Evaluation of a Fast Test Based on Biometric Signals to Assess Mental Fatigue at the Workplace—A Pilot Study
Abstract
:1. Introduction
2. Data
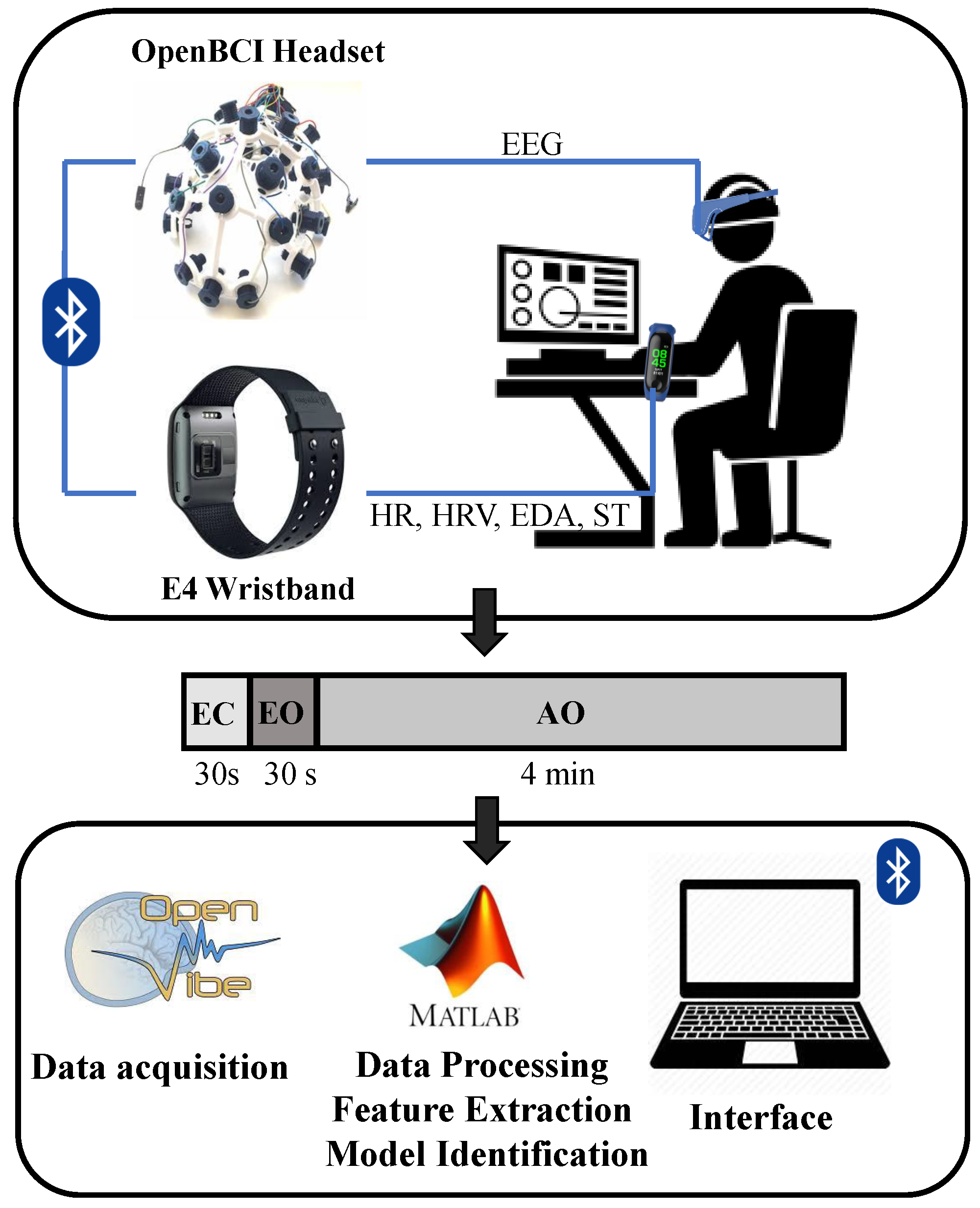
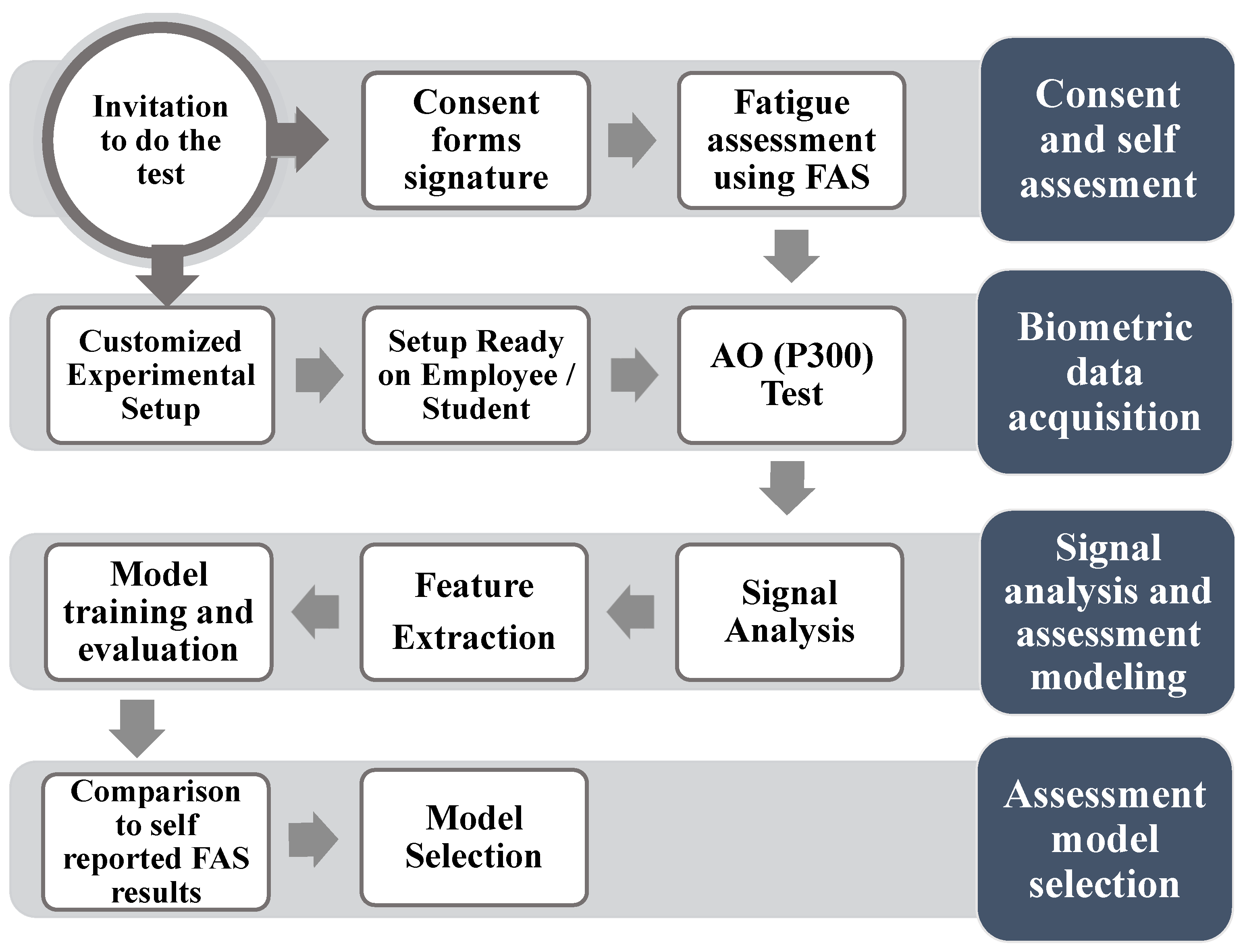
2.1. Experimental Design
2.2. EEG Acquisition
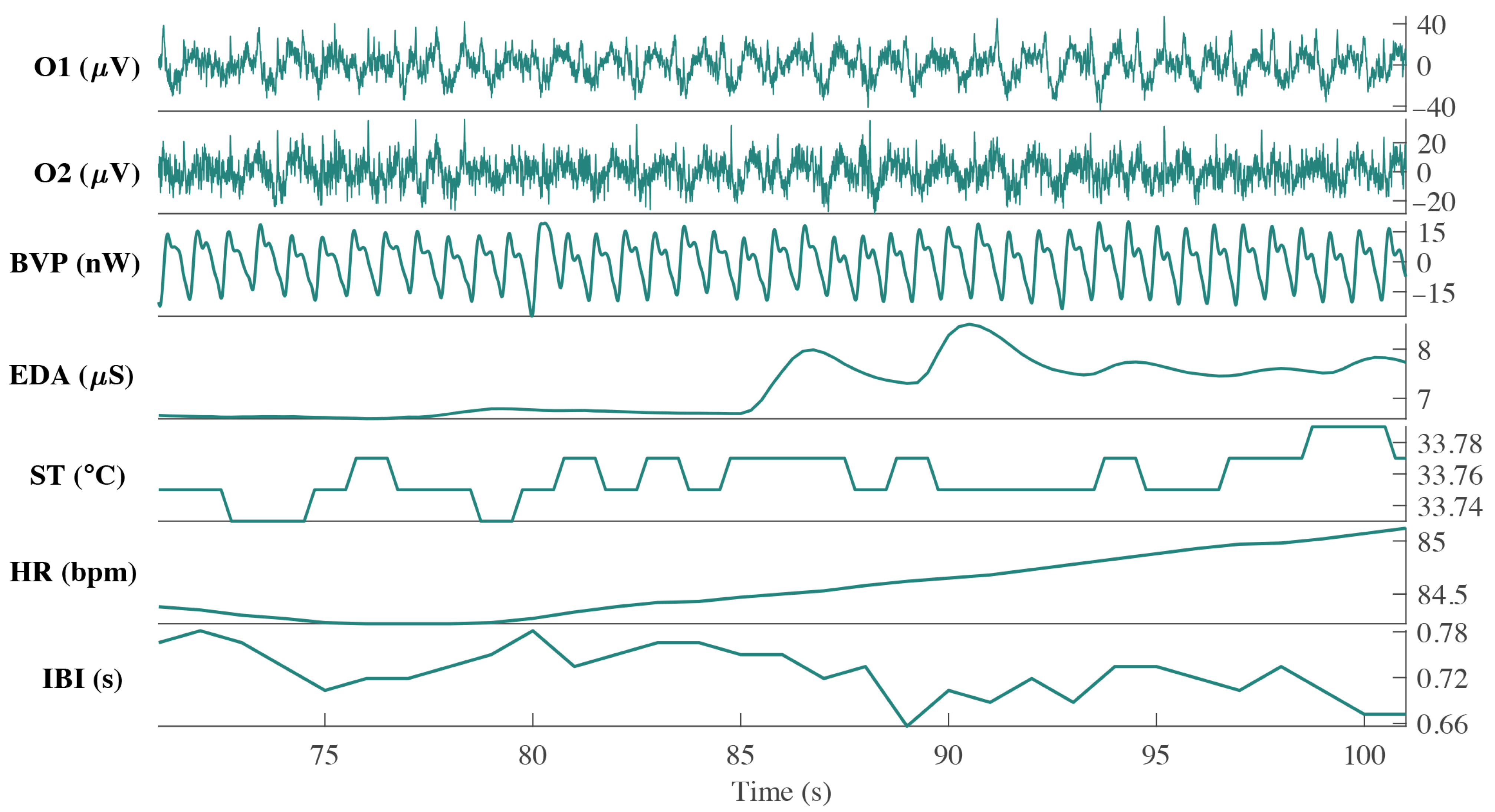
2.3. Biometric Signals Acquisition
2.4. Fatigue Assessment Scale
3. Methods
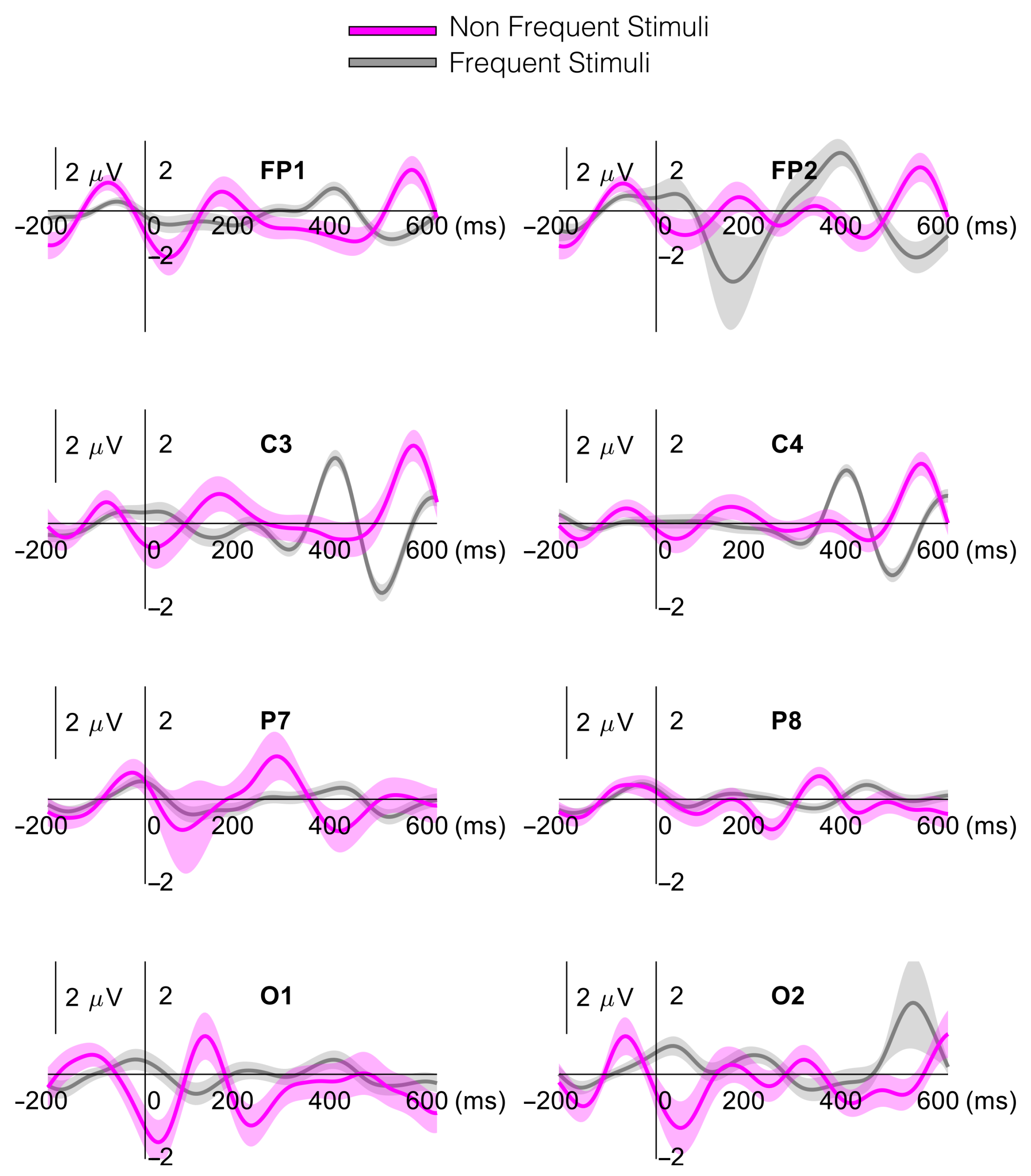
3.1. EEG Temporal (P300) Analysis
3.2. EEG Spectral Analysis
3.3. Empatica E4 Analysis
3.4. Feature Selection
3.5. Model Training and Evaluation
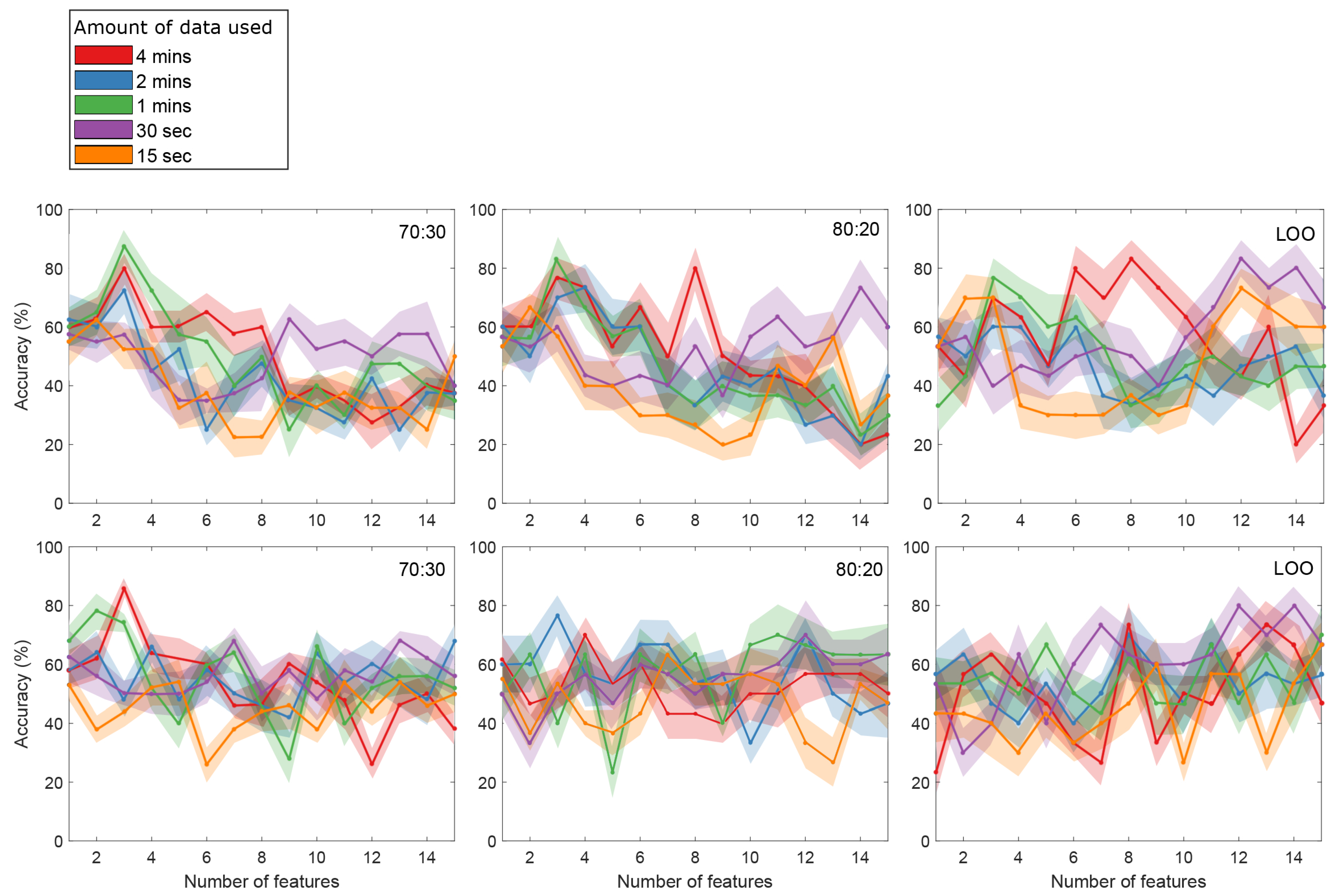
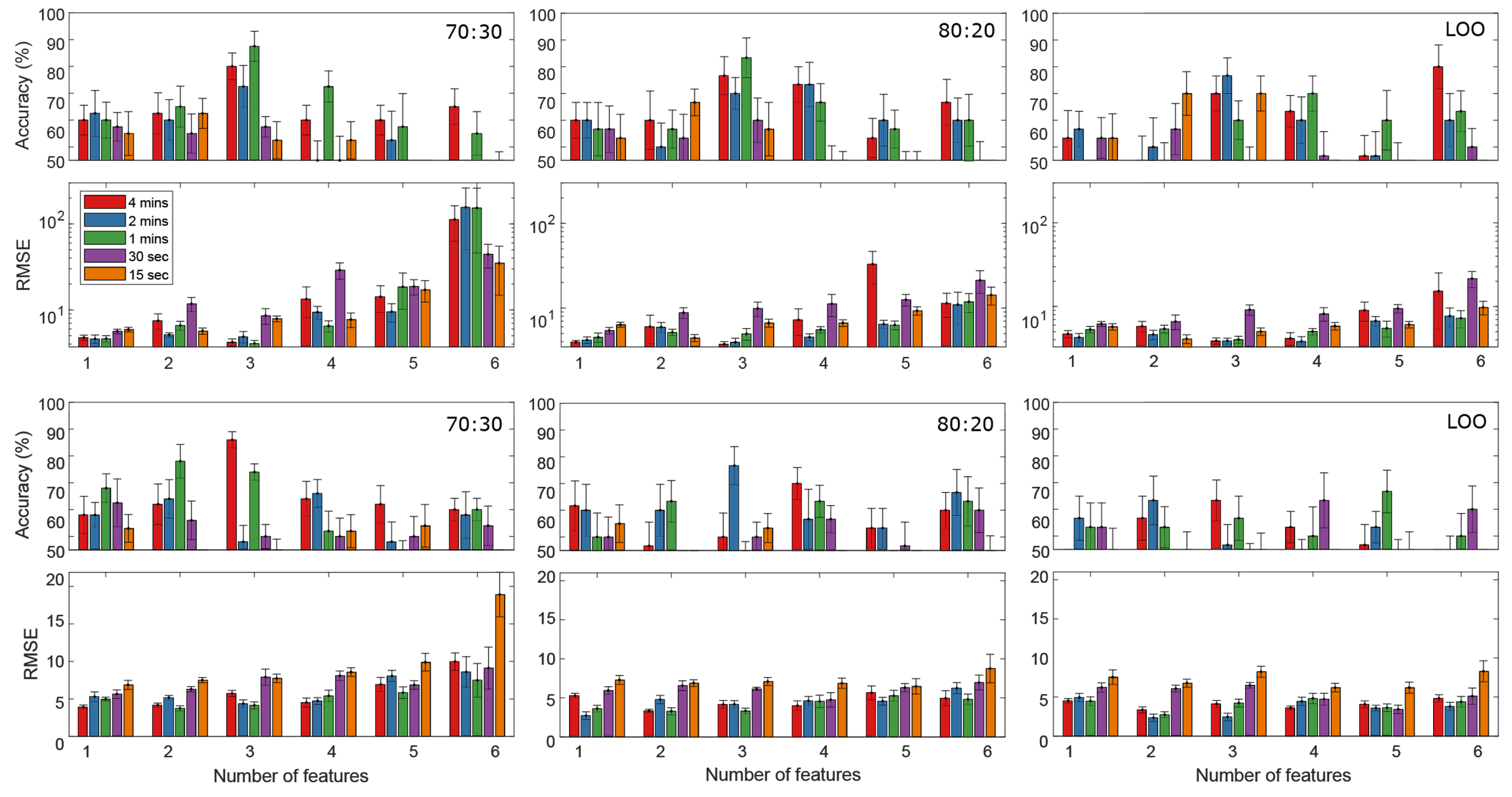
4. Results
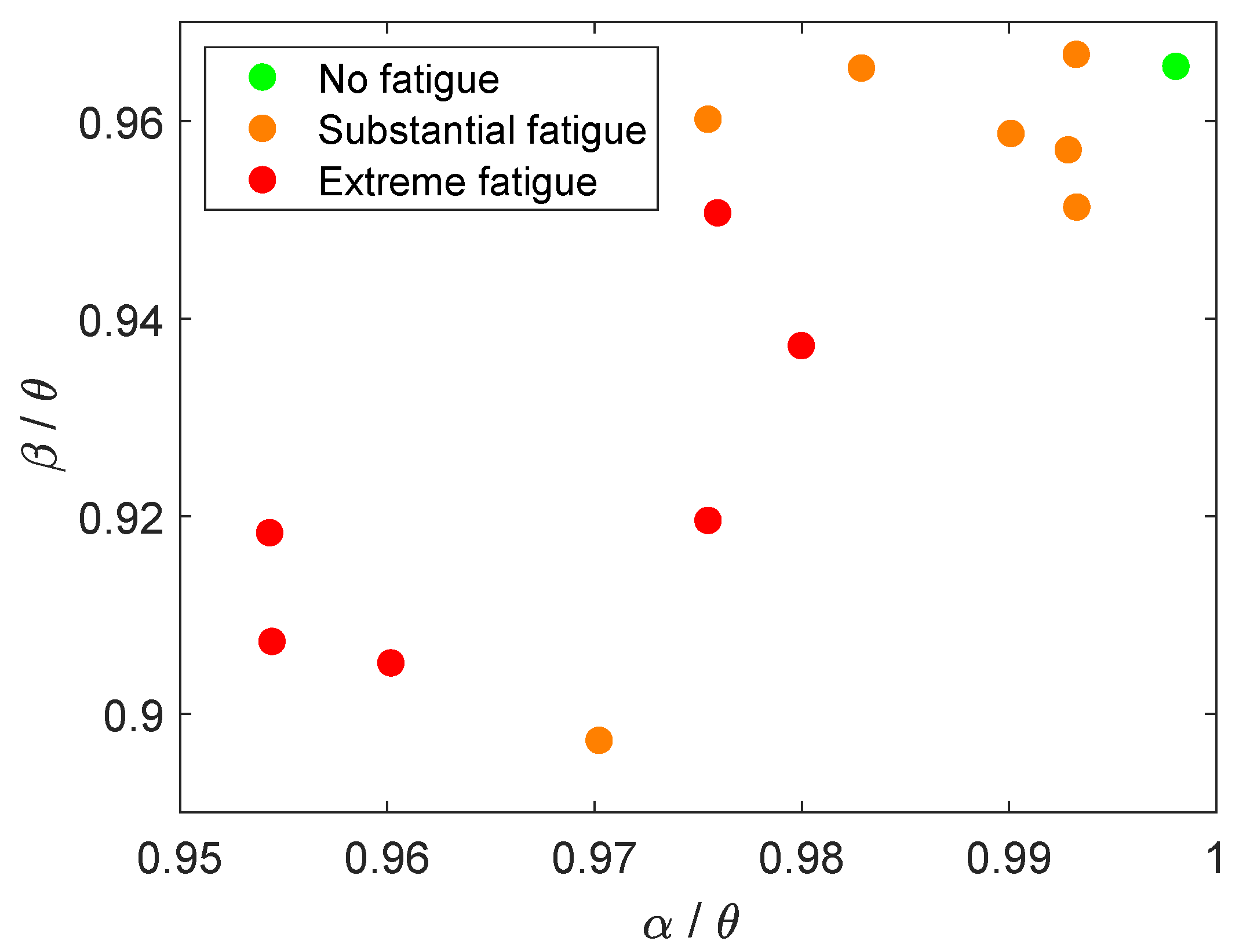
5. Discussion
5.1. Feature Analysis
5.2. Model Evaluation
5.3. Limitations
5.4. Final Remarks
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, K.; Meeusen, R.; Thompson, K.G.; Keegan, R.; Rattray, B. Mental Fatigue Impairs Endurance Performance: A Physiological Explanation. Sports Med. 2018, 48, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Vries, J.D.; Michielsen, H.J.; Heck, G.L.V. Assessment of fatigue among working people: A comparison of six questionnaires. Occup. Environ. Med. 2003, 60, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.J.; Lakhiani, S.D.; “Trey” Morrison, D.R. Staying Alert: Incorporating Human Fatigue in Risk Management. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2019, 63, 1819–1823. [Google Scholar] [CrossRef]
- Ko, L.W.; Komarov, O.; Hairston, W.D.; Jung, T.P.; Lin, C.T. Sustained attention in real classroom settings: An EEG study. Front. Hum. Neurosci. 2017, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Gharagozlou, F.; Saraji, G.N.; Mazloumi, A.; Nahvi, A.; Nasrabadi, A.M.; Foroushani, A.R.; Kheradmand, A.A.; Ashouri, M.; Samavati, M. Detecting driver mental fatigue based on EEG alpha power changes during simulated driving. Iran. J. Public Health 2015, 44, 1693–1700. [Google Scholar] [PubMed]
- Pires, F.O.; Silva-Júnior, F.L.; Brietzke, C.; Franco-Alvarenga, P.E.; Pinheiro, F.A.; de França, N.M.; Teixeira, S.; Santos, T.M. Mental fatigue alters cortical activation and psychological responses, impairing performance in a distance-based cycling trial. Front. Physiol. 2018, 9, 277. [Google Scholar] [CrossRef]
- Lal, S.K.; Craig, A. Driver fatigue: Electroencephalography and psychological assessment. Psychophysiology 2002, 39, 313–321. [Google Scholar] [CrossRef]
- Leso, V.; Fontana, L.; Caturano, A.; Vetrani, I.; Fedele, M.; Iavicoli, I. Impact of Shift Work and Long Working Hours on Worker Cognitive Functions: Current Evidence and Future Research Needs. Int. J. Environ. Res. Public Health 2021, 18, 6540. [Google Scholar] [CrossRef] [PubMed]
- Al-Mekhlafi, A.B.A.; Isha, A.S.N.; Chileshe, N.; Abdulrab, M.; Saeed, A.A.H.; Kineber, A.F. Modelling the Relationship between the Nature of Work Factors and Driving Performance Mediating by Role of Fatigue. Int. J. Environ. Res. Public Health 2021, 18, 6752. [Google Scholar] [CrossRef]
