Mitochondrial DNA Copy Number Adaptation as a Biological Response Derived from an Earthquake at Intrauterine Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Mitochondrial DNA Copy Number Quantification
2.3. Covariates
2.4. Statistical Analysis
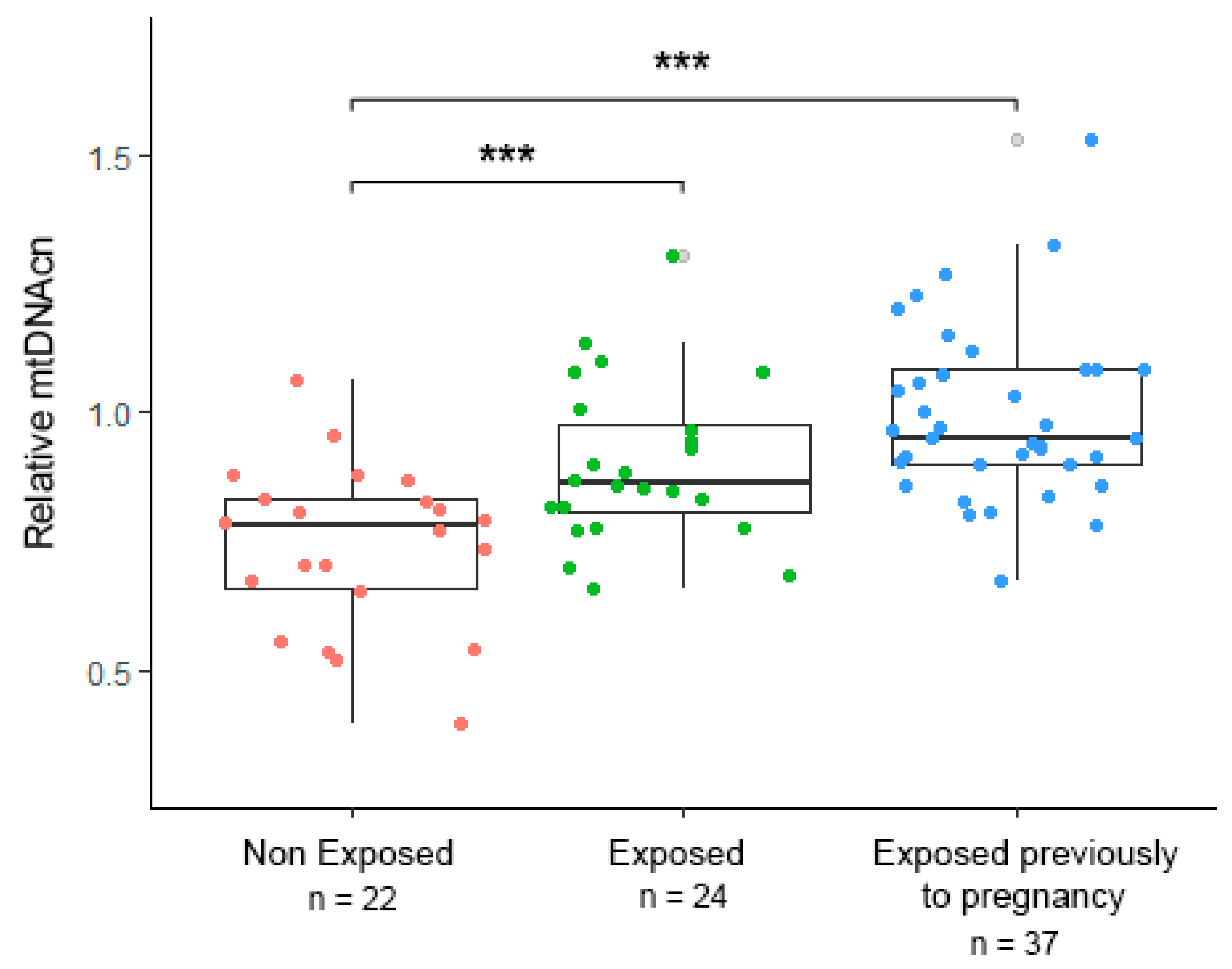
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morén, C.; Hernández, S.; Guitart-Mampel, M.; Garrabou, G. Mitochondrial Toxicity in Human Pregnancy: An Update on Clinical and Experimental Approaches in the Last 10 Years. Int. J. Environ. Res. Public Health 2014, 11, 9897–9918. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Higgins, J.S.; Vaughan, O.R.; Murray, A.J.; Fowden, A.L. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc. Natl. Acad. Sci. USA 2019, 116, 1621–1626. [Google Scholar] [CrossRef]
- Holland, O.; Dekker Nitert, M.; Gallo, L.A.; Vejzovic, M.; Fisher, J.J.; Perkins, A.V. Review: Placental mitochondrial function and structure in gestational disorders. Placenta 2017, 54, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 259, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Vatish, M.; Alam, M.T.; Ott, S.; Kumar, S.; Saravanan, P. Low vitamin B12 in pregnancy is associated with adipose-derived circulating miRs targeting PPARg and insulin resistance. J. Clin. Endocrinol. Metab. 2017, 102, 4200–4209. [Google Scholar] [CrossRef] [PubMed]
- Clay Montier, L.L.; Deng, J.J.; Bai, Y. Number matters: Control of mammalian mitochondrial DNA copy number. J. Genet. Genom. 2009, 36, 125–131. [Google Scholar] [CrossRef]
- Li, Z.Z.; Fu, J.; Li, Z.Z.; Tang, Y.; Hua, Q.; Liu, L.; Zhao, J. Air pollution and placental mitochondrial DNA copy number: Mechanistic insights and epidemiological challenges. Environ. Pollut. 2019, 255, 113266. [Google Scholar] [CrossRef]
