Assessment of Trace Elements Supply in Canned Tuna Fish Commercialized for Human Consumption in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tuna Fish Samples Acquisition and Preparation
2.2. Microwave-Assisted Digestion Procedure, Inductively Coupled Plasma–Optical Emission Spectrometry (ICP OES) Elemental Analysis, and Calibration Curves
2.3. Human Health Risk Assessment and Pollution Index
2.4. Statistical Analysis
3. Results
3.1. Canned Tuna Content and Intake Limits
3.2. Health Risk Assessment
4. Discussion
4.1. Canned Tuna Content and Intake Limits
4.2. Health Risk Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machate, D.J.; Melo, E.S.d.P.; Arakaki, D.G.; Guimarães, R.d.C.A.; Hiane, P.A.; Bogo, D.; Pott, A.; Nascimento, V.A.d. High Concentration of Heavy Metal and Metalloid Levels in Edible Campomanesia Adamantium Pulp from Anthropic Areas. Int. J. Environ. Res. Public Health 2021, 18, 5503. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic Exposure: A Public Health Problem Leading to Several Cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Withanachchi, S.S.; Ghambashidze, G.; Kunchulia, I.; Urushadze, T.; Ploeger, A. Water Quality in Surface Water: A Preliminary Assessment of Heavy Metal Contamination of the Mashavera River, Georgia. Int. J. Environ. Res. Public Health 2018, 15, 621. [Google Scholar] [CrossRef] [Green Version]
- Custodio, M.; Cuadrado, W.; Peñaloza, R.; Montalvo, R.; Ochoa, S.; Quispe, J. Human Risk from Exposure to Heavy Metals and Arsenic in Water from Rivers with Mining Influence in the Central Andes of Peru. Water 2020, 12, 1946. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Medeiros, C.O.; Bernardo, G.L.; Ebone, M.V.; Di Pietro, P.F.; de Assis, M.A.A.; Vasconcelos, F.d.A.G.d. Benefits and Risks of Fish Consumption for the Human Health. Rev. Nutr. 2012, 25, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Ma, X.; Xie, J. Review on Natural Preservatives for Extending Fish Shelf Life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R.d.C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; de Oliveira, L.C.S.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. In Brief: Sustainability in Action; FAO: Rome, Italy, 2020; ISBN 978-92-5-132773-9. [Google Scholar]
- Ababneh, F.A.; Al-Momani, I.F. Levels of Mercury, Cadmium, Lead and Other Selected Elements in Canned Tuna Fish Commercialised in Jordan. Int. J. Environ. Anal. Chem. 2013, 93, 755–766. [Google Scholar] [CrossRef]
- Al Ghoul, L.; Abiad, M.G.; Jammoul, A.; Matta, J.; El Darra, N. Zinc, Aluminium, Tin and Bis-Phenol a in Canned Tuna Fish Commercialized in Lebanon and Its Human Health Risk Assessment. Heliyon 2020, 6, e04995. [Google Scholar] [CrossRef]
- Alva, C.V.; Mársico, E.T.; Ribeiro, R.d.O.R.; da Carneiro, C.S.; Simões, J.S.; da Ferreira, M.S. Concentrations and Health Risk Assessment of Total Mercury in Canned Tuna Marketed in Southest Brazil. J. Food Compos. Anal. 2020, 88, 103357. [Google Scholar] [CrossRef]
- Andayesh, S.; Hadiani, M.R.; Mousavi, Z.; Shoeibi, S. Lead, Cadmium, Arsenic and Mercury in Canned Tuna Fish Marketed in Tehran, Iran. Food Addit. Contam. Part B 2015, 8, 93–98. [Google Scholar] [CrossRef]
- Boufleur, L.A.; dos Santos, C.E.I.; Debastiani, R.; Yoneama, M.L.; Amaral, L.; Dias, J.F. Elemental Characterization of Brazilian Canned Tuna Fish Using Particle Induced X-ray Emission (PIXE). J. Food Compos. Anal. 2013, 30, 19–25. [Google Scholar] [CrossRef] [Green Version]
- de Paiva, E.L.; Morgano, M.A.; Milani, R.F. Cadmium, Lead, Tin, Total Mercury, and Methylmercury in Canned Tuna Commercialised in São Paulo, Brazil. Food Addit. Contam. Part B 2017, 10, 185–191. [Google Scholar] [CrossRef]
- Idriss, A.A.; Ahmad, A.K. Heavy Metal Concentrations in Fishes from Juru River, Estimation of the Health Risk. Bull. Environ. Contam. Toxicol. 2015, 94, 204–208. [Google Scholar] [CrossRef]
- Ikem, A.; Egiebor, N.O. Assessment of Trace Elements in Canned Fishes (Mackerel, Tuna, Salmon, Sardines and Herrings) Marketed in Georgia and Alabama (United States of America). J. Food Compos. Anal. 2005, 18, 771–787. [Google Scholar] [CrossRef]
- Mol, S. Levels of Selected Trace Metals in Canned Tuna Fish Produced in Turkey. J. Food Compos. Anal. 2011, 24, 66–69. [Google Scholar] [CrossRef]
