Associations between Weather, Air Quality and Moderate Extreme Cancer-Related Mortality Events in Augsburg, Southern Germany
Abstract
:1. Introduction
2. Materials and Methods
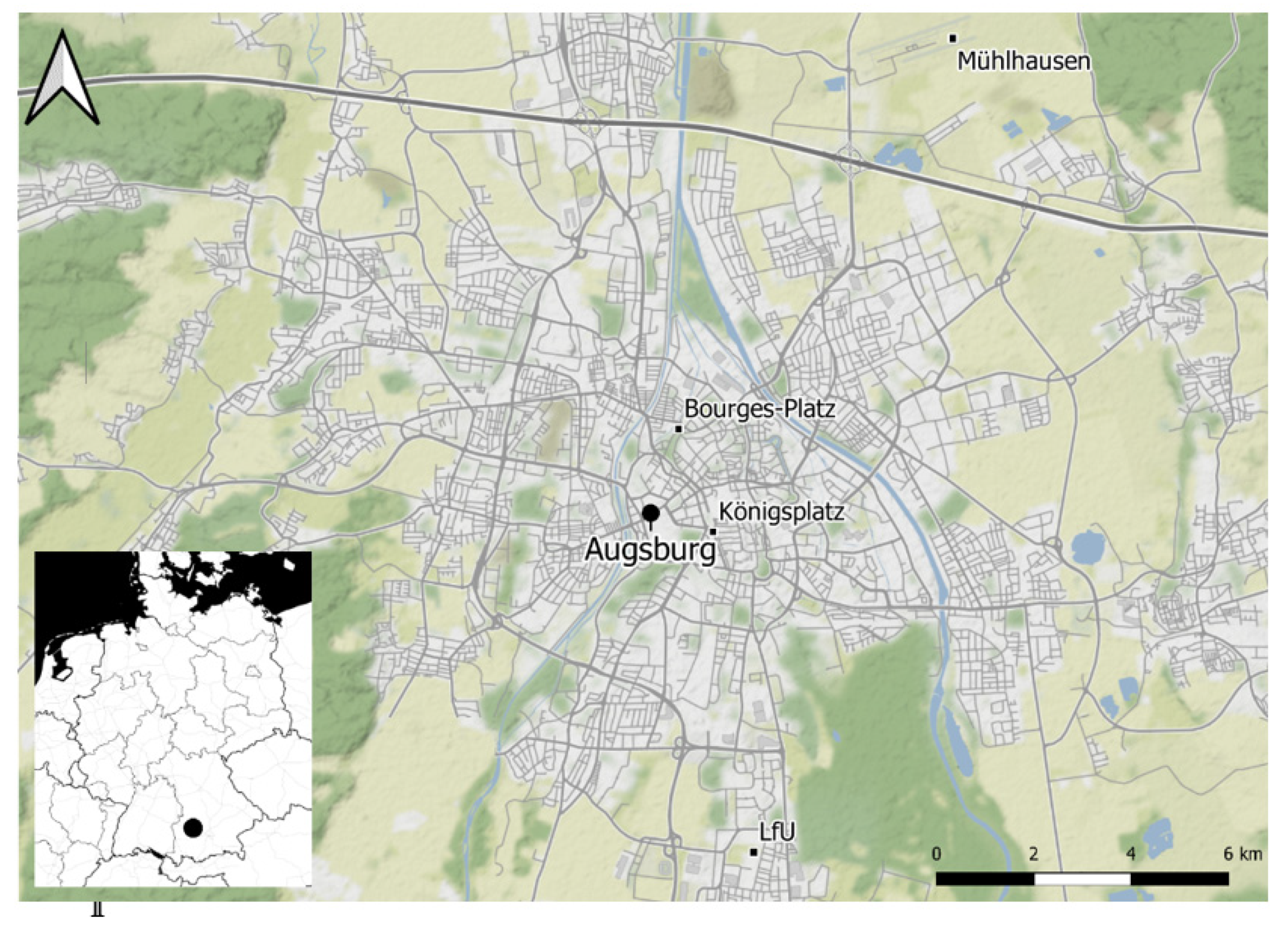
2.1. Air Quality and Meteorology Data
2.2. Cancer Mortality Data
2.3. SLP Reanalysis Data
2.4. Trends and Seasonality
2.5. Mann–Whitney U Test and Predictor Correlations
2.6. Case-Crossover
2.7. Principal Component Analysis (PCA)
3. Results
3.1. Cancer-Related High-Mortality Events
3.2. Relations to Weather and Air Quality
3.3. PCA Results
3.3.1. PC 1
3.3.2. PC 2
3.3.3. PC 4
3.3.4. PC 5
3.4. Relationship of Cancer-Related Mortality Events to Non-Cancer-Related Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masson-Delmotte, V.P.; Zhai, A.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L. (Eds.) International panel on climate change; summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 18 August 2021).
