Illegal Drug Use and Risk of Hearing Loss in the United States: A National Health and Nutrition Examination Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
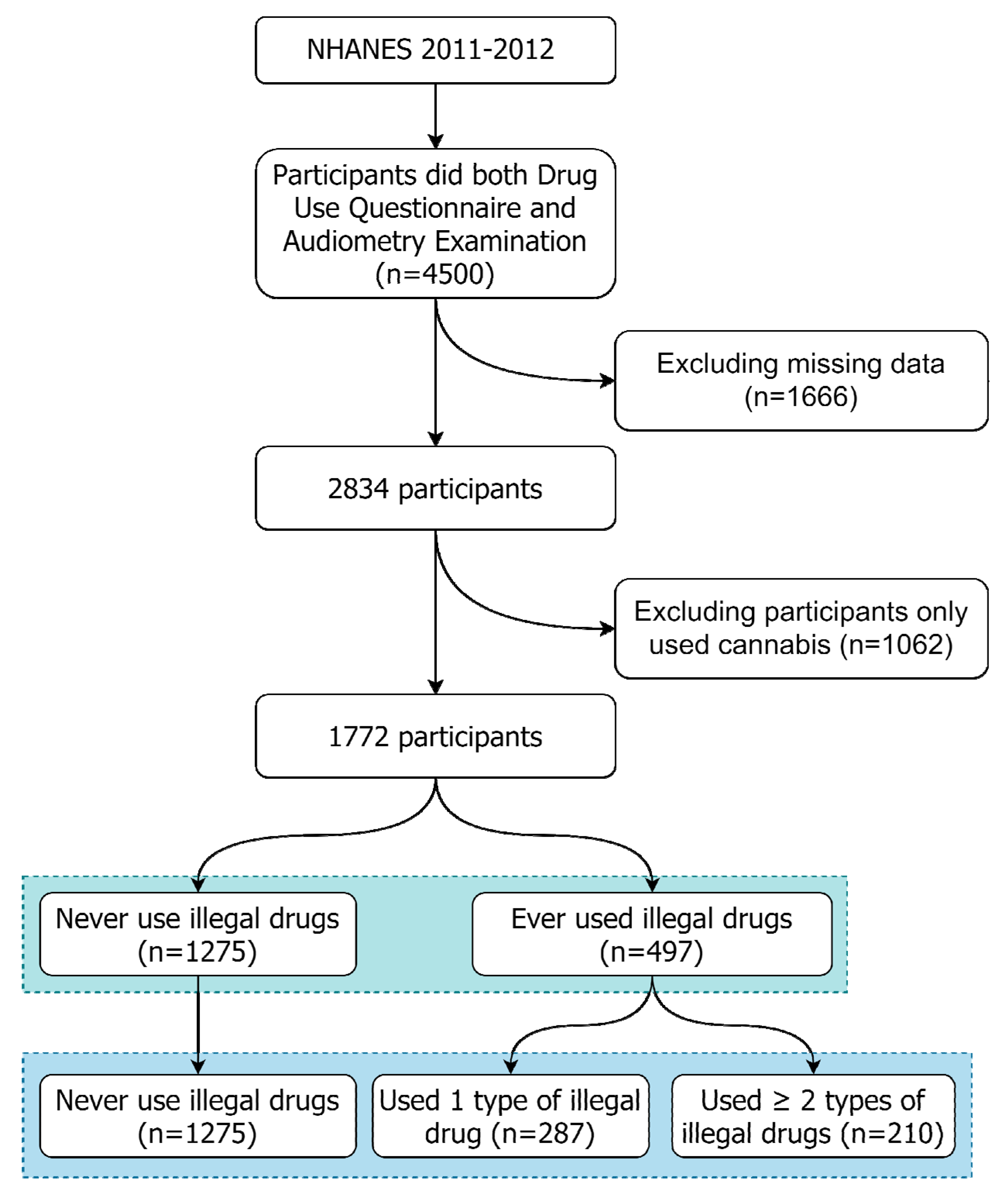
2.2. Study Sample Selection
2.3. Outcome Measurement
2.4. Covariate Measurement
2.5. Statistical Analyses
3. Results
3.1. The Demographics and Comorbidities of the Selected Participants
3.2. Prevalence, Odds Ratios, and 95% Confidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieman, C.L.; Reed, N.S.; Lin, F.R. Otolaryngology for the internist: Hearing loss. Med. Clin. N. Am. 2018, 102, 977–992. [Google Scholar] [CrossRef]
- World Health Organization Deafness and Hearing Loss. Available online: https://www.who.int/en/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 12 November 2021).