- Russo, R.; Lo Voi, A.; De Simone, A.; Serpe, F.P.; Anastasio, A.; Pepe, T.; Cacace, D.; Severino, L. Heavy Metals in Canned Tuna from Italian Markets. J. Food Prot. 2013, 76, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.V.; Sobhanardakani, S.; Tayebi, L. Heavy Metals Contamination of Canned Fish and Related Health Implications in Iran. Turk. J. Fish. Aquat. Sci. 2018, 18, 951–957. [Google Scholar]
- Tuzen, M.; Soylak, M. Determination of Trace Metals in Canned Fish Marketed in Turkey. Food Chem. 2007, 101, 1378–1382. [Google Scholar] [CrossRef]
- De Lima, N.V.; Melo, E.S.d.P.; Arakaki, D.G.; Tschinkel, P.F.S.; de Souza, I.D.; Ulbrecht, M.O.d.O.; Mendes dos Reis, F.J.; Rosa, A.C.G.; Rosa, R.H.; Aragão do Nascimento, V. Data on Metals, Nonmetal, and Metalloid in the Samples of the Canned Tuna and Canned Sardines Sold in Brazil. Data Brief 2021, 35, 106865. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, K.; Weistenhöfer, W.; Neff, F.; Hartwig, A.; van Thriel, C.; Drexler, H. The Health Effects of Aluminum Exposure. Dtsch. Arztebl. Int. 2017, 114, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Inan-Eroglu, E.; Ayaz, A. Is Aluminum Exposure a Risk Factor for Neurological Disorders? J. Res. Med. Sci 2018, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Roney, N. Toxicological Profile for Zinc; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- Wessling-Resnick, M. Excess Iron: Considerations Related to Development and Early Growth. Am. J. Clin. Nutr. 2017, 106, 1600S–1605S. [Google Scholar] [CrossRef]
- Petrović, M. Selenium: Widespread yet Scarce, Essential yet Toxic. ChemTexts 2021, 7, 11. [Google Scholar] [CrossRef]
- Peana, M.; Medici, S.; Dadar, M.; Zoroddu, M.A.; Pelucelli, A.; Chasapis, C.T.; Bjørklund, G. Environmental Barium: Potential Exposure and Health-Hazards. Arch. Toxicol. 2021, 95, 2605–2612. [Google Scholar] [CrossRef]
- Song, W.; Qi, R.; Zhao, L.; Xue, N.; Wang, L.; Yang, Y. Bacterial Community Rather than Metals Shaping Metal Resistance Genes in Water, Sediment and Biofilm in Lakes from Arid Northwestern China. Environ. Pollut. 2019, 254, 113041. [Google Scholar] [CrossRef]
- Qu, B.; Song, J.; Yuan, H.; Li, X.; Li, N.; Duan, L.; Liang, X. Historical Evolutions of Sediment Quality in Bays under Serious Anthropogenic Influences in China, Basing on Fuzzy Comprehensive Assessment of Heavy Metals. Environ. Sci. Pollut. Res. 2020, 27, 25933–25942. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization. Evaluation of Certain Food Additives and Contaminants: Fifty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization. Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2004; Volume 61, ISBN 92-4-120922-4. [Google Scholar]
- National Academies of Sciences, E.; Oria, M.; Harrison, M.; Stallings, V.A. Dietary Reference Intakes (DRIs): Tolerable Upper Intake Levels, Elements, Food and Nutrition Board, National Academies. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545442/table/appJ_tab9/ (accessed on 5 October 2020).
- WHO; JECFA Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=298 (accessed on 6 October 2020).
- World Health Organization. Barium in Drinking-Water. Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2004; Available online: https://www.who.int/water_sanitation_health/dwq/GDWQ2004web.pdf (accessed on 20 September 2021).
- World Health Organization. Principles and Methods for the Risk Assessment of Chemicals in Food; World Health Organization: Geneva, Switzerland, 2009; ISBN 92-4-157240-X. [Google Scholar]
- AOAC. Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC: Arlington, VA, USA, 2002. [Google Scholar]
- Mermet, J.-M.; Poussel, E. ICP Emission Spectrometers: 1995 Analytical Figures of Merit. Appl. Spectrosc. AS 1995, 49, 12A–18A. [Google Scholar] [CrossRef]
- FDA, E. 06/09/2014: FDA and EPA Issue Updated Draft Advice for Fish Consumption/Advice Encourages Pregnant Women and Breastfeeding Mothers to Eat More Fish That Are Lower in Mercury. Available online: https://archive.epa.gov/epapages/newsroom_archive/newsreleases/b8edc480d8cfe29b85257cf20065f826.html (accessed on 17 August 2021).
- Means, B. Risk-Assessment Guidance for Superfund. Volume 1. Human Health Evaluation Manual. Part A. Interim Report (Final); Environmental Protection Agency: Washington, DC, USA, 1989.