- Sarabia-Cobo, C.; Pérez, V.; de Lorena, P.; Fernández-Rodríguez, Á.; González-López, J.R.; González-Vaca, J. Burnout, Compassion Fatigue and Psychological Flexibility among Geriatric Nurses: A Multicenter Study in Spain. Int. J. Environ. Res. Public Health 2021, 18, 7560. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, J.; Kim, H.R.; Kang, M.Y. Health-Related Productivity Loss According to Health Conditions among Workers in South Korea. Int. J. Environ. Res. Public Health 2021, 18, 7589. [Google Scholar] [CrossRef]
- Keller, S.M.; Berryman, P.; Lukes, E. Effects of Extended Work Shifts and Shift Work on Patient Safety, Productivity, and Employee Health. AAOHN J. 2009, 57, 497–504. [Google Scholar] [CrossRef]
- Lee, S.; Seong, S.; Park, S.; Lim, J.; Hong, S.; Cho, Y.; Kim, H. Korean Version of the Swedish Occupational Fatigue Inventory among Construction Workers: Cultural Adaptation and Psychometric Evaluation. Int. J. Environ. Res. Public Health 2021, 18, 4302. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Pons, D.; Pearse, J. A Methodology for Harmonizing Safety and Health Scales in Occupational Risk Assessment. Int. J. Environ. Res. Public Health 2021, 18, 4849. [Google Scholar] [CrossRef] [PubMed]
- Rey-Merchán, M.d.C.; Gómez-de Gabriel, J.M.; López-Arquillos, A.; Fernández-Madrigal, J.A. Virtual Fence System Based on IoT Paradigm to Prevent Occupational Accidents in the Construction Sector. Int. J. Environ. Res. Public Health 2021, 18, 6839. [Google Scholar] [CrossRef]
- Yedla, A.; Kakhki, F.D.; Jannesari, A. Predictive Modeling for Occupational Safety Outcomes and Days Away from Work Analysis in Mining Operations. Int. J. Environ. Res. Public Health 2020, 17, 7054. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mazumder, N.U.S.; Agnew, R.J.; Grover, I.B.; Song, G.; Li, R. Using Artificial Neural Network Modeling to Analyze the Thermal Protective and Thermo-Physiological Comfort Performance of Textile Fabrics Used in Oilfield Workers’ Clothing. Int. J. Environ. Res. Public Health 2021, 18, 6991. [Google Scholar] [CrossRef]
- Pishgar, M.; Issa, S.F.; Sietsema, M.; Pratap, P.; Darabi, H. REDECA: A Novel Framework to Review Artificial Intelligence and Its Applications in Occupational Safety and Health. Int. J. Environ. Res. Public Health 2021, 18, 6705. [Google Scholar] [CrossRef]
- Mónica, G.; María, R.; Aide, M.; Jaime, S.; Jorge, D.L.R. Work Stress and Psychosocial Factors in the Manufacturing Industry: A Literature Review. In Evaluating Mental Workload for Improved Workplace Performance; Real, A., Ed.; IGI Global: London, UK, 2020; pp. 24–48. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X. Estimating mental fatigue Based on electroencephalogram and heart rate variability. Pol. J. Med. Phys. Eng. 2009, 16, 67–84. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Hsur, H.T. Mental Fatigue Measurement Using EEG. In Risk Management Trends; Intech Open: Rijeka, Croatia, 2011; pp. 203–228. [Google Scholar] [CrossRef]