- Malik, A.N.; Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Brunst, K.J.; Baccarelli, A.A.; Wright, R.J. Integrating mitochondriomics in children’s environmental health. J. Appl. Toxicol. 2015, 35, 976–991. [Google Scholar] [CrossRef]
- Brunst, K.J.; Sanchez-Guerra, M.; Chiu, Y.H.M.; Wilson, A.; Coull, B.A.; Kloog, I.; Schwartz, J.; Brennan, K.J.; Bosquet Enlow, M.; Wright, R.O.; et al. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environ. Int. 2018, 112, 49–58. [Google Scholar] [CrossRef]
- Moss, K.M.; Simcock, G.; Cobham, V.; Kildea, S.; Elgbeili, G.; Laplante, D.P.; King, S. A potential psychological mechanism linking disaster-related prenatal maternal stress with child cognitive and motor development at 16 months: The QF2011 Queensland flood study. Dev. Psychol. 2017, 53, 629–641. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Laplante, D.P.; Elgbeili, G.; Dawson, P.A.; Kildea, S.; King, S.; Vaillancourt, C. Natural disaster-related prenatal maternal stress is associated with alterations in placental glucocorticoid system: The QF2011 Queensland Flood Study. Psychoneuroendocrinology 2018, 94, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Carruthers, C.K.; Harris, M.C. Maternal stress and birth outcomes: Evidence from the 1994 Northridge earthquake. J. Econ. Behav. Organ. 2017, 140, 354–373. [Google Scholar] [CrossRef]
- Palmeiro-Silva, Y.K.; Orellana, P.; Venegas, P.; Monteiro, L.; Varas-Godoy, M.; Norwitz, E.; Rice, G.; Osorio, E.; Illanes, S.E. Effects of earthquake on perinatal outcomes: A Chilean register-based study. PLoS ONE 2018, 13, e0191340. [Google Scholar] [CrossRef] [PubMed]
- Harville, E.W.; Do, M. Reproductive and Birth Outcomes in Haiti before and after the 2010 Earthquake. Disaster Med. Public Health Prep. 2016, 10, 59–66. [Google Scholar] [CrossRef]
- Los 10 Terremotos más Potentes y Mortíferos de la Historia en América Latina—BBC News Mundo. Available online: https://www.bbc.com/mundo/noticias-america-latina-41343606 (accessed on 29 April 2021).