- Heffernan, E.; Coulson, N.S.; Henshaw, H.; Barry, J.G.; Ferguson, M.A. Understanding the psychosocial experiences of adults with mild-moderate hearing loss: An application of Leventhal’s self-regulatory model. Int. J. Audiol. 2016, 55, S3–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, A.; O’Loughlin, K.; Davis, A.; Kendig, H. Hearing loss and paid employment: Australian population survey findings. Int. J. Audiol. 2009, 48, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Noble, W. Assessing binaural hearing: Results using the speech, spatial and qualities of hearing scale. J. Am. Acad. Audiol. 2010, 21, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Bhattacharyya, N. Association of hearing loss with decreased employment and income among adults in the United States. Ann. Otol. Rhinol. Laryngol. 2012, 121, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Boi, R.; Racca, L.; Cavallero, A.; Carpaneto, V.; Racca, M.; Dall’ Acqua, F.; Ricchetti, M.; Santelli, A.; Odetti, P. Hearing loss and depressive symptoms in elderly patients. Geriatr. Gerontol. Int. 2012, 12, 440–445. [Google Scholar] [CrossRef]
- Goman, A.M.; Lin, F.R. Prevalence of hearing loss by severity in the United States. Am. J. Public Health 2016, 106, 1820–1822. [Google Scholar] [CrossRef]
- McKee, M.M.; Meade, M.A.; Zazove, P.; Stewart, H.J.; Jannausch, M.L.; Ilgen, M.A. The relationship between hearing loss and substance use disorders among adults in the U.S. Am. J. Prev. Med. 2019, 56, 586–590. [Google Scholar] [CrossRef]
- Maclean, J.C.; Saloner, B. The effect of public insurance expansions on substance use disorder treatment: Evidence from the affordable care act. J. Policy Anal. Manag. 2019, 38, 366–393. [Google Scholar] [CrossRef] [Green Version]
- Schulte, M.T.; Hser, Y.I. Substance use and associated health conditions throughout the lifespan. Public Health Rev. 2014. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, L.; Stockings, E.; Patton, G.; Hall, W.D.; Lynskey, M. The increasing global health priority of substance use in young people. Lancet Psychiatry 2016, 3, 251–264. [Google Scholar] [CrossRef]
- United Nations World Drug Report. 2020. Available online: https://wdr.unodc.org/wdr2020/index.html (accessed on 12 November 2021).
- Noll, G.; Wenzel, R.R.; Binggeli, C.; Corti, C.; Luscher, T.F. Role of sympathetic nervous system in hypertension and effects of cardiovascular drugs. Eur. Heart J. 1998, 19, F32–F38. [Google Scholar] [PubMed]
- Heien, M.L.; Khan, A.S.; Ariansen, J.L.; Cheer, J.F.; Phillips, P.E.; Wassum, K.M.; Wightman, R.M. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natl. Acad. Sci. USA 2005, 102, 10023–10028. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.H.; Wang, C.H.; Chen, H.C.; Li, I.H.; Cheng, C.Y.; Liu, R.S.; Huang, W.S.; Shiue, C.Y.; Ma, K.H. Investigating the effects of noise-induced hearing loss on serotonin transporters in rat brain using 4-[18F]-ADAM/small animal PET. Neuroimage 2013, 75, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Q.; Trussell, L.O. Serotonergic modulation of sensory representation in a central multisensory circuit Is pathway specific. Cell Rep. 2017, 20, 1844–1854. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A. A case of sensorineural deafness following ingestion of Ecstasy. J. Laryngol. Otol. 2001, 115, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Bovo, R.; Prosser, S.; Martini, A. Considerations on the physiopathological mechanism of inner ear damage induced by intravenous cocaine abuse: Cues from a case report. Auris Nasus Larynx 2009, 36, 213–217. [Google Scholar] [CrossRef]