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A.; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Khorrami, Z.; Pourkhosravani, M.; Rezapour, M.; Etemad, K.; Taghavi-Shahri, S.M.; Künzli, N.; Amini, H.; Khanjani, N. Multiple air pollutant exposure and lung cancer in Tehran, Iran. Sci. Rep. 2021, 11, 9239. [Google Scholar] [CrossRef]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.C.; Krewski, D.; Diver, W.R.; Pope, C.A.; Burnett, R.T.; Jerrett, M.; Marshall, J.D.; Gapstur, S.M. Ambient air pollution and cancer mortality in the cancer prevention study II. Environ. Health Perspect. 2017, 125, 087013. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.C.; Gracia-Lavedan, E.; Cirac, M.; Castaño-Vinyals, G.; Malats, N.; Tardon, A.; Garcia-Closas, R.; Serra, C.; Carrato, A.; Jones, R.R.; et al. Ambient air pollution and incident bladder cancer risk: Updated analysis of the Spanish Bladder Cancer Study. Int. J. Cancer 2019, 145, 894–900. [Google Scholar] [CrossRef]
- Tagliabue, G.; Borgini, A.; Tittarelli, A.; van Donkelaar, A.; Martin, R.V.; Bertoldi, M.; Fabiano, S.; Maghini, A.; Codazzi, T.; Scaburri, A.; et al. Atmospheric fine particulate matter and breast cancer mortality: A population-based cohort study. BMJ Open 2016, 6, e012580. [Google Scholar] [CrossRef] [Green Version]
- White, A.J.; Keller, J.P.; Zhao, S.; Carroll, R.; Kaufman, J.D.; Sandler, D.P. Air pollution, clustering of particulate matter components, and breast cancer in the sister study: A U.S.-wide cohort. Environ. Health Perspect. 2019, 127, 107002. [Google Scholar] [CrossRef]
- Hwang, J.; Bae, H.; Choi, S.; Yi, H.; Ko, B.; Kim, N. Impact of air pollution on breast cancer incidence and mortality: A nationwide analysis in South Korea. Sci. Rep. 2020, 10, 5392. [Google Scholar] [CrossRef]
- Kazemiparkouhi, F.; Eum, K.-D.; Wang, B.; Manjourides, J.; Suh, H.H. Long-term ozone exposures and cause-specific mortality in a US Medicare cohort. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Lequy, E.; Siemiatycki, J.; de Hoogh, K.; Vienneau, D.; Dupuy, J.-F.; Garès, V.; Hertel, O.; Christensen, J.H.; Zhivin, S.; Goldberg, M.; et al. Contribution of long-term exposure to outdoor black carbon to the carcinogenicity of air pollution: Evidence regarding risk of cancer in the gazel cohort. Environ. Health Perspect. 2021, 129, 37005. [Google Scholar] [CrossRef]
- Bayerisches Landesamt für Umwelt. Messwertarchiv [Archive of Measurement Values]. Available online: https://www.lfu.bayern.de/luft/immissionsmessungen/messwertarchiv/index.htm (accessed on 10 October 2020).
- Moberg, A.; Jones, P.D.; Lister, D.; Walther, A.; Brunet, M.; Jacobeit, J.; Alexander, L.; Della-Marta, P.M.; Luterbacher, J.; Yiou, P.; et al. Indices for daily temperature and precipitation extremes in Europe analyzed for the period 1901–2000. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Wijngaard, J.B.; Tank, A.K.; Können, G.P. Homogeneity of 20th century European daily temperature and precipitation series. Int. J. Clim. 2003, 23, 679–692. [Google Scholar] [CrossRef]
- Morfeld, P.; Groneberg, D.A.; Spallek, M.F. Effectiveness of low emission zones: Large scale analysis of changes in environmental NO2, NO and NOX concentrations in 17 German cities. PLoS ONE 2014, 9, e102999. [Google Scholar] [CrossRef] [Green Version]
- Climate Data Center. Available online: https://opendata.dwd.de/climate_environment/ (accessed on 19 May 2021).
- Bayerisches Krebsregister. Regionalzentrum Augsburg. Available online: https://www.lgl.bayern.de/gesundheit/krebsregister/index.htm (accessed on 17 August 2021).