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- USEPA. Integrated Risk Information System. Available online: https://www.epa.gov/iris (accessed on 13 February 2021).
- USEPA. Guidance Manual for Assessing Human Health Risks from Chemically Contaminated, Fish and Shellfish; USEPA: Washington, DC, USA, 1989.
- Adebiyi, F.M.; Ore, O.T.; Ogunjimi, I.O. Evaluation of Human Health Risk Assessment of Potential Toxic Metals in Commonly Consumed Crayfish (Palaemon Hastatus) in Nigeria. Heliyon 2020, 6, e03092. [Google Scholar] [CrossRef] [Green Version]
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood Consumption and Components for Health. Glob. J. Health Sci. 2012, 4, 72–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations. Codex Nutrient Reference Values: Especially for Vitamins, Minerals and Protein; Food & Agriculture ORG: Rome, Italy, 2019; ISBN 978-92-5-131957-4. [Google Scholar]
- USEPA. Program Information about the Integrated Risk Information System: Chronic Oral Reference Dose (RfD). Available online: https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments (accessed on 11 September 2021).
- Liang, Y.; Yi, X.; Dang, Z.; Wang, Q.; Luo, H.; Tang, J. Heavy Metal Contamination and Health Risk Assessment in the Vicinity of a Tailing Pond in Guangdong, China. Int. J. Environ. Res. Public Health 2017, 14, 1557. [Google Scholar] [CrossRef] [Green Version]
- ANVISA. Resolução RDC No. 42 de 29 de Agosto de 2013, Dispõe Sobre o Regulamento Técnico MERCOSUL Sobre Limites Máximos de Contaminantes Inorgânicos Em Alimentos. 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2013/rdc0042_29_08_2013.html#:~:text=Disp%C3%B5e%20sobre%20o%20Regulamento%20T%C3%A9cnico,III%20e%20IV%2C%20do%20art (accessed on 19 August 2021).
- Joint FAO/WHO. WHO Food Standards Programme Codex Committee on Contaminants in Foods. 2011. Available online: https://www.fao.org/fao-who-codexalimentarius/committees/committee/en/?committee=CCCF (accessed on 20 September 2021).
- NRS National Residue Survey (NRS) Annual Report 2010–11. Available online: https://nla.gov.au/nla.obj-763108559 (accessed on 30 August 2021).
- European Comission. Commission Regulation (EC) No 1881/2006. Off. J. Eur. Union 2006, 49, 5–24. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 20 September 2021).
- Türkmen, A.; Türkmen, M.; Tepe, Y.; Akyurt, İ. Heavy Metals in Three Commercially Valuable Fish Species from İskenderun Bay, Northern East Mediterranean Sea, Turkey. Food Chem. 2005, 91, 167–172. [Google Scholar] [CrossRef]
- Dordevic, D.; Buchtova, H.; Jancikova, S.; Macharackova, B.; Jarosova, M.; Vitez, T.; Kushkevych, I. Aluminum Contamination of Food during Culinary Preparation: Case Study with Aluminum Foil and Consumers’ Preferences. Food Sci. Nutr. 2019, 7, 3349–3360. [Google Scholar] [CrossRef]