- Los 8 Sismos más Devastadores en la Historia de México. Available online: https://www.forbes.com.mx/los-8-sismos-mas-catastroficos-en-la-historia-de-mexico/ (accessed on 29 April 2021).
- Janssen, B.G.; Munters, E.; Pieters, N.; Smeets, K.; Cox, B.; Cuypers, A.; Fierens, F.; Penders, J.; Vangronsveld, J.; Gyselaers, W.; et al. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ. Health Perspect. 2012, 120, 1346–1352. [Google Scholar] [CrossRef]
- Hou, L.; Zhu, Z.Z.; Zhang, X.; Nordio, F.; Bonzini, M.; Schwartz, J.; Hoxha, M.; Dioni, L.; Marinelli, B.; Pegoraro, V.; et al. Airborne particulate matter and mitochondrial damage: A cross-sectional study. Environ. Health Glob. Access Sci. Source 2010, 9, 1–9. [Google Scholar] [CrossRef]
- Ingram, P.B.; Clarke, E.; Lichtenberg, J.W. Confirmatory Factor Analysis of the Perceived Stress Scale-4 in a Community Sample. Stress Health 2016, 32, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef]
- Wachsmuth, M.; Hübner, A.; Li, M.; Madea, B.; Stoneking, M. Age-Related and Heteroplasmy-Related Variation in Human mtDNA Copy Number. PLoS Genet. 2016, 12, 1005939. [Google Scholar] [CrossRef] [PubMed]
- Bijnens, E.M.; Derom, C.; Weyers, S.; Janssen, B.G.; Thiery, E.; Nawrot, T.S. Placental mitochondrial DNA content is associated with childhood intelligence. J. Transl. Med. 2019, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Geary, D.C. Efficiency of mitochondrial functioning as the fundamental biological mechanism of general intelligence (g). Psychol. Rev. 2018, 125, 1028. [Google Scholar] [CrossRef] [PubMed]
- Ridout, K.K.; Parade, S.H.; Kao, H.T.; Magnan, S.; Seifer, R.; Porton, B.; Price, L.H.; Tyrka, A.R. Childhood maltreatment, behavioral adjustment, and molecular markers of cellular aging in preschool-aged children: A cohort study. Psychoneuroendocrinology 2019, 107, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Tyrka, A.R.; Parade, S.H.; Price, L.H.; Kao, H.T.; Porton, B.; Philip, N.S.; Welch, E.S.; Carpenter, L.L. Alterations of Mitochondrial DNA Copy Number and Telomere Length with Early Adversity and Psychopathology. Biol. Psychiatry 2016, 79, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Yao, Y.; Wu, G.; Lv, T.; Luo, L.; Song, Y. The Plasma Mitochondrial DNA Is an Independent Predictor for Post-Traumatic Systemic Inflammatory Response Syndrome. PLoS ONE 2013, 8, e72834. [Google Scholar] [CrossRef]
- Edwards, A.C.; Aggen, S.H.; Cai, N.; Bigdeli, T.B.; Peterson, R.E.; Docherty, A.R.; Webb, B.T.; Bacanu, S.A.; Flint, J.; Kendler, K.S. Chronicity of depression and molecular markers in a large sample of han chinese women. Depress. Anxiety 2016, 33, 1048–1054. [Google Scholar] [CrossRef]
- Chung, J.K.; Lee, S.Y.; Park, M.; Joo, E.J.; Kim, S.A. Investigation of mitochondrial DNA copy number in patients with major depressive disorder. Psychiatry Res. 2019, 282, 112616. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Izumi, T.; Boku, S.; Kimura, A.; Zhang, Y.; Mouri, K.; Okazaki, S.; Shiroiwa, K.; Takahashi, M.; Ueno, Y.; et al. Aberrant telomere length and mitochondrial DNA copy number in suicide completers. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, Y.; Chang, S.; Liang, J.; Lin, C.; Zhang, X.; Liang, L.; Hu, J.; Chan, W.; Kendler, K.S.; et al. Genetic Control over mtDNA and Its Relationship to Major Depressive Disorder. Curr. Biol. 2015, 25, 3170–3177. [Google Scholar] [CrossRef]