- Jeong, J.H.; Yu, B.W.; Lee, D.H.; Lee, S.W. Classification of drowsiness levels based on a deep spatio-temporal convolutional bidirectional lstm network using electroencephalography signals. Brain Sci. 2019, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.K.; Li, Y.L.; Yang, Y.X.; Ma, C. A recurrence network-based convolutional neural network for fatigue driving detection from EEG. Chaos 2019, 29, 113126. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, C.; Zhao, M.; Tu, Y.; Liu, J. Multivariate autoregressive models and kernel learning algorithms for classifying driving mental fatigue based on electroencephalographic. Expert Syst. Appl. 2011, 38, 1859–1865. [Google Scholar] [CrossRef]
- Tseng, V.W.S.; Valliappan, N.; Ramachandran, V.; Choudhury, T.; Navalpakkam, V. Digital biomarker of mental fatigue. NPJ Digit. Med. 2021, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, X.; Yang, Y.; Mu, C.; Cai, Q.; Dang, W.; Zuo, S. EEG-Based Spatio-Temporal Convolutional Neural Network for Driver Fatigue Evaluation. IEEE Trans. Neural Netw. Learn. Syst. 2019, 30, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Trejo, L.J.; Kubitz, K.; Rosipal, R.; Kochavi, R.L.; Montgomery, L.D. EEG-Based Estimation and Classification of Mental Fatigue. Psychology 2015, 6, 572–589. [Google Scholar] [CrossRef]
- Luo, H.; Lee, P.A.; Clay, I.; Jaggi, M.; De Luca, V. Assessment of Fatigue Using Wearable Sensors: A Pilot Study. Digit. Biomarkers 2020, 4, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Sulaiman, N.; Abdul Majeed, A.P.P.; Musa, R.M.; Ahmad, A.F.; Bari, B.S.; Khatun, S. Current Status, Challenges, and Possible Solutions of EEG-Based Brain-Computer Interface: A Comprehensive Review. Front. Neurorobotics 2020, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- di Fronso, S.; Fiedler, P.; Tamburro, G.; Haueisen, J.; Bertollo, M.; Comani, S. Dry EEG in Sports Sciences: A Fast and Reliable Tool to Assess Individual Alpha Peak Frequency Changes Induced by Physical Effort. Front. Neurosci. 2019, 13, 982. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dragomir, A.; Abbasi, N.I.; Li, J.; Thakor, N.V.; Bezerianos, A. A novel real-time driving fatigue detection system based on wireless dry EEG. Cogn. Neurodynamics 2018, 12, 365–376. [Google Scholar] [CrossRef]
- Lim, W.L.; Sourina, O.; Wang, L.P. STEW: Simultaneous task EEG workload data set. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2106–2114. [Google Scholar] [CrossRef]
- Lin, S.; Liu, J.; Li, W.; Wang, D.; Huang, Y.; Jia, C.; Li, Z.; Murtaza, M.; Wang, H.; Song, J.; et al. A Flexible, Robust, and Gel-Free Electroencephalogram Electrode for Noninvasive Brain-Computer Interfaces. Nano Lett. 2019, 19, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Kosmyna, N.; Maes, P. Attentivu: An EEG-based closed-loop biofeedback system for real-time monitoring and improvement of engagement for personalized learning. Sensors 2019, 19, 5200. [Google Scholar] [CrossRef] [PubMed]