- International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: https://icd.who.int/browse10/2010/en (accessed on 17 September 2021).
- Grundmann, N.; Meisinger, C.; Trepel, M.; Müller-Nordhorn, J.; Schenkirsch, G.; Linseisen, J. Trends in cancer incidence and survival in the Augsburg study region—Results from the Augsburg cancer registry. BMJ Open 2020, 10, e036176. [Google Scholar] [CrossRef]
- Natürliche Bevölkerungsbewegung—Sterbefälle. Available online: https://www.statistik.bayern.de/statistik/gebiet_bevoelkerung/bevoelkerungsbewegung/index.html#link_2 (accessed on 18 August 2021).
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horanyi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Botlaguduru, V.S.V.; Kommalapati, R.R. Meteorological detrending of long-term (2003–2017) ozone and precursor concentrations at three sites in the Houston Ship Channel Region. J. Air Waste Manag. Assoc. 2019, 70, 93–107. [Google Scholar] [CrossRef]
- Sohail, H.; Kollanus, V.; Tiittanen, P.; Schneider, A.; Lanki, T. Heat, heatwaves and cardiorespiratory hospital admissions in Helsinki, Finland. Int. J. Environ. Res. Public Health 2020, 17, 7892. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N.E.; Long, S.R.; Peng, C.-K. On the trend, detrending, and variability of nonlinear and nonstationary time series. Proc. Natl. Acad. Sci. USA 2007, 104, 14889–14894. [Google Scholar] [CrossRef] [Green Version]
- Iler, A.M.; Inouye, D.W.; Schmidt, N.M.; Høye, T.T. Detrending phenological time series improves climate–phenology analyses and reveals evidence of plasticity. Ecology 2017, 98, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Tai, A.P.K.; Mickley, L.J.; Jacob, D.J. Correlations between fine particulate matter (PM2.5) and meteorological variables in the United States: Implications for the sensitivity of PM2.5 to climate change. Atmos. Environ. 2010, 44, 3976–3984. [Google Scholar] [CrossRef]
- Gosling, S.N.; McGregor, G.R.; Páldy, A. Climate change and heat-related mortality in six cities Part 1: Model construction and validation. Int. J. Biometeorol. 2007, 51, 525–540. [Google Scholar] [CrossRef]
- Rooney, C.; McMichael, A.J.; Kovats, R.S.; Coleman, M.P. Excess mortality in England and Wales, and in Greater London, during the 1995 heatwave. J. Epidemiol. Community Health 1998, 52, 482–486. [Google Scholar] [CrossRef] [Green Version]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Pinheiro, S.L.L.A.; Saldiva, P.H.N.; Schwartz, J.; Zanobetti, A. Isolated and synergistic effects of PM10 and average temperature on cardiovascular and respiratory mortality. Rev. Saúde Pública 2014, 48, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Philipp, A. Comparison of principal component and cluster analysis for classifying circulation pattern sequences for the European domain. Theor. Appl. Clim. 2008, 96, 31–41. [Google Scholar] [CrossRef]
- Huth, R. Properties of the circulation classification scheme based on the rotated principal component analysis. Meteorol. Atmos. Phys. 1996, 59, 217–233. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Severi, G.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Boutron-Ruault, M.-C.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; et al. Long-term low-level ambient air pollution exposure and risk of lung cancer—A pooled analysis of 7 European cohorts. Environ. Int. 2021, 146, 106249. [Google Scholar] [CrossRef]
- Santovito, A.; Gendusa, C.; Cervella, P.; Traversi, D. In vitro genomic damage induced by urban fine particulate matter on human lymphocytes. Sci. Rep. 2020, 10, 8853. [Google Scholar] [CrossRef]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Influenza and pneumonia-attributed deaths among cancer patients in the United States; a population-based study. Expert Rev. Respir. Med. 2021, 15, 393–401. [Google Scholar] [CrossRef]
- Elfaituri, M.K.; Morsy, S.; Tawfik, G.M.; Abdelaal, A.; El-Qushayri, A.E.; Faraj, H.A.; Hieu, T.H.; Huy, N.T. Incidence of infection-related mortality in cancer patients: Trend and survival analysis. J. Clin. Oncol. 2019, 37, e23095. [Google Scholar] [CrossRef]