- Kupsco, A.; Sanchez-Guerra, M.; Amarasiriwardena, C.; Brennan, K.J.M.; Estrada-Gutierrez, G.; Svensson, K.; Schnaas, L.; Pantic, I.; Téllez-Rojo, M.M.; Baccarelli, A.A.; et al. Prenatal manganese and cord blood mitochondrial DNA copy number: Effect modification by maternal anemic status. Environ. Int. 2019, 126, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wahlberg, K.; Love, T.M.; Watson, G.E.; Yeates, A.J.; Mulhern, M.S.; McSorley, E.M.; Strain, J.J.; Davidson, P.W.; Shamlaye, C.F.; et al. Associations of blood mercury and fatty acid concentrations with blood mitochondrial DNA copy number in the Seychelles Child Development Nutrition Study. Environ. Int. 2019, 124, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kaali, S.; Jack, D.; Delimini, R.; Hu, L.; Burkart, K.; Opoku-Mensah, J.; Quinn, A.; Ae-Ngibise, K.; Wylie, B.; Boamah-Kaali, E.; et al. Prenatal Household Air Pollution Alters Cord Blood Mononuclear Cell Mitochondrial DNA Copy Number: Sex-Specific Associations. Int. J. Environ. Res. Public Health 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, B.; Wang, L.; Wu, M.; Zhang, L.; Liu, Y.; Bi, J.; Yang, S.; Zhang, B.; Xia, W.; et al. Exposure to arsenic during pregnancy and newborn mitochondrial DNA copy number: A birth cohort study in Wuhan, China. Chemosphere 2020, 243, 125335. [Google Scholar] [CrossRef]
- Wu, M.; Shu, Y.; Song, L.; Liu, B.; Zhang, L.; Wang, L.; Liu, Y.; Bi, J.; Xiong, C.; Cao, Z.; et al. Prenatal exposure to thallium is associated with decreased mitochondrial DNA copy number in newborns: Evidence from a birth cohort study. Environ. Int. 2019, 129, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Tillett, T. Potential mechanism for PM10 effects on birth outcomes: In utero exposure linked to mitochondrial DNA damage. Environ. Health Perspect. 2012, 120, a363. [Google Scholar] [CrossRef][Green Version]
- Bersani, F.S.; Morley, C.; Lindqvist, D.; Epel, E.S.; Picard, M.; Yehuda, R.; Flory, J.; Bierer, L.M.; Makotkine, I.; Abu-Amara, D.; et al. Mitochondrial DNA copy number is reduced in male combat veterans with PTSD. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Z.; Zhang, W.; Wen, Q.; Li, X.; Zhou, J.; Wu, X.; Guo, Y.; Liu, Y.; Wei, C.; et al. The memory of neuronal mitochondrial stress is inherited transgenerationally via elevated mitochondrial DNA levels. Nat. Cell Biol. 2021, 23, 870–880. [Google Scholar] [CrossRef]
- Cao-Lei, L.; Massart, R.; Suderman, M.J.; Machnes, Z.; Elgbeili, G.; Laplante, D.P.; Szyf, M.; King, S. DNA Methylation Signatures Triggered by Prenatal Maternal Stress Exposure to a Natural Disaster: Project Ice Storm. PLoS ONE 2014, 9, e107653. [Google Scholar]
- Dobrovolsky, I.P.; Gershenzon, N.I.; Gokhberg, M.B. Theory of electrokinetic effects occurring at the final stage in the preparation of a tectonic earthquake. Phys. Earth Planet. Inter. 1989, 57, 144–156. [Google Scholar] [CrossRef]