- Stenner, M.; Sturmer, K.; Beutner, D.; Klussmann, J.P. Sudden bilateral sensorineural hearing loss after intravenous cocaine injection: A case report and review of the literature. Laryngoscope 2009, 119, 2441–2443. [Google Scholar] [CrossRef]
- Kao, L.T.; Li, I.H.; Wang, W.M.; Lee, H.C.; Kao, H.H.; Pan, K.T. Illicit drugs, cannabis, and psoriasis in the United States: National health and nutrition examination survey. J. Am. Acad. Dermatol. 2020, 82, 1514–1517. [Google Scholar] [CrossRef]
- Shih, J.H.; Li, I.H.; Pan, K.T.; Wang, C.H.; Chen, H.C.; Fann, L.Y.; Tseng, J.H.; Kao, L.T. Association between anemia and auditory threshold shifts in the US population: National health and nutrition examination survey. Int. J. Environ. Res. Public Health 2020, 17, 3916. [Google Scholar] [CrossRef]
- Shargorodsky, J.; Curhan, S.G.; Curhan, G.C.; Eavey, R. Change in prevalence of hearing loss in US adolescents. JAMA 2010, 304, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curhan, S.G.; Eavey, R.; Shargorodsky, J.; Curhan, G.C. Prospective study of alcohol use and hearing loss in men. Ear Heart 2011, 32, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Dawes, P.; Cruickshanks, K.J.; Moore, D.R.; Edmondson-Jones, M.; McCormack, A.; Fortnum, H.; Munro, K.J. Cigarette smoking, passive smoking, alcohol consumption, and hearing loss. J. Assoc. Res. Otolaryngol. 2014, 15, 663–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Sasaki, N.; Ogasawara, T.; Nagahama, S.; Akter, S.; Kuwahara, K.; Kochi, T.; Eguchi, M.; Kashino, I.; Murakami, T.; et al. Smoking, smoking cessation, and the risk of hearing loss: Japan epidemiology collaboration on occupational health study. Nicotine Tob. Res. 2019, 21, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.M.; Wang, M.; Stankovic, K.M.; Eavey, R.; McKenna, M.J.; Curhan, G.C.; Curhan, S.G. Cigarette smoking, smoking cessation, and risk of hearing loss in women. Am. J. Med. 2020, 133, 1180–1186. [Google Scholar] [CrossRef]
- Aulet, R.M.; Flis, D.; Sillman, J. A case of heroin induced sensorineural hearing loss. Case Rep. Otolaryngol. 2014, 2014, 962759. [Google Scholar] [CrossRef] [Green Version]
- Weich, T.M.; Tochetto, T.M.; Seligman, L. Brain stem evoked response audiometry of former drug users. Braz. J. Otorhinolaryngol. 2012, 78, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Mokhtarinejad, F.; Peyvandi, A.A.; Shadnia, S.; Peyvandi, H.; Rezvani, M.; Khoshsirat, S.; Oroei, M. Hearing status in patients with overdose of illicit drugs. Med. J. Islam. Repub. Iran 2021, 35, 56. [Google Scholar] [CrossRef]
- Gittelman, J.X.; Perkel, D.J.; Portfors, C.V. Dopamine modulates auditory responses in the inferior colliculus in a heterogeneous manner. J. Assoc. Res. Otolaryngol. 2013, 14, 719–729. [Google Scholar] [CrossRef]