- Bondy, S.C. Prolonged Exposure to Low Levels of Aluminum Leads to Changes Associated with Brain Aging and Neurodegeneration. Toxicology 2014, 315, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Falk, S.; Rohrbeck, A.; Georgii, S.; Herzog, C.; Wiegand, A.; Hotz, S.; Boschek, B.; Zorn, H.; Brunn, H. Migration of Aluminum from Food Contact Materials to Food—a Health Risk for Consumers? Part I of III: Exposure to Aluminum, Release of Aluminum, Tolerable Weekly Intake (TWI), Toxicological Effects of Aluminum, Study Design, and Methods. Environ. Sci. Eur. 2017, 29, 19. [Google Scholar] [CrossRef]
- Ciminelli, V.S.T.; Gasparon, M.; Ng, J.C.; Silva, G.C.; Caldeira, C.L. Dietary Arsenic Exposure in Brazil: The Contribution of Rice and Beans. Chemosphere 2017, 168, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, D.H.; Lee, Y.S.; Cho, S.-Y.; Chung, M.-S.; Cho, M.; Kang, Y.; Kim, H.; Kim, D.; Lee, K.-W. Monitoring of Arsenic Contents in Domestic Rice and Human Risk Assessment for Daily Intake of Inorganic Arsenic in Korea. J. Food Compos. Anal. 2018, 69, 25–32. [Google Scholar] [CrossRef]
- Roya, A.Q.; Ali, M.S. Heavy Metals in Rice Samples on the Torbat-Heidarieh Market, Iran. Food Addit. Contam. Part B 2017, 10, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Bolger, P.M. Dietary Arsenic Intakes in the United States: FDA Total Diet Study, September 1991–December 1996. Food Addit. Contam. 2010, 16, 465–472. [Google Scholar] [CrossRef]
- Huq, S.M.I.; Joardar, J.C.; Parvin, S.; Correll, R.; Naidu, R. Arsenic Contamination in Food-Chain: Transfer of Arsenic into Food Materials through Groundwater Irrigation. J. Health Popul. Nutr. 2006, 24, 305–316. [Google Scholar]
- Santra, S.C.; Samal, A.C.; Bhattacharya, P.; Banerjee, S.; Biswas, A.; Majumdar, J. Arsenic in Foodchain and Community Health Risk: A Study in Gangetic West Bengal. Procedia Environ. Sci. 2013, 18, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Shanker, U. Shikha Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, e304524. [Google Scholar] [CrossRef]
- Pearson, A.J.; Ashmore, E. Risk Assessment of Antimony, Barium, Beryllium, Boron, Bromine, Lithium, Nickel, Strontium, Thallium and Uranium Concentrations in the New Zealand Diet. Food Addit. Contam. Part A 2020, 37, 451–464. [Google Scholar] [CrossRef]
- Montanari, A. Inorganic Contaminants of Food as a Function of Packaging Features. In Food Packaging Hygiene; Barone, C., Bolzoni, L., Caruso, G., Montanari, A., Parisi, S., Steinka, I., Eds.; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–41. ISBN 978-3-319-14827-4. [Google Scholar]
- European Commission. Commission Regulation (EU) No. 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off J. Eur. Union 2011, L12, 1–89. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:0089:en:PDF (accessed on 20 September 2021).
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, A.; Khaled, A. Determination of Metals in Tuna Species and Bivalves from Alexandria, Egypt. Egypt J. Aquat. Res. 2014, 40, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Gonoodi, K.; Moslem, A.; Darroudi, S.; Ahmadnezhad, M.; Mazloum, Z.; Tayefi, M.; Zadeh, S.A.T.; Eslami, S.; Shafiee, M.; Khashayarmanesh, Z.; et al. Serum and Dietary Zinc and Copper in Iranian Girls. Clin. Biochem. 