- Vicedo-Cabrera, A.M.; Scovronick, N.; Sera, F.; Royé, D.; Schneider, R.; Tobias, A.; Astrom, C.; Guo, Y.; Honda, Y.; Hondula, D.M.; et al. The burden of heat-related mortality attributable to recent human-induced climate change. Nat. Clim. Chang. 2021, 11, 492–500. [Google Scholar] [CrossRef]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobías, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Kloog, I.; Ridgway, B.; Koutrakis, P.; Coull, B.A.; Schwartz, J.D. Long- and short-term exposure to PM2.5 and mortality: Using novel exposure models. Epidemiology 2013, 24, 555–561. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Gasparrini, A.; Armstrong, B.; Valois, M.-F. The short-term influence of temperature on daily mortality in the temperate climate of Montreal, Canada. Environ. Res. 2011, 111, 853–860. [Google Scholar] [CrossRef]
- Touloumi, G.; Pocock, S.J.; Katsouyanni, K.; Trichopoulos, D. Short-term effects of air pollution on daily mortality in athens: A time-series analysis. Int. J. Epidemiol. 1994, 23, 957–967. [Google Scholar] [CrossRef]
- Slama, A.; Śliwczyński, A.; Woźnica-Pyzikiewicz, J.; Zdrolik, M.; Wiśnicki, B.; Kubajek, J.; Turżańska-Wieczorek, O.; Studnicki, M.; Wierzba, W.; Franek, E. The short-term effects of air pollution on respiratory disease hospitalizations in 5 cities in Poland: Comparison of time-series and case-crossover analyses. Environ. Sci. Pollut. Res. 2020, 27, 24582–24590. [Google Scholar] [CrossRef]
- Mokoena, K.; Ethan, C.J.; Yu, Y.; Shale, K.; Liu, F. Ambient air pollution and respiratory mortality in Xi’an, China: A time-series analysis. Respir. Res. 2019, 20, 139. [Google Scholar] [CrossRef]
- Zhu, F.; Ding, R.; Lei, R.; Cheng, H.; Liu, J.; Shen, C.; Zhang, C.; Xu, Y.; Xiao, C.; Li, X.; et al. The short-term effects of air pollution on respiratory diseases and lung cancer mortality in Hefei: A time-series analysis. Respir. Med. 2019, 146, 57–65. [Google Scholar] [CrossRef]
- Meng, X.; Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Milojevic, A.; Guo, Y.; Tong, S.; de Sousa Zanotti Stagliorio Coelho, M.; Saldiva, P.H.N.; et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: Multilocation analysis in 398 cities. BMJ 2021, 372, n534. [Google Scholar] [CrossRef]
- Ren, M.; Li, N.; Wang, Z.; Liu, Y.; Chen, X.; Chu, Y.; Li, X.; Zhu, Z.; Tian, L.; Xiang, H. The short-term effects of air pollutants on respiratory disease mortality in Wuhan, China: Comparison of time-series and case-crossover analyses. Sci. Rep. 2017, 7, 40482. [Google Scholar] [CrossRef]
- Xue, X.; Chen, J.; Sun, B.; Zhou, B.; Li, X. Temporal trends in respiratory mortality and short-term effects of air pollutants in Shenyang, China. Environ. Sci. Pollut. Res. 2018, 25, 11468–11479. [Google Scholar] [CrossRef] [Green Version]
- Breitner, S.; Wolf, K.; Devlin, R.B.; Diaz-Sanchez, D.; Peters, A.; Schneider, A. Short-term effects of air temperature on mortality and effect modification by air pollution in three cities of Bavaria, Germany: A time-series analysis. Sci. Total Environ. 2014, 485–486, 49–61. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, W.; Lei, Y.; Zhao, Y.; Cheng, S.; Ren, Z.; Huang, Q. Impact of meteorological conditions on PM2.5 pollution in China during winter. Atmosphere 2018, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Hay, J.E.; Fisher, G.W. Effects of meteorological conditions on concentrations of nitrogen oxides in Auckland. Weather Clim. 2005, 24, 15–34. [Google Scholar] [CrossRef]
- Heintzenberg, J.; Birmili, W.; Hellack, B.; Spindler, G.; Tuch, T.; Wiedensohler, A. Aerosol pollution maps and trends over Germany with hourly data at four rural background stations from 2009 to 2018. Atmos. Chem. Phys. Discuss. 2020, 20, 10967–10984. [Google Scholar] [CrossRef]