- Waldhoer, M.; Bartlett, S.E.; Whistler, J.L. Opioid receptors. Annu. Rev. Biochem. 2004, 73, 953–990. [Google Scholar] [CrossRef] [Green Version]
- Robles, L.; Ruggero, M.A. Mechanics of the mammalian cochlea. Physiol. Rev. 2001, 81, 1305–1352. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Full Cohort | Numbers of Illegal Drug Use | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Illegal Drug Users (n = 497) | Nonusers (n = 1275) | p Value | Used 1 Type of Illegal Drug (n = 287) | Used ≥ 2 Types of Illegal Drugs (n = 210) | Nonusers (n = 1275) | p Value | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| Age (Mean ± SD) | 41.9 ± 10.7 | 39.2 ± 11.6 | 0.012 | 42.0 ± 11.0 | 41.8 ± 10.2 | 39.2 ± 11.6 | <0.001 | ||||||
| Sex | <0.001 | <0.001 | |||||||||||
| Male | 301 | 60.6 | 564 | 44.2 | 169 | 58.9 | 132 | 62.9 | 564 | 44.2 | |||
| Female | 196 | 39.4 | 711 | 55.8 | 118 | 41.1 | 78 | 37.1 | 711 | 55.8 | |||
| Race | <0.001 | <0.001 | |||||||||||
| Mexican American | 57 | 11.5 | 163 | 12.8 | 32 | 11.1 | 25 | 11.9 | 163 | 12.8 | |||
| Other Hispanic | 33 | 6.6 | 146 | 11.5 | 24 | 8.4 | 9 | 4.3 | 146 | 11.5 | |||
| Non-Hispanic White | 267 | 53.7 | 345 | 27.1 | 134 | 46.7 | 133 | 63.3 | 345 | 27.1 | |||
| Non-Hispanic Black | 91 | 18.3 | 312 | 24.5 | 66 | 23 | 25 | 11.9 | 312 | 24.5 | |||
| Other race | 49 | 9.9 | 309 | 24.2 | 31 | 10.8 | 18 | 8.6 | 309 | 24.2 | |||
| Hypertension a | <0.001 | <0.001 | |||||||||||
| Yes | 149 | 30 | 276 | 21.7 | 74 | 25.9 | 75 | 35.7 | 276 | 21.7 | |||
| No | 347 | 70 | 998 | 78.3 | 212 | 74.1 | 135 | 64.3 | 998 | 78.3 | |||
| Ear infection a | <0.001 | <0.001 | |||||||||||
| Yes | 142 | 29.2 | 237 | 19.1 | 79 | 28.2 | 63 | 30.6 | 237 | 19.1 | |||
| No | 344 | 70.8 | 1001 | 80.9 | 201 | 71.8 | 143 | 69.4 | 1001 | 80.9 | |||
| Diabetes a | 0.954 | 0.917 | |||||||||||
| Yes | 36 | 7.5 | 93 | 7.6 | 22 | 7.9 | 14 | 6.9 | 93 | 7.6 | |||
| No | 443 | 92.5 | 1131 | 92.4 | 255 | 92.1 | 188 | 93.1 | 1131 | 92.4 | |||
| Variables | Illegal Drug Users (n = 497) | Nonusers (n = 1275) | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Low-Frequency Hearing Loss | |||||
| Yes | 138 | 27.8 | 324 | 25.4 | |
| No | 359 | 72.2 | 951 | 74.6 | |
| Crude OR (95% CI) a | 1.13 (0.89–1.43) | Ref. | |||
| Adjusted OR (95% CI) a,b | 0.90 (0.69–1.19) | Ref. | |||
| High-Frequency Hearing Loss | |||||
| Yes | 341 | 68.6 | 720 | 56.5 | |
| No | 156 | 31.4 | 555 | 43.5 | |
| Crude OR (95% CI) a | 1.69 *** (1.35–2.10) | Ref. | |||
| Adjusted OR (95% CI) a,b | 1.32 * (1.00–1.73) | Ref. | |||
| Overall Hearing Loss | |||||
| Yes | 348 | 70 | 739 | 58 | |
| No | 149 | 30 | 536 | 42 | |
| Crude OR (95% CI) a | 1.69 *** (1.36–2.12) | Ref. | |||
| Adjusted OR (95% CI) a,b | 1.38 * (1.05–1.82) | Ref. | |||
| Variables | Illegal Drugs Users | Nonusers (n = 1275) | |||||
|---|---|---|---|---|---|---|---|
| Used 1 Type of Illegal Drug (n = 287) | Used ≥ 2 Types of Illegal Drugs (n = 210) | ||||||
| No. | % | No. | % | No. | % | ||
| Low-Frequency Hearing Loss | |||||||
| Yes | 74 | 25.8 | 64 | 30.5 | 324 | 25.4 | |
| No | 213 | 74.2 | 146 | 69.5 | 951 | 74.6 | |
| Crude OR (95% CI) a | 1.02 (0.76–1.37) | 1.29 (0.94–1.77) | Ref. | ||||
| Adjusted OR (95% CI) a,b | 0.81 (0.58–1.13) | 1.06 (0.73–1.54) | Ref. | ||||