2018, 54, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary Copper and Human Health: Current Evidence and Unresolved Issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Templeton, D.M.; O’Brien, P.J. Mitochondrial Involvement in Genetically Determined Transition Metal Toxicity: II. Copper Toxicity. Chem. Biol. Interact. 2006, 163, 77–85. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper-2 Ingestion, Plus Increased Meat Eating Leading to Increased Copper Absorption, Are Major Factors Behind the Current Epidemic of Alzheimer’s Disease. Nutrients 2015, 7, 10053–10064. [Google Scholar] [CrossRef] [Green Version]
- De Romaña, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and Benefits of Copper in Light of New Insights of Copper Homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef]
- Rahmani, J.; Fakhri, Y.; Shahsavani, A.; Bahmani, Z.; Urbina, M.A.; Chirumbolo, S.; Keramati, H.; Moradi, B.; Bay, A.; Bjørklund, G. A Systematic Review and Meta-Analysis of Metal Concentrations in Canned Tuna Fish in Iran and Human Health Risk Assessment. Food Chem. Toxicol. 2018, 118, 753–765. [Google Scholar] [CrossRef]
- Girolametti, F.; Annibaldi, A.; Carnevali, O.; Pignalosa, P.; Illuminati, S.; Truzzi, C. Potential Toxic Elements (PTEs) in Wild and Farmed Atlantic Bluefin Tuna (Thunnus Thynnus) from Mediterranean Sea: Risks and Benefits for Human Consumption. Food Control 2021, 125, 108012. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Pasricha, S.-R.; Low, M.; Thompson, J.; Farrell, A.; De-Regil, L.-M. Iron Supplementation Benefits Physical Performance in Women of Reproductive Age: A Systematic Review and Meta-Analysis. J. Nutr. 2014, 144, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996; ISBN 92-4-156173-4. [Google Scholar]
- Rayman, M.P. Selenium Intake, Status, and Health: A Complex Relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranges, S.; Marshall, J.; Natarajan, R.; Donahue, R.; Trevisan, M.; Combs, G.; Cappuccio, F.; Ceriello, A.; Reid, M. Effects of Long-Term Selenium Supplementation on the Incidence of Type 2 Diabetes: A Randomized Trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Behne, D.; Weiler, H.; Kyriakopoulos, A. Effects of Selenium Deficiency on Testicular Morphology and Function in Rats. Reproduction 1996, 106, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.E.; Zhou, J.; McMahan, W.J.; Motley, A.K.; Burk, R.F. Neurological Dysfunction Occurs in Mice with Targeted Deletion of the Selenoprotein P Gene. J. Nutr. 2004, 134, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Vézina, D.; Mauffette, F.; Roberts, K.D.; Bleau, G. Selenium-Vitamin E Supplementation in Infertile Men. Biol. Trace Elem. Res. 1996, 53, 65–83. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Han, J.-L.; Pan, X.-D.; Chen, Q.; Huang, B.-F. Health Risk Assessment of Heavy Metals in Marine Fish to the Population in Zhejiang, China. Sci. Rep. 2021, 11, 11079. [Google Scholar] [CrossRef]
- Ashraf, W. Levels of Selected Heavy Metals in Tuna Fish. Arab. J. Sci. Eng. 2006, 31, 89. [Google Scholar]
- Eriksson, B.K. Long-Term Changes in Macroalgal Vegetation on the Swedish Coast: An Evaluation of Eutrophication Effects with Special Emphasis on Increased Organic Sedimentation. Ph.D. Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2002. [Google Scholar]
