm Formation Reactors [32,33,34,70,72,73,74] | ✓ | ✓ | ✓ | High biomass concentration Smaller reactor volumes Low energy requirements | Limitation on mass transfer with increasing biomass concentrations |
| Rotating Packed Bed Biofilm Reactors [35,43,44] | ✓ | Efficient mass transfer from bulk gas to cell surface | The rate-limiting step is the diffusion across gas–liquid interface Maintaining optimum rotation needs careful operation | ||
| Monolithic Biofilm Reactor [36,37,38] | ✓ | Prevents biomass wash out at greater dilution rates Large pore size Specific surface area Great mechanical strength | Dependence on channel geometry Low flow rate of gas | ||
| Membrane Bioreactor [40,41,77,78] | ✓ | ✓ | Suitable for poorly water soluble gases Flexible application | Membrane wetting and biofouling | |
| Trickle Bed Reactor [45,46,47] | ✓ | Large volume/surface area No need for mechanical agitation Control on superficial gas velocity | Inconsistent irrigation of packing material | ||
| Bubble Column Reactor [49,50,51,52,53] | ✓ | Low maintenance and operational costs No need for mechanical mixing Operation in different modes | Optimization of bubble size for a successful mass transfer | ||
| Hollow Fiber Reactors [42,65] | ✓ | ✓ | Improved production rates Lower investment costs Resistance to washout of microorganisms | Uncontrolled thickness of biomass can limit mass transfer | |
| Carrier Bed Reactors [75,76,77,78,79] | ✓ | Different types of carriers can be used such as biochar, polyurethane foam, etc. | Need for mechanical agitation | ||
| Fixed Bed Reactors [76] | ✓ | Low operation costs Low reactor size Improved biomass concentrations | Gas–liquid mass transfer limitations Channeling | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayol, A.; Peixoto, L.; Keskin, T.; Abubackar, H.N. Reactor Designs and Configurations for Biological and Bioelectrochemical C1 Gas Conversion: A Review. Int. J. Environ. Res. Public Health 2021, 18, 11683. https://doi.org/10.3390/ijerph182111683
Ayol A, Peixoto L, Keskin T, Abubackar HN. Reactor Designs and Configurations for Biological and Bioelectrochemical C1 Gas Conversion: A Review. International Journal of Environmental Research and Public Health. 2021; 18(21):11683. https://doi.org/10.3390/ijerph182111683
Chicago/Turabian StyleAyol, Azize, Luciana Peixoto, Tugba Keskin, and Haris Nalakath Abubackar. 2021. "Reactor Designs and Configurations for Biological and Bioelectrochemical C1 Gas Conversion: A Review" International Journal of Environmental Research and Public Health 18, no. 21: 11683. https://doi.org/10.3390/ijerph182111683
APA StyleAyol, A., Peixoto, L., Keskin, T., & Abubackar, H. N. (2021). Reactor Designs and Configurations for Biological and Bioelectrochemical C1 Gas Conversion: A Review. International Journal of Environmental Research and Public Health, 18(21), 11683. https://doi.org/10.3390/ijerph182111683