| High-Frequency Hearing Loss | |||||||
| Yes | 190 | 66.2 | 151 | 71.9 | 720 | 56.5 | |
| No | 97 | 33.8 | 59 | 28.1 | 555 | 43.5 | |
| Crude OR (95% CI) a | 1.51 ** (1.15–1.98) | 1.97 *** (1.43–2.72) | Ref. | ||||
| Adjusted OR (95% CI) a,b | 1.18 (0.84–1.65) | 1.57 * (1.06–2.32) | Ref. | ||||
| Overall Hearing Loss | |||||||
| Yes | 195 | 67.9 | 153 | 72.9 | 739 | 58 | |
| No | 92 | 32.1 | 57 | 27.1 | 536 | 42 | |
| Crude OR (95% CI) a | 1.54 ** (1.17–2.02) | 1.95 *** (1.41–2.69) | Ref. | ||||
| Adjusted OR (95% CI) a,b | 1.26 (0.90–1.76) | 1.60 * (1.08–2.37) | Ref. | ||||
| Variables | Illegal Drugs Users | Nonusers (n = 1275) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ever Used Cocaine (n = 472) | Ever Used Heroin (n = 56) | Ever Used Methamphetamine (n = 209) | |||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Low-Frequency Hearing Loss | |||||||||
| Yes | 132 | 28 | 21 | 37.5 | 59 | 28.2 | 324 | 25.4 | |
| No | 340 | 72 | 35 | 62.5 | 150 | 71.8 | 951 | 74.6 | |
| Crude OR (95% CI) a | 1.14 (0.90–1.45) | 1.76 * (1.01–3.07) | 1.16 (0.83–1.60) | Ref. | |||||
| Adjusted OR (95% CI) a,b | 0.88 (0.67–1.17) | 1.03 (0.53–2.02) | 1.08 (0.74–1.57) | Ref. | |||||
| High-Frequency Hearing Loss | |||||||||
| Yes | 328 | 69.5 | 45 | 80.4 | 142 | 67.9 | 720 | 56.5 | |
| No | 144 | 30.5 | 11 | 19.6 | 67 | 32.1 | 555 | 43.5 | |
| Crude OR (95% CI) a | 1.76 *** (1.40–2.20) | 3.15 *** (1.62–6.15) | 1.63 ** (1.20–2.23) | Ref. | |||||
| Adjusted OR (95% CI) a,b | 1.34 * (1.01–1.77) | 1.91 (0.87–4.19) | 1.43 (0.98–2.11) | Ref. | |||||
| Overall Hearing Loss | |||||||||
| Yes | 333 | 70.6 | 46 | 82.1 | 146 | 69.9 | 739 | 58 | |
| No | 139 | 29.4 | 10 | 17.9 | 63 | 30.1 | 536 | 42 | |
| Crude OR (95% CI) a | 1.74 *** (1.39–2.18) | 3.33 *** (1.67–6.67) | 1.68 *** (1.23–2.31) | Ref. | |||||
| Adjusted OR (95% CI) a,b | 1.38 * (1.04–1.82) | 2.17 (0.97–4.84) | 1.54 * (1.05–2.27) | Ref. | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-T.; Li, I.-H.; Yang, H.-W.; Chiang, K.-W.; Wang, C.-H.; Kao, L.-T. Illegal Drug Use and Risk of Hearing Loss in the United States: A National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2021, 18, 11945. https://doi.org/10.3390/ijerph182211945
Lin P-T, Li I-H, Yang H-W, Chiang K-W, Wang C-H, Kao L-T. Illegal Drug Use and Risk of Hearing Loss in the United States: A National Health and Nutrition Examination Survey. International Journal of Environmental Research and Public Health. 2021; 18(22):11945. https://doi.org/10.3390/ijerph182211945
Chicago/Turabian StyleLin, Po-Ting, I-Hsun Li, Hui-Wen Yang, Kuan-Wei Chiang, Chih-Hung Wang, and Li-Ting Kao. 2021. "Illegal Drug Use and Risk of Hearing Loss in the United States: A National Health and Nutrition Examination Survey" International Journal of Environmental Research and Public Health 18, no. 22: 11945. https://doi.org/10.3390/ijerph182211945
APA StyleLin, P.-T., Li, I.-H., Yang, H.-W., Chiang, K.-W., Wang, C.-H., & Kao, L.-T. (2021). Illegal Drug Use and Risk of Hearing Loss in the United States: A National Health and Nutrition Examination Survey. International Journal of Environmental Research and Public Health, 18(22), 11945. https://doi.org/10.3390/ijerph182211945