- Kojadinovic, J.; Potier, M.; Le Corre, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of Trace Elements in Pelagic Fish from the Western Indian Ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, W.A.; Saleh, Y.S.; Marie, M.-A.S. Integrating Multiple Fish Biomarkers and Risk Assessment as Indicators of Metal Pollution along the Red Sea Coast of Hodeida, Yemen Republic. Ecotoxicol. Environ. Saf. 2014, 110, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hydes, D.J. Dissolved Aluminium Concentration in Sea Water. Nature 1977, 268, 136–137. [Google Scholar] [CrossRef]
- Hydes, D.J. Aluminum in Seawater: Control by Inorganic Processes. Science 1979, 205, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Simal-Gándara, J. Selection of Can Coatings for Different Applications. Food Rev. Int. 1999, 15, 121–137. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Grabarić, Z.; Pezzani, A.; Squitieri, G.; Fasanaro, G.; Impembo, M. Corrosion Behaviour of Tinplate Cans in Contact with Tomato Purée and Protective (Inhibiting) Substances. Food Addit. Contam. Part A 2009, 26, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Lemly, A.D. Guidelines for Evaluating Selenium Data from Aquatic Monitoring and Assessment Studies. Environ. Monit. Assess. 1993, 28, 83–100. [Google Scholar] [CrossRef]
- Hamilton, S.J. Review of Selenium Toxicity in the Aquatic Food Chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Neff, J.M. Ecotoxicology of Arsenic in the Marine Environment. Environ. Toxicol. Chem. 1997, 16, 917–927. [Google Scholar] [CrossRef]
- UN. The Second World Ocean Assessment. Available online: https://www.un.org/regularprocess/woa2launch (accessed on 2 September 2021).
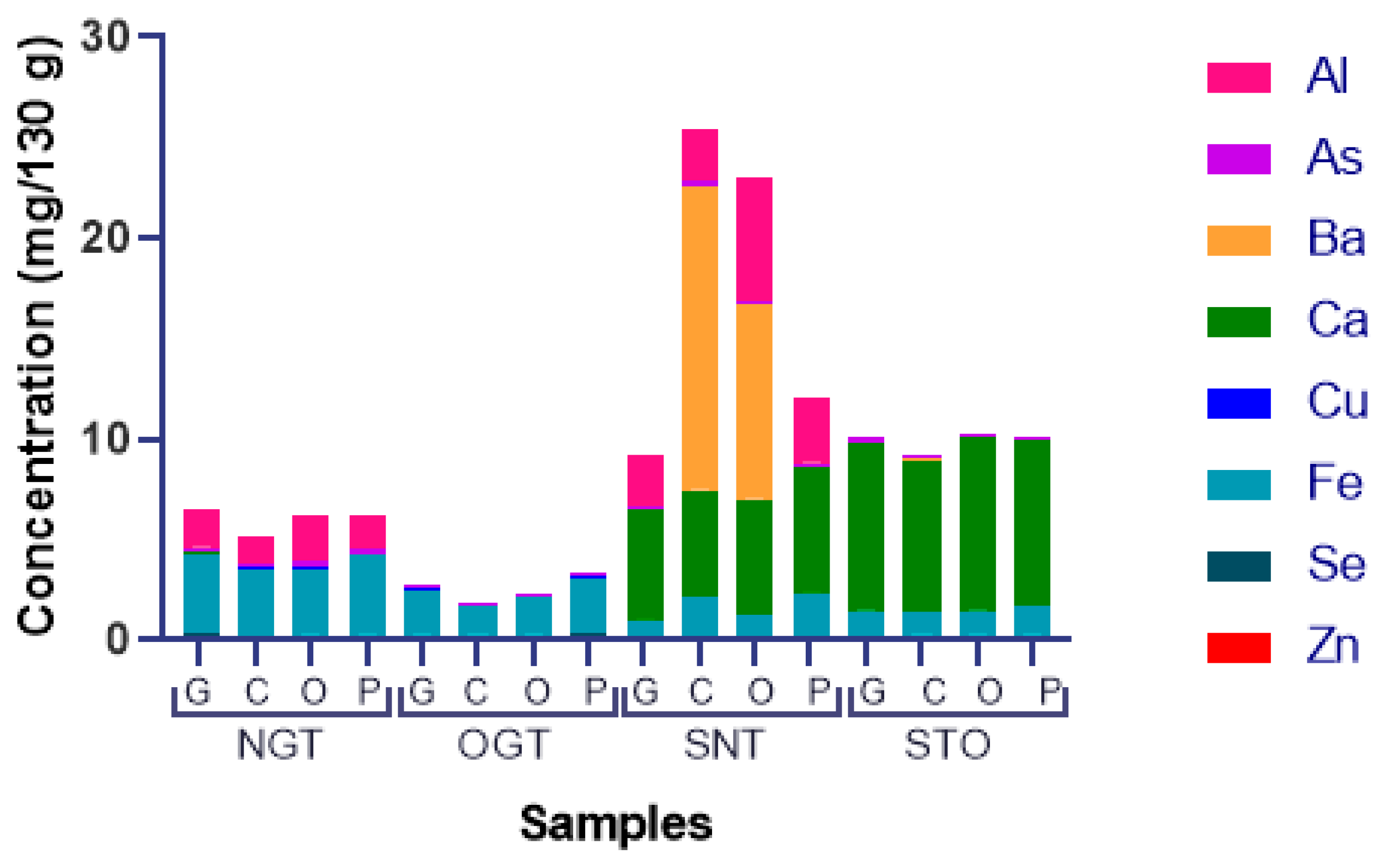

| Element | Natural Grated Tuna—NGT (mg/130 g) | Oil Grated Tuna—OGT (mg/130 g) | Solid Natural Tuna—SNT (mg/130 g) | Solid Oil Tuna—STO (mg/130 g) | Reference for 70 kg Adults (mg/day) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NGT-G | NGT-C | NGT-O | NGT-P | OGT-G | OGT-C | OGT-O | OGT-P | SNT-G | SNT-C | SNT-O | SNT-P | STO-G | STO-C | STO-O | STO-P | ||
| Al | 1.9 ± 0.4 | 1.3 ± 0.003 | 2.4 ± 0.4 | 1.7 ± 0.3 | <LOD | <LOD | <LOD | <LOD | 2.6 ± 0.03 | 2.7 ± 0.4 | 6.1 ± 0.4 | 3.3 ± 0.5 | <LOD | <LOD | <LOD | 0.00065 ± 1.3 × 10−5 | 19.95 [37] |
| As | 0.3 ± 0.01 | 0.2 ± 0.007 | 0.2 ± 0.004 | 0.2 ± 0.01 | 0.2 ± 0.001 | 0.2 ± 0.01 | 0.2 ± 0.006 | 0.2 ± 0.03 | 0.2 ± 0.002 | 0.2 ± 0.01 | 0.2 ± 0.003 | 0.2 ± 0.006 | 0.2 ± 0.006 | 0.2 ± 0.001 | 0.2 ± 0.01 | 0.2 ± 0.02 | 0.147 [39] |
| Ba | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.004 ± 0.02 | 0.002 ± 0.0003 | <LOD | 15 ± 4 | 10 ± 4.9 | <LOD | 0.008 ± 0.003 | 0.03 ± 0.007 | 0.08 ± 0.03 | 0.02 ± 0.004 | 1.4 [38] |
| Ca | 0.07 ± 0.03 | 0.01 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.006 | <LOD | <LOD | <LOD | 0.04 ± 0.005 | 5.4 ± 0.2 | 5.2 ± 0.03 | 5.8 ± 0.1 | 6.2 ± 0.04 | 9 ± 0.2 | 8.2 ± 0.4 | 9.2 ± 2.6 | 8.9 ± 0.4 | 2000–2500 [36] |