- EEA. Air Quality in Europe—2020 Report; EEA Report, No. 09/2020; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Eeftens, M.; Tsai, M.-Y.; Ampe, C.; Anwander, B.; Beelen, R.; Bellander, T.; Cesaroni, G.; Cirach, M.; Cyrys, J.; De Hoogh, K.; et al. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2—Results of the ESCAPE project. Atmos. Environ. 2012, 62, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-B.; Pan, X.-F.; Chen, J.; Cao, A.; Zhang, Y.-G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef]
- Karavasiloglou, N.; Pestoni, G.; Wanner, M.; Faeh, D.; Rohrmann, S. Healthy lifestyle is inversely associated with mortality in cancer survivors: Results from the third national health and nutrition examination survey (NHANES III). PLoS ONE 2019, 14, e0218048. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wan, X.; Yang, G.; Zou, X. Traffic-related air pollution and lung cancer: A meta-analysis. Thorac. Cancer 2015, 6, 307–318. [Google Scholar] [CrossRef]
- Hertig, E.; Russo, A.; Trigo, R.M. Heat and ozone pollution waves in central and South Europe—Characteristics, weather types, and association with mortality. Atmosphere 2020, 11, 1271. [Google Scholar] [CrossRef]
- Hertig, E. Health-relevant ground-level ozone and temperature events under future climate change using the example of Bavaria, Southern Germany. Air Qual. Atmos. Health 2020, 13, 435–446. [Google Scholar] [CrossRef] [Green Version]






| Abb. | Variable | Unit | Measuring Site | Measuring Entity |
|---|---|---|---|---|
| NO | nitric oxide | µg/m3 | A-Bourges-Platz | LfU Bayern |
| NO2 | nitrogen dioxide | µg/m3 | A-Bourges-Platz | LfU Bayern |
| PM10 | particulate matter | µg/m3 | A-LfU | LfU Bayern |
| PM2.5 | particulate matter | µg/m3 | A-LfU | LfU Bayern |
| O3 | ozone | µg/m3 | A-LfU | LfU Bayern |
| TN | minimum temperature | °C | A-Mühlhausen | DWD |
| TM | mean temperature | °C | A-Mühlhausen | DWD |
| TX | maximum temperature | °C | A-Mühlhausen | DWD |
| RH | relative humidity | % | A-Mühlhausen | DWD |
| FM | mean wind speed | ms−1 | A-Mühlhausen | DWD |
| FX | maximum wind speed | ms−1 | A-Mühlhausen | DWD |
| R | rainfall amount | mm | A-Mühlhausen | DWD |
| P | Mean sea level pressure | hPa | A-Mühlhausen | DWD |
| Composite | Months | Lead-In Days | Above Average | Below Average |
|---|---|---|---|---|
| 1 | February–April | 12–13 | NO2, PM2.5 | TN |
| 2 | February–May | 4–5 | NO2 | TN |
| 3 | February–May | 10–13 | NO2, PM2.5 | |
| 4 | April–June | 6–9 | NO2 | TM, TX |
| 5 | June–September | 3–8 | TM, TX | |
| 6 | August–October | 7–10 | TM, TX | TN |
| 7 | September–November | 10–13 | O3, TX | |
| 8 | November–February | 3–8 | TN, TM, TX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olschewski, P.; Kaspar-Ott, I.; Koller, S.; Schenkirsch, G.; Trepel, M.; Hertig, E. Associations between Weather, Air Quality and Moderate Extreme Cancer-Related Mortality Events in Augsburg, Southern Germany. Int. J. Environ. Res. Public Health 2021, 18, 11737. https://doi.org/10.3390/ijerph182211737
Olschewski P, Kaspar-Ott I, Koller S, Schenkirsch G, Trepel M, Hertig E. Associations between Weather, Air Quality and Moderate Extreme Cancer-Related Mortality Events in Augsburg, Southern Germany. International Journal of Environmental Research and Public Health. 2021; 18(22):11737. https://doi.org/10.3390/ijerph182211737
Chicago/Turabian StyleOlschewski, Patrick, Irena Kaspar-Ott, Stephanie Koller, Gerhard Schenkirsch, Martin Trepel, and Elke Hertig. 2021. "Associations between Weather, Air Quality and Moderate Extreme Cancer-Related Mortality Events in Augsburg, Southern Germany" International Journal of Environmental Research and Public Health 18, no. 22: 11737. https://doi.org/10.3390/ijerph182211737
APA StyleOlschewski, P., Kaspar-Ott, I., Koller, S., Schenkirsch, G., Trepel, M., & Hertig, E. (2021). Associations between Weather, Air Quality and Moderate Extreme Cancer-Related Mortality Events in Augsburg, Southern Germany. International Journal of Environmental Research and Public Health, 18(22), 11737. https://doi.org/10.3390/ijerph182211737