- Krigolson, O.E.; Williams, C.C.; Norton, A.; Hassall, C.D.; Colino, F.L. Choosing MUSE: Validation of a low-cost, portable EEG system for ERP research. Front. Neurosci. 2017, 11, 109. [Google Scholar] [CrossRef]
- Ravindran, A.S.; Mobiny, A.; Cruz-Garza, J.G.; Paek, A.; Kopteva, A.; Contreras Vidal, J.L. Assaying neural activity of children during video game play in public spaces: A deep learning approach. J. Neural Eng. 2019, 16, 036028. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Chung, W.Y. A single-channel SSVEP-based BCI speller using deep learning. IEEE Access 2019, 7, 1752–1763. [Google Scholar] [CrossRef]
- Ge, S.; Yang, Q.; Wang, R.; Lin, P.; Gao, J.; Leng, Y.; Yang, Y.; Wang, H. A Brain-Computer Interface Based on a Few-Channel EEG-fNIRS Bimodal System. IEEE Access 2017, 5, 208–218. [Google Scholar] [CrossRef]
- Roy, R.N.; Bonnet, S.; Charbonnier, S.; Campagne, A. Mental fatigue and working memory load estimation: Interaction and implications for EEG-based passive BCI. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Osaka, Japan, 3–7 July 2013; pp. 6607–6610. [Google Scholar] [CrossRef]
- Shen, K.Q.; Li, X.P.; Ong, C.J.; Shao, S.Y.; Wilder-Smith, E.P. EEG-based mental fatigue measurement using multi-class support vector machines with confidence estimate. Clin. Neurophysiol. 2008, 119, 1524–1533. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, Z.; Cui, J.; Sourina, O.; Muller-Wittig, W. EEG-Based cross-subject mental fatigue recognition. In Proceedings of the 2019 International Conference on Cyberworlds, CW 2019, Kyoto, Japan, 2–4 October 2019; pp. 247–252. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, Z.; Cui, J.; Sourina, O.; Müller-Wittig, W. Inter-subject transfer learning for EEG-based mental fatigue recognition. Adv. Eng. Inform. 2020, 46, 101157. [Google Scholar] [CrossRef]
- Das Chakladar, D.; Dey, S.; Roy, P.P.; Dogra, D.P. EEG-based mental workload estimation using deep BLSTM-LSTM network and evolutionary algorithm. Biomed. Signal Process. Control 2020, 60, 101989. [Google Scholar] [CrossRef]
- Charbonnier, S.; Roy, R.N.; Bonnet, S.; Campagne, A. EEG index for control operators’ mental fatigue monitoring using interactions between brain regions. Expert Syst. Appl. 2016, 52, 91–98. [Google Scholar] [CrossRef]
- Eoh, H.J.; Chung, M.K.; Kim, S.H. Electroencephalographic study of drowsiness in simulated driving with sleep deprivation. Int. J. Ind. Ergon. 2005, 35, 307–320. [Google Scholar] [CrossRef]
- Alexander, J.E.; Polich, J.; Bloom, F.E.; Bauer, L.O.; Kupermanc, S.; Rohrbaugh, O.; Morzorati, S.; O’Connor, S.J.; Porjesz, B.; Begleiter, H. P300 from an auditory oddball task: Inter-laboratory consistency. Int. J. Psychophysiol. 1994, 17, 35–46. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, C.; Luo, H.; Zhang, W. The Effect of Mental Fatigue on Response Processes: An ERP Study in Go/NoGo Task. In Advances in Neuroergonomics and Cognitive Engineering; Springer: Berlin/Heidelberg, Germany, 2018; pp. 49–60. [Google Scholar] [CrossRef]