| Cu | 0.08 ± 0.004 | 0.07 ± 0.03 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.03 ± 0.005 | 0.04 ± 0.006 | 0.1 ± 0.013 | <LOD | 0.008 ± 0.002 | <LOD | 0.02 ± 0.004 | 0.04 ± 0.004 | 0.02 ± 0.003 | 0.03 ± 0.005 | 0.03 ± 0.004 | 3 [35] |
| Fe | 3.9 ± 0.5 | 3.3 ± 0.03 | 3.3 ± 0.06 | 4 ± 0.2 | 2.3 ± 0.6 | 1.4 ± 0.1 | 1.8 ± 0.7 | 2.7 ± 0.4 | 1 ± 0.059 | 2 ± 0.04 | 1 ± 0.06 | 2.2 ± 0.2 | 1.2 ± 0.02 | 1.1 ± 0.04 | 1 ± 0.009 | 1.4 ± 0.106 | 17 [34] |
| Se | 0.3 ± 0.02 | 0.2 ± 0.003 | 0.3 ± 0.005 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.05 | 0.3 ± 0.03 | 0.2 ± 0.001 | 0.2 ± 0.01 | 0.2 ± 0.001 | 0.2 ± 0.005 | 0.2 ± 0.01 | 0.2 ± 0.005 | 0.26 ± 0.01 | 0.3 ± 0.02 | 0.04 [36] |
| Zn | 0.05 ± 0.005 | 0.02 ± 0.0003 | 0.04 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.01 ± 0.001 | 0.03 ± 0.004 | 0.05 ± 0.006 | 0.01 ± 4 × 10−6 | 0.02 ± 0.001 | 0.01 ± 0.0006 | 0.02 ± 0.001 | 0.02 ± 0.0002 | 0.01 ± 0.001 | 0.02 ± 0.002 | 0.013 ± 0.001 | 70 [35] |
| Sample | Cancer Risk Arsenic (As) | ||
|---|---|---|---|
| 8 Years Old | 18 Years Old | 30 Years Old | |
| NGT-G | 0.0062 | 0.0026 | 0.0023 |
| NGT-C | 0.0051 | 0.0022 | 0.0019 |
| NGT-O | 0.0053 | 0.0022 | 0.0020 |
| NGT-P | 0.0047 | 0.0020 | 0.0018 |
| OGT-G | 0.0057 | 0.0024 | 0.0013 |
| OGT-C | 0.0054 | 0.0023 | 0.0020 |
| OGT-O | 0.0044 | 0.0019 | 0.0016 |
| OGT-P | 0.0052 | 0.0022 | 0.0019 |
| SNT-G | 0.0043 | 0.0018 | 0.0016 |
| SNT-C | 0.0049 | 0.0021 | 0.0018 |
| SNT-O | 0.0041 | 0.0017 | 0.0015 |
| SNT-P | 0.0054 | 0.0022 | 0.0012 |
| STO-G | 0.0060 | 0.0025 | 0.0022 |
| STO-C | 0.0054 | 0.0023 | 0.0020 |
| STO-O | 0.0056 | 0.0024 | 0.0021 |
| STO-P | 0.0060 | 0.0025 | 0.0022 |
| Age (Years) | Samples | HQ | HI | Samples | HQ | HI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | As | Ba | Cu | Fe | Se | Zn | Al | As | Ba | Cu | Fe | Se | Zn | |||||
| 8 | NGT-G | 0.03 | 13.84 | 0.00 | 0.03 | 0.09 | 1.11 | 0.003 | 15.11 | SNT-G | 0.04 | 9.47 | 0.00 | 0.00 | 0.02 | 0.54 | 0.0007 | 10.08 |
| NGT-C | 0.02 | 11.48 | 0.00 | 0.03 | 0.08 | 0.74 | 0.001 | 12.35 | SNT-C | 0.04 | 10.96 | 1.25 | 0.003 | 0.05 | 0.62 | 0.001 | 11.91 | |
| NGT-O | 0.04 | 11.70 | 0.00 | 0.04 | 0.08 | 0.85 | 0.002 | 12.75 | SNT-O | 0.10 | 9.10 | 0.80 | 0.00 | 0.03 | 0.63 | 0.0006 | 10.64 | |
| NGT-P | 0.03 | 10.50 | 0.00 | 0.02 | 0.09 | 0.81 | 0.002 | 11.44 | SNT-P | 0.05 | 11.90 | 0.00 | 0.007 | 0.05 | 0.54 | 0.0009 | 12.54 | |
| OGT-G | 0.00 | 12.70 | 0.00 | 0.02 | 0.05 | 0.74 | 0.002 | 13.52 | STO-G | 0.00 | 13.36 | 0.0006 | 0.018 | 0.03 | 0.66 | 0.001 | 14.05 | |
| OGT-C | 0.00 | 12.06 | 0.00 | 0.01 | 0.03 | 0.83 | 0.0008 | 12.93 | STO-C | 0.00 | 11.99 | 0.003 | 0.01 | 0.03 | 0.85 | 0.0008 | 12.88 | |
| OGT-O | 0.00 | 9.82 | 0.0003 | 0.02 | 0.04 | 0.87 | 0.001 | 10.75 | STO-O | 0.00 | 12.51 | 0.001 | 0.01 | 0.03 | 0.85 | 0.0009 | 13.40 | |
| OGT-P | 0.00 | 11.47 | 0.0001 | 0.04 | 0.07 | 0.84 | 0.003 | 12.41 | STO-P | 0.00001 | 13.23 | 0.001 | 0.01 | 0.03 | 0.88 | 0.0007 | 14.19 | |
| 18 | NGT-G | 0.01 | 5.80 | 0.00 | 0.01 | 0.04 | 0.47 | 0.001 | 6.33 | SNT-G | 0.02 | 3.97 | 0.00 | 0.00 | 0.01 | 0.23 | 0.0003 | 4.23 |