- Igarashi, G.; Saeki, S.; Takahata, N.; Sumikawa, K.; Tasaka, S.; Sasaki, Y.; Takahashi, M.; Sano, Y. Ground-water radon anomaly before the kobe earthquake in Japan. Science 1995, 269, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Freund, F.; Stolc, V. Nature of pre-earthquake phenomena and their effects on living organisms. Animals 2013, 3, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Palgi, Y. Subjective age and perceived distance-to-death moderate the association between posttraumatic stress symptoms and posttraumatic growth among older adults. Aging Ment. Health 2016, 20, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Meli, L.; Kautz, M.; Julian, J.; Edmondson, D.; Sumner, J.A. The role of perceived threat during emergency department cardiac evaluation and the age-posttraumatic stress disorder link. J. Behav. Med. 2018, 41, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.J.; Just, A.C.; Guerra, M.S.; Kloog, I.; Hsu, H.H.L.; Brennan, K.J.; García, A.M.; Coull, B.; Wright, R.J.; Téllez Rojo, M.M.; et al. Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ. Int. 2017, 98, 198–203. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Virgolini, M.B.; Rossi-George, A.; Thiruchelvam, M.; Lisek, R.; Weston, D. Lifetime Consequences of Combined Maternal Lead and Stress. Basic Clin. Pharmacol. Toxicol. 2008, 102, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Dolcini, J.; Kioumourtzoglou, M.-A.; Cayir, A.; Sanchez-Guerra, M.; Brennan, K.J.; Dereix, A.E.; Coull, B.A.; Spiro, A.; Vokonas, P.; Schwartz, J.; et al. Age and mitochondrial DNA copy number influence the association between outdoor temperature and cognitive function. Environ. Epidemiol. 2020, 4, e0108. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.A.; Longchamps, R.J.; Sumpter, J.A.; Newcomb, C.E.; Lane, J.A.; Grove, M.L.; Bressler, J.; Brody, J.A.; Floyd, J.S.; Bartz, T.M.; et al. Mitochondrial DNA copy number can influence mortality and cardiovascular disease via methylation of nuclear DNA CpGs. Genome Med. 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, H. Mitochondrial DNA Copy Number and Developmental Origins of Health and Disease (DOHaD). Int. J. Mol. Sci. 2021, 22, 6634. [Google Scholar] [CrossRef]

| Characteristic | Non-Exposed (n = 22) | Exposed during Pregnancy (n = 24) | Exposed after Pregnancy (n = 37) | p-Value |
|---|---|---|---|---|
| Maternal age (years) | 30 ± 7 | 29 ± 4 | 30 ± 5 | 0.817 |
| Maternal pregestational body mass index (kg/m2) | 26.35 ± 4.03 | 26.86 ± 4.36 | 27.3 ± 6.53 | 0.8 |
| Low socioeconomic status | 5 (22.7) | 4 (16.6) | 6 (16.2) | 0.819 |
| Middle socioeconomic status | 13 (59.1) | 12 (50) | 19 (51.3) | 0.376 |
| High socioeconomic status | 4 (18.2) | 8 (33.3) | 12 (32.4) | 0.135 |
| 1st trimester pre-existing stress | 9.86 ± 4.58 | 10.8 ± 3.66 | 9.03 ± 3.17 | 0.205 |
| 3rd trimester pre-existing stress | 8.55 ± 3.19 | 8.68 ± 3.33 | 8.21 ± 3.03 | 0.879 |
| Preeclampsia | 3 (13.6) | 4 (16.6) | 4 (10.8) | 0.913 |
| Gestational diabetes | 1 (4.5) | 2 (8.3) | 2 (5.4) | 0.819 |
| Newborns | ||||
| Gestational age at birth (weeks) | 37.6 ± 2.1 | 38.5 ± 1.2 | 38.6 ± 1.4 | 0.156 |
| Weight (g) | 2755 ± 643 | 2938 ± 340 | 2837 ± 333 | 0.37 |