- Fazel-Rezai, R.; Allison, B.Z.; Guger, C.; Sellers, E.W.; Kleih, S.C.; Kübler, A. P300 brain computer interface: Current challenges and emerging trends. Front. Neuroeng. 2012, 5, 14. [Google Scholar] [CrossRef]
- Mak, J.N.; McFarland, D.J.; Vaughan, T.M.; McCane, L.M.; Tsui, P.Z.; Zeitlin, D.J.; Sellers, E.W.; Wolpaw, J.R. EEG correlates of P300-based brain–computer interface (BCI) performance in people with amyotrophic lateral sclerosis. J. Neural Eng. 2012, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.R.; Gutiérrez, D. Evaluating a Semiautonomous Brain-Computer Interface Based on Conformal Geometric Algebra and Artificial Vision. Comput. Intell. Neurosci. 2019, 2019, 9374802. [Google Scholar] [CrossRef]
- Donchin, E.; Kubovy, M.; Kutas, M.; Jonhson, R.; Herning, R.I. Graded changes in evoked response (P300) amplitude as a function of cognitive activity. Percept. Psychophys. 1973, 14, 319–324. [Google Scholar] [CrossRef]
- Rutkowski, T. Robotic and virtual reality BCIs using spatial tactile and auditory oddball paradigms. Front. Neurorobotics 2016, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Sugiyama, Y.; Mano, T.; Okada, H.; Matsukawa, T.; Iwase, S. Skin sympathetic nerve activity and event-related potentials during auditory oddball paradigms. J. Auton. Nerv. Syst. 1996, 60, 129–135. [Google Scholar] [CrossRef]
- Isreal, J.B.; Chesney, G.L.; Wickens, C.D.; Donchin, E. P300 and tracking difficulty: Evidence for multiple resources in dual-task performance. Psychophysiology 1980, 17, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Uetake, A.; Takasawa, Y. Evaluation of mental fatigue using feature parameter extracted from event-related potential. Int. J. Ind. Ergon. 2005, 35, 761–770. [Google Scholar] [CrossRef]
- Iragui, V.; Kutas, M.; Mitchiner, M.R.; Hillyard, S.A. Effects of aging on event-related potentials and reaction times in an auditory oddball task. Psychophysiology 1993, 30, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Sang-Joong, J.; Heung-Sub, S.; Wan-Young, C. Driver fatigue and drowsiness monitoring system with embedded electrocardiogram sensor on steering wheel. Inst. Eng. Technol. 2014, 8, 43–50. [Google Scholar] [CrossRef]
- Kurian, D.; Joseph, P.J.; Radhakrishnan, K.; Balakrishnan, A.A. Drowsiness Detection Using Photoplethysmography Signal. In Proceedings of the 2014 Fourth IEEE International Conference on Advances in Computing and Communications, Kochi, India, 27–29 August 2014. [Google Scholar] [CrossRef]
- Miró, E.; Cano-Lozano, M.C.; Buela-Casal, G. Electrodermal activity during total sleep deprivation and its relationship with other activation and performance measures. J. Sleep Res. 2002, 11, 105–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Pan, Z. A Deep Temporal Model for Mental Fatigue Detection. In Proceedings of the 2018 IEEE International Conference on Systems, Man, and Cybernetics, SMC 2018, Miyazaki, Japan, 7–10 October 2019; pp. 1879–1884. [Google Scholar] [CrossRef]