| NGT-C | 0.01 | 4.82 | 0.00 | 0.01 | 0.03 | 0.31 | 0.0005 | 5.18 | SNT-C | 0.02 | 4.60 | 0.52 | 0.001 | 0.02 | 0.26 | 0.0005 | 5.41 | |
| NGT-O | 0.02 | 4.90 | 0.00 | 0.01 | 0.03 | 0.36 | 0.0008 | 5.30 | SNT-O | 0.04 | 3.82 | 0.33 | 0.00 | 0.01 | 0.26 | 0.0003 | 4.46 | |
| NGT-P | 0.01 | 4.40 | 0.00 | 0.006 | 0.04 | 0.34 | 0.0007 | 4.80 | SNT-P | 0.02 | 4.99 | 0.00 | 0.003 | 0.021 | 0.23 | 0.0004 | 5.26 | |
| OGT-G | 0.00 | 5.33 | 0.00 | 0.01 | 0.02 | 0.31 | 0.0007 | 5.67 | STO-G | 0.00 | 5.60 | 0.0003 | 0.008 | 0.012 | 0.28 | 0.0005 | 5.90 | |
| OGT-C | 0.00 | 5.06 | 0.00 | 0.01 | 0.01 | 0.35 | 0.0003 | 5.42 | STO-C | 0.00 | 5.03 | 0.0010 | 0.004 | 0.01 | 0.36 | 0.0003 | 5.40 | |
| OGT-O | 0.00 | 4.12 | 0.0001 | 0.01 | 0.02 | 0.37 | 0.0006 | 4.50 | STO-O | 0.00 | 5.25 | 0.0006 | 0.004 | 0.01 | 0.36 | 0.0004 | 5.60 | |
| OGT-P | 0.00 | 4.81 | 0.00006 | 0.02 | 0.027 | 0.35 | 0.001 | 5.21 | STO-P | 0.000004 | 5.57 | 0.0006 | 0.0047 | 0.0140 | 0.37 | 0.0003 | 5.95 | |
| 30 | NGT-G | 0.01 | 5.14 | 0.00 | 0.01 | 0.03 | 0.41 | 0.0011 | 5.61 | SNT-G | 0.01 | 3.51 | 0.00 | 0.00 | 0.01 | 0.20 | 0.0003 | 3.74 |
| NGT-C | 0.01 | 4.27 | 0.00 | 0.01 | 0.03 | 0.27 | 0.0004 | 4.58 | SNT-C | 0.02 | 4.07 | 0.46 | 0.0013 | 0.02 | 0.23 | 0.0004 | 4.80 | |
| NGT-O | 0.01 | 4.34 | 0.00 | 0.01 | 0.03 | 0.31 | 0.0007 | 4.71 | SNT-O | 0.04 | 3.38 | 0.29 | 0.00 | 0.01 | 0.23 | 0.0002 | 3.96 | |
| NGT-P | 0.01 | 3.90 | 0.00 | 0.01 | 0.03 | 0.30 | 0.0007 | 4.24 | SNT-P | 0.01 | 2.65 | 0.00 | 0.002 | 0.01 | 0.12 | 0.0002 | 2.79 | |
| OGT-G | 0.00 | 2.83 | 0.00 | 0.01 | 0.01 | 0.17 | 0.0004 | 3.01 | STO-G | 0.00 | 4.96 | 0.0002 | 0.007 | 0.01 | 0.24 | 0.0004 | 5.22 | |
| OGT-C | 0.00 | 4.48 | 0.00 | 0.004 | 0.01 | 0.31 | 0.0003 | 4.80 | STO-C | 0.00 | 4.45 | 0.0009 | 0.003 | 0.01 | 0.32 | 0.0003 | 4.78 | |
| OGT-O | 0.00 | 3.65 | 0.00012 | 0.006 | 0.02 | 0.32 | 0.0005 | 3.99 | STO-O | 0.00 | 4.65 | 0.0005 | 0.004 | 0.01 | 0.32 | 0.0003 | 4.98 | |
| OGT-P | 0.00 | 4.26 | 0.00005 | 0.01 | 0.02 | 0.31 | 0.001 | 4.61 | STO-P | 0.000004 | 4.93 | 0.0005 | 0.004 | 0.01 | 0.32 | 0.0003 | 4.65 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, N.V.; Granja Arakaki, D.; Melo, E.S.d.P.; Machate, D.J.; do Nascimento, V.A. Assessment of Trace Elements Supply in Canned Tuna Fish Commercialized for Human Consumption in Brazil. Int. J. Environ. Res. Public Health 2021, 18, 12002. https://doi.org/10.3390/ijerph182212002
de Lima NV, Granja Arakaki D, Melo ESdP, Machate DJ, do Nascimento VA. Assessment of Trace Elements Supply in Canned Tuna Fish Commercialized for Human Consumption in Brazil. International Journal of Environmental Research and Public Health. 2021; 18(22):12002. https://doi.org/10.3390/ijerph182212002
Chicago/Turabian Stylede Lima, Nayara Vieira, Daniela Granja Arakaki, Elaine Silva de Pádua Melo, David Johane Machate, and Valter Aragão do Nascimento. 2021. "Assessment of Trace Elements Supply in Canned Tuna Fish Commercialized for Human Consumption in Brazil" International Journal of Environmental Research and Public Health 18, no. 22: 12002. https://doi.org/10.3390/ijerph182212002