| Length (cm) | 45.4 ± 2.8 | 46.7 ± 1.4 | 47.3 ± 1.8 | 0.06 |
| Head circumference (cm) | 33 ± 2.1 | 33.3 ± 1.3 | 33.4 ± 1.1 | 0.529 |
| Sex | ||||
| Female | 8 (36.4) | 12 (50) | 22 (59.5) | 0.07 |
| Male | 14 (63.6) | 12 (50) | 15 (40.5) | 0.843 |
| Statistical Model | Earthquake during Gestation (n = 24) (Regression Coefficient) | 95% Confidence Interval | Gestation after the Earthquake (n = 37) (β (SE)) | 95% Confidence Interval | AIC |
|---|---|---|---|---|---|
| mtDNAcn~Status | 0.159 | (0.072, 0.241) | 0.255 | (0.176, 0.344) | −60.34 |
| mtDNAcn~Status + Sex | 0.174 | (0.086, 0.253) | 0.275 | (0.196, 0.363) | −62.73 |
| mtDNAcn~Status + Sex + BMI | 0.174 | (0.083, 0.252) | 0.274 | (0.194, 0.362) | −60.75 |
| mtDNAcn~Status + Sex + BMI + Age | 0.175 | (0.083, 0.253) | 0.276 | (0.194, 0.363) | −58.83 |
| mtDNAcn~Status + Sex + BMI + Age + CoMo | 0.175 | (0.087, 0.265) | 0.277 | (0.190, 0.362) | −56.31 |
| mtDNAcn~Status + Sex + BMI + Age + CoMo + SES | 0.170 | (0.07, 0.265) | 0.270 | (0.174, 0.346) | −56.84 |
| mtDNAcn~Status + Sex + BMI + Age + CoMo + SES + Stress 1T | 0.168 | (0.079, 0.276) | 0.260 | (0.178, 0.359) | −53.83 |
| mtDNAcn~Status + Sex + BMI + Age + CoMo + SES + Stress 3T | 0.186 | (0.93, 0.296) | 0.266 | (0.181, 0.367) | −48.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Ortega, J.A.; Reyes-Muñoz, E.; Nava-Salazar, S.; Rodríguez-Martínez, S.; Parra-Hernández, S.B.; Schnaas, L.; Suárez-Rico, B.V.; Torres-Olascoaga, L.A.; Baccarelli, A.A.; Wright, R.J.; et al. Mitochondrial DNA Copy Number Adaptation as a Biological Response Derived from an Earthquake at Intrauterine Stage. Int. J. Environ. Res. Public Health 2021, 18, 11771. https://doi.org/10.3390/ijerph182211771
Mendoza-Ortega JA, Reyes-Muñoz E, Nava-Salazar S, Rodríguez-Martínez S, Parra-Hernández SB, Schnaas L, Suárez-Rico BV, Torres-Olascoaga LA, Baccarelli AA, Wright RJ, et al. Mitochondrial DNA Copy Number Adaptation as a Biological Response Derived from an Earthquake at Intrauterine Stage. International Journal of Environmental Research and Public Health. 2021; 18(22):11771. https://doi.org/10.3390/ijerph182211771
Chicago/Turabian StyleMendoza-Ortega, Jonatan A., Enrique Reyes-Muñoz, Sonia Nava-Salazar, Sandra Rodríguez-Martínez, Sandra B. Parra-Hernández, Lourdes Schnaas, Blanca Vianey Suárez-Rico, Libni A. Torres-Olascoaga, Andrea A. Baccarelli, Rosalind J. Wright, and et al. 2021. "Mitochondrial DNA Copy Number Adaptation as a Biological Response Derived from an Earthquake at Intrauterine Stage" International Journal of Environmental Research and Public Health 18, no. 22: 11771. https://doi.org/10.3390/ijerph182211771
APA StyleMendoza-Ortega, J. A., Reyes-Muñoz, E., Nava-Salazar, S., Rodríguez-Martínez, S., Parra-Hernández, S. B., Schnaas, L., Suárez-Rico, B. V., Torres-Olascoaga, L. A., Baccarelli, A. A., Wright, R. J., Wright, R. O., Estrada-Gutierrez, G., & Tamayo-Ortiz, M. (2021). Mitochondrial DNA Copy Number Adaptation as a Biological Response Derived from an Earthquake at Intrauterine Stage. International Journal of Environmental Research and Public Health, 18(22), 11771. https://doi.org/10.3390/ijerph182211771