- Debener, S.; Makeig, S.; Delorme, A.; Engel, A.K. What is novel in the novelty oddball paradigm? Functional significance of the novelty P3 event-related potential as revealed by independent component analysis. Cogn. Brain Res. 2005, 22, 309–321. [Google Scholar] [CrossRef]
- Renard, Y.; Lotte, F.; Gibert, G.; Congedo, M.; Maby, E.; Delannoy, V.; Bertrand, O.; Lécuyer, A. OpenViBE: An Open-Source Software Platform to Design, Test, and Use Brain–Computer Interfaces in Real and Virtual Environments. Presence 2010, 19, 35–53. [Google Scholar] [CrossRef]
- Aldridge, A.; Barnes, E.; Bethel, C.L.; Carruth, D.W.; Kocturova, M.; Pleva, M.; Juhar, J. Accessible Electroencephalograms (EEGs): A Comparative Review with OpenBCI’s Ultracortex Mark IV Headset. In Proceedings of the 2019 29th IEEE International Conference Radioelektronika (RADIOELEKTRONIKA), Pardubice, Czech Republic, 16–18 April 2019. [Google Scholar] [CrossRef]
- Jiang, X.; Bian, G.B.; Tian, Z. Removal of artifacts from EEG signals: A review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Onton, J.A.; Kang, D.Y.; Coleman, T.P. Visualization of whole-night sleep EEG from 2-channel mobile recording device reveals distinct deep sleep stages with differential electrodermal activity. Front. Hum. Neurosci. 2016, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, H.J.; Vries, J.D.; Heck, G.L.V. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J. Psychosom. Res. 2003, 54, 345–352. [Google Scholar] [CrossRef]
- Zeba, M.Z.; Friganocić, K.; Palmovic, M.; Isgum, V.; Cifrek, M. Assessment of mental fatigue during examination period with P300 oddball paradigm. In Proceedings of the 42nd IEEE International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 20–24 May 2019; pp. 1479–1484. [Google Scholar] [CrossRef]
- Bråndal, A.; Eriksson, M.; Wester, P.; Lundin-Olsson, L. Reliability and validity of the Swedish Fatigue Assessment Scale when self-administrered by persons with mild to moderate stroke. Top. Stroke Rehabil. 2016, 23, 90–97. [Google Scholar] [CrossRef]
- Horisberger, A.; Courvoisier, D.; Ribi, C. The Fatigue Assessment Scale as a simple and reliable tool in systemic lupus erythematosus: A cross-sectional study. Arthritis Res. Ther. 2019, 21, 80. [Google Scholar] [CrossRef]
- Cano-Climent, A.; Oliver-Roig, A.; Cabrero-García, J.; de Vries, J.; Richart-Martínez, M. The Spanish version of the Fatigue Assessment Scale: Reliability and validity assessment in postpartum women. PeerJ 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, J.M.; Muñoz-Vega, P.; Alberca, S.B.; Peral-Pacheco, D. Health-Related Quality of Life and Fatigue after Transient Ischemic Attack and Minor Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Hsu, H.S.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic EEG Artifact Removal. In Proceedings of the IEEE Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Honolulu, HI, USA, 17–21 July 2018. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Citherlet, D.; Boucher, O.; Tremblay, J.; Robert, M.; Gallagher, A.; Bouthillier, A.; Lepore, F.; Nguyen, D.K. Role of the insula in top–down processing: An intracranial EEG study using a visual oddball detection paradigm. Brain Struct. Funct. 2019, 224, 2045–2059. [Google Scholar] [CrossRef]
- Luo, S.; Duh, H.B.; Zhou, J.; Cheng, F. BVP signal feature analysis for intelligent user interface. In Proceedings of the Conference on Human Factors in Computing Systems—Proceedings, Denver, CO, USA, 6–11 May 2017. [Google Scholar] [CrossRef]
- Kumar, R.; Kalra, P.; Lall, A.K. Mental Fatigue Quantification by Physiological and Neurophysiological Techniques: An Overview. Ergon. Caring People 2018, 327–336. [Google Scholar] [CrossRef]
- Plesinger, F.; Viscor, I.; Nejedly, P.; Andrla, P.; Halamek, J.; Jurak, P. Automated Sleep Arousal Detection Based on EEG Envelograms. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; Volume 45, pp. 1–4. [Google Scholar]
- Ashford, J.; Bird, J.J.; Campelo, F.; Faria, D.R. Classification of EEG Signals Based on Image Representation of Statistical Features. In Advances in Computational Intelligence Systems; Ju, Z., Yang, L., Yang, C., Gegov, A., Zhou, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 449–460. [Google Scholar]
- Kowal, R. Characteristics and Properties of a Simple Linear Regression Model. Folia Oeconomica Stetin. 2017, 16, 248–263. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, N.; Zhang, X.; Yue, K.; Li, W.; Kumar, D. Machine learning based resource allocation of cloud computing in auction. Comput. Mater. Contin. 2018, 56, 123–135. [Google Scholar] [CrossRef]
- Uetake, A.; Murata, A. Assessment of mental fatigue during VDT task using event-related potential (P300). In Proceedings of the IEEE International Workshop on Robot and Human Interactive Communication, Osaka, Japan, 27–29 September 2000; pp. 235–240. [Google Scholar] [CrossRef]
- Intriligator, J.; Polich, J. On the relationship between EEG and ERP variability. Int. J. Psychophysiol. 1995, 20, 59–74. [Google Scholar] [CrossRef]
- Aryal, A.; Ghahramani, A.; Becerik-Gerber, B. Monitoring fatigue in construction workers using physiological measurements. Autom. Constr. 2017, 82, 154–165. [Google Scholar] [CrossRef]
- Géron, A. Hands-On Machine Learning with Scikit-Learn & TensorFlow, 1st ed.; O’Reilly Media: Sebastopol, CA, USA, 2019. [Google Scholar]
- Spreng, R.N.; Wojtowicz, M.; Grady, C.L. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neurosci. Biobehav. Rev. 2010, 34, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, H.; Yan, S.; Pang, G.; Gui, G. Fatigue EEG Feature Extraction Based on Tasks with Different Physiological States for Ubiquitous Edge Computing. IEEE Access 2019, 7, 73057–73064. [Google Scholar] [CrossRef]
- Verleger, R.; Śmigasiewicz, K. Do rare stimuli evoke large P3S by being unexpected? A comparison of oddball effects between Standard-Oddball and Prediction-Oddball tasks. Adv. Cogn. Psychol. 2016, 12, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, M.A.; Díaz-Padilla, M.; Valenzuela-Gómez, K.D.; Vargas-Martínez, A.; Tudón-Martínez, J.C.; Morales-Menendez, R.; Ramírez-Mendoza, R.A.; Pérez-Henríquez, B.L.; Lozoya-Santos, J.d.J. EEG-Based Tool for Prediction of University Students’ Cognitive Performance in the Classroom. Brain Sci. 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
| Feature | p-Value | r | Feature | p-Value | r | Feature | p-Value | r | Feature | p-Value | r |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (C3) | (O1) | Latency (P7) | (FP1) | ||||||||
| (C3) | (O1) | (FP1) | (C4) | ||||||||
| (O2) | (C3) | (P8) | (C4) | ||||||||
| (O2) | (O2) | (C3) | (O1) | ||||||||
| (C3) | (O1) | (C3) | (C3) | ||||||||
| (C3) | (O2) | (C4) | (FP1) | ||||||||
| (O1) | (O2) | (O1) | ST (E4) | ||||||||
| (O1) | (FP1) | (O1) | LF (E4) | ||||||||
| (O1) | (O2) | (O1) | TP (E4) | ||||||||
| (O1) | (C3) | (FP1) | HF (E4) | ||||||||
| (O2) | (C3) | (FP1) | LFN (E4) | ||||||||
| (O2) | (FP1) | (FP1) | LF/HF (E4) | ||||||||
| (O1) | (P8) | (C3) | |||||||||
| (O2) | Latency (C3) | (O2) | |||||||||
| (O2) | (C3) | (O2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Moreno, M.A.; Carrillo-Tijerina, P.; Candela-Leal, M.O.; Alanis-Espinosa, M.; Tudón-Martínez, J.C.; Roman-Flores, A.; Ramírez-Mendoza, R.A.; Lozoya-Santos, J.d.J. Evaluation of a Fast Test Based on Biometric Signals to Assess Mental Fatigue at the Workplace—A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 11891. https://doi.org/10.3390/ijerph182211891
Ramírez-Moreno MA, Carrillo-Tijerina P, Candela-Leal MO, Alanis-Espinosa M, Tudón-Martínez JC, Roman-Flores A, Ramírez-Mendoza RA, Lozoya-Santos JdJ. Evaluation of a Fast Test Based on Biometric Signals to Assess Mental Fatigue at the Workplace—A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(22):11891. https://doi.org/10.3390/ijerph182211891
Chicago/Turabian StyleRamírez-Moreno, Mauricio A., Patricio Carrillo-Tijerina, Milton Osiel Candela-Leal, Myriam Alanis-Espinosa, Juan Carlos Tudón-Martínez, Armando Roman-Flores, Ricardo A. Ramírez-Mendoza, and Jorge de J. Lozoya-Santos. 2021. "Evaluation of a Fast Test Based on Biometric Signals to Assess Mental Fatigue at the Workplace—A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 22: 11891. https://doi.org/10.3390/ijerph182211891
APA StyleRamírez-Moreno, M. A., Carrillo-Tijerina, P., Candela-Leal, M. O., Alanis-Espinosa, M., Tudón-Martínez, J. C., Roman-Flores, A., Ramírez-Mendoza, R. A., & Lozoya-Santos, J. d. J. (2021). Evaluation of a Fast Test Based on Biometric Signals to Assess Mental Fatigue at the Workplace—A Pilot Study. International Journal of Environmental Research and Public Health, 18(22), 11891. https://doi.org/10.3390/ijerph182211891